Contents
Introduction
The role of chemokine networks in cancer
Atypical chemokine receptors
Conclusion and future perspectives
Introduction
Chemokines are small heparin-binding proteins (∼8-
to 17-kDa long) with multiple activities. Since their main function
is to regulate the migration of cells, particularly leukocytes, to
the sites of inflammation, they are known as chemoattractant
cytokines. Chemokine biological activities are regulated at several
levels and their production can be constitutive or induced by
environmental stimuli. Based on this, chemokines are subdivided
into homeostatic and inflammatory subsets, with constitutive
chemokines usually regulating the homeostatic trafficking of
leukocytes and lymphocyte recirculation under normal or steady
state conditions, while inflammatory chemokines are produced in
response to inflammatory and immune stimuli and direct leukocytes
to inflamed peripheral tissues (1).
Chemokines are differentially produced in particular tissues either
constitutively or after an appropriate stimulus and attract
receptor-bearing cells to these locations. It is thought that this
process occurs by forming extracellular chemotactic or haptotactic
gradients depending on whether they are in solution or bound to
extracellular matrix components, respectively (2). Previously, the roles of chemokines and
chemokine receptors were identified: i) during leukocyte migration,
by acting on firm adhesion, locomotion, diapedesis and chemotaxis;
ii) during development, through the regulation of hematopoiesis,
cardiogenesis as well as vascular and cerebellar development; and
iii) during tumor biology, by controlling angiogenesis, metastasis
and cell proliferation (3).
Chemokine structure (Fig. 1) comprises an N-terminal loop
region, three-strand antiparallel β-sheets forming the typical core
fold of the chemokines and a C-terminal α helix which overlays the
β-sheet. The major receptor-binding site is the N-loop region that
follows the first two cysteines and connects the N-terminus to the
β-sheet region, with the sequence therein conferring receptor
specificity (4). Based on the
variations in the configuration of a conserved amino- proximal
cysteine-containing motif, chemokines are divided into four
subfamilies, known as CC, CXC, XC and CX3C (where X is any amino
acid). Generally, chemokines belonging to the CC family act
primarily on monocytes, although they are also capable of
attracting lymphocytes, basophils and eosinophils, whereas CXC
chemokines attract neutrophils and lymphocytes. Additionally, XC
and CX3C chemokines act on lymphocytes only and on
monocytes/lymphocytes, respectively.
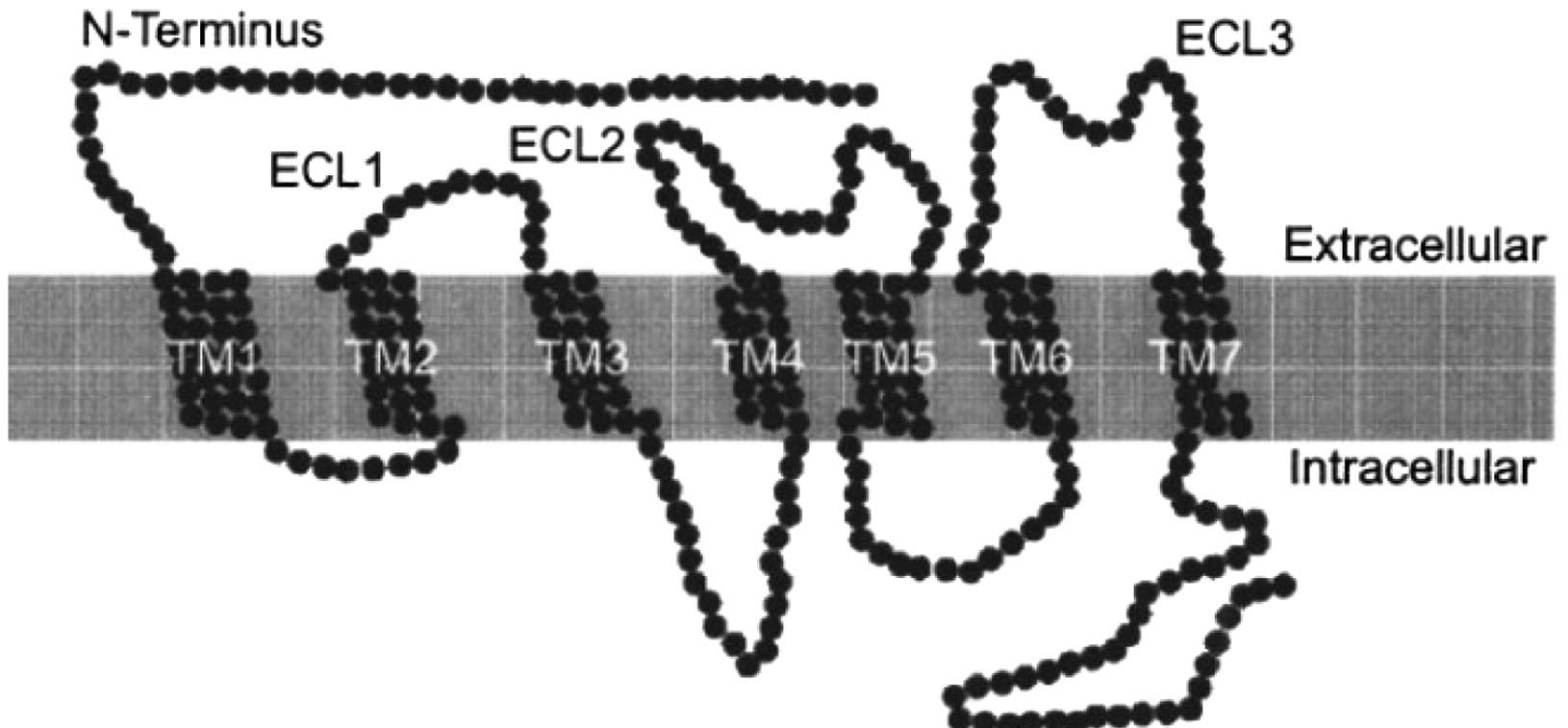
Chemokine receptors are known to be embedded in the
lipid bilayer of the cell surface and also to possess
seven-transmembrane domains (TM) (Fig.
2) (5). Chemokines bind to the
seven-TM spanning G-protein-coupled receptors (GPCRs) to exert
their actions and the receptors are classified according to the
chemokines they bind. There are eleven CCRs (receptors for CC
chemokines) and seven CXCRs (receptors for CXC chemokines) in
addition to XCR1 and CX3CR1. Some of these receptors, including
CXCR2, CCR1 and CCR3, are highly promiscuous and are crucial during
inflammation, while others such as CXCR4, CCR7 and CCR9, are more
selective and perform critical homeostatic functions (6). These chemokine receptors are located
on the surface of leukocytes and other cell types. Moreover, they
sense the extracellular chemokine environment and transmit signals
to change cell behaviour. Chemokine responsiveness is generally
determined by the expression of these receptors, converting induced
or constitutive tissue chemokine expression into appropriate
biological responses (6). A typical
receptor specifically binds to its ligand leading to typical
signalling pathways. Following the chemokine-driven activation of
chemokine receptors, these receptors initiate a wide range of
intracellular signalling cascades, including those involving
G-proteins, phosphoinositide-3-kinases, MAP kinases and small
GTPases (7) and typically lead to
cell polarisation and directed motility.
However, certain chemokines are subject to natural
modulation of their concentrations by proteins to which they bind
without leading to typical signalling (8). These proteins may be endogenously
encoded or expressed by exogenous sources to modulate chemokine
functions. These ‘scavenger’ or ‘decoy’ proteins act as
‘interceptors’ (intercepting receptors) that neutralize the action
of the chemokine or by transporting chemokines across endothelial
barriers. These decoy receptors, which bind ligands with high
affinity but do not elicit signal transduction, include D6, Duffy
antigen receptor for chemokines (DARC), ChemoCentryx chemokine
receptor (CCX-CKR) and CXCR7 (9).
Chemokines and chemokine receptors play many key
roles in physiological and pathological activities in infectious
and inflammatory diseases, in the modulation of angiogenesis, in
tumor growth as well as stem cell proliferation and have been
widely reported to participate in the process of malignant
progression (10–13). Cancer cells have been found to
produce chemokines and chemokine receptors which are able to
respond specifically to these chemokines, thus forming a complex
chemokine network which is involved in influencing tumor cell
survival, spreading and growth (14).
The role of chemokine networks in
cancer
Although the major function of chemokines is the
coordination of leukocyte recruitment, their involvement is not
limited to inflammation and immunity. They are produced by
different cell types, including tumor cells, and mediate other
biological activities, including the regulation of cell
differentiation, proliferation, survival and senescence. The
expression of chemokines and their receptors is relevant in several
types of human pathologies, including cancer, since the
identification of CCL2 in culture supernatants of tumor cell lines
(15). In cancer biology, their
roles expand from the regulation of leukocyte attraction within the
tumor mass to the promotion of tumor cell survival, proliferation
and dissemination. Tumors are major producers of chemokines and are
an invaluable source for chemokine identification and
characterization.
Although inflammation ensures effective host defence
and tissue repair, chronic inflammation has been connected to the
development of cancer in mice and humans (16,17).
Established tumors develop the mechanisms to autonomously dictate
the nature of their inflammatory infiltrate, harnessing
pro-tumorigenic leukocyte activity to help tumor survival and
progression. In the tumor microenvironment, chemokines are crucial
regulators of the levels of tumor-infiltrating leukocytes,
especially of macrophages. Strong evidence has suggested that
tumor-associated macrophages may promote tumor progression by
releasing angiogenic factors, proteolytic enzymes and
immunosuppressive molecules, which would enhance tumor growth,
dissemination and evasion from immune control. Preneoplastic to
neoplastic transformation, tumor growth, invasion as well as
metastases depend on the establishment of a proangiogenic
environment (18). Angiogenesis is
a crucial step in tumor growth and progression. Net angiogenesis in
the local microenvironment is determined by the imbalance in the
overexpression of angiogenic factors, as compared to angiostatic
factors (18). Inflammatory
leukocytes are thought to create a supportive environment for early
tumor development, possibly through the production of cytokines,
proteases and angiogenic factors. Thus, prompt resolution of
inflammation prevents the establishment of an inflammatory
environment conducive to tumorigenesis. A complex network of
chemokines and receptors exists in the tumor microenvironment and
affects tumor development. Modification of these chemokine networks
modulates leukocyte recruitment, angiogenesis and tumor growth.
The chemokine system is also used by cancer cells to
promote cell proliferation, tumor survival and neovascularization,
or to establish metastases at distant but non-random sites
(19). These mediators (chemokines)
control a variety of biological activities, such as the production
and deposition of collagen, the activation of matrix-digesting
enzymes, the stimulation of cell growth, the inhibition of
apoptosis and the promotion of neo-angiogenesis. Chemokines are
powerful inducers of enzymes and receptors that degrade the
extracellular matrix and facilitate tumor invasion (20). Tumor-derived proteases are able to
cleave the extracellular matrix molecules and lead to the
dissolution of the basement membrane, thus facilitating the process
of tumor cell invasion. These proteolytic enzymes include the
tissue-type plasminogen activator (t-PA), the urokinase-type
plasminogen activators (u-PA) and the large family of
matrix-metalloproteinases (MMPs). Therefore, the expression of
chemokines is of potential advantage for tumor cells, rendering
them capable of proliferation and dissemination (21).
Tumors are composed of cancer and stromal cells,
where, besides fibroblasts and endothelial cells, leukocytes
(macrophages and T lymphocytes, in particular) are the most
represented cell types. In the tumor microenvironment, chemokines
are produced both by stromal cells (fibroblasts, endothelial cells
and infiltrating leukocytes) and by the tumor itself. Immune cells
are actively recruited at the tumor site by the chemokines produced
by neoplastic and stromal cells, leading to the recruitment of
tumor-associated macrophages (TAMs), tumor-associated neutrophils
(TANs), lymphocytes, cancer-associated fibroblasts (CAFs),
mesenchymal stem cells (MSCs) and endothelial cells into the tumor
microenvironment. These infiltrating cells provide a secondary
source of chemokines that may affect tumor growth, cell survival,
senescence, angiogenesis and metastasis. For instance, TAMs produce
a host of growth factors which affect tumor cell proliferation,
angiogenesis, as well as the deposition and dissolution of
connective tissues. Uneven vascularisation and hypoxia are
characteristics of neoplastic tissues. TAMs accumulate
preferentially in the poorly-vascularized regions of tumors with
low oxygen tension. Under hypoxic conditions, TAMs are stimulated
to express hypoxia-inducible genes, such as the vascular
endothelial growth factor (VEGF), the basic fibroblast growth
factor (bFGF) and CXCL8. In various studies, TAM accumulation in
human cancer has been associated with high neovascularisation and
with the production of angiogenic factors such as VEGF,
platelet-derived endothelial cell growth factor, fibroblast,
epidermal growth factor (EGF), fibroblast growth factor (FGF) and
chemokines (22,23).
A subgroup of chemokines, the CXC family, is
important in angiogenesis, as well as in physiologic and pathologic
contexts, including chronic inflammation, fibrosis and malignancy
(24). With regard to tumor growth,
chemokines of the CXC branch have been shown to exert either
angiogenic or angiostatic activities depending on whether or not
amino acid sequence Glu-Leu-Arg (ELR motif) is present or absent
(25). The NH2 terminus
of several CXC chemokines contain three amino acid residues
(Glu-Leu-Arg or ELR motif), which precede the first cysteine amino
acid residue of the primary structure of these chemokines.
Depending on whether or not the ELR motif is present or absent
prior to the first cysteine residue in their structure, CXC
chemokines are subdivided into ELR+ and
ELR–(24). On the basis
of their structure and receptor binding, individual ligands exhibit
either angiogenic or angiostatic biological activities in the
regulation of angiogenesis. The balance of
ELR+/angiogenic vs. non-ELR/angiostatic chemokines
produced in the tumor microenvironment is likely to determine the
degree of angiogenesis surrounding and inside the tumor tissue, and
the consequent tumor progression (21).
Chemokines may be involved in tumor progression
through the direct stimulation of neoplastic growth, promotion of
inflammation and induction of angiogenesis. Apart from indirectly
recruiting leukocytes that provide angiogenic factors, chemokines
regulate angiogenesis directly, through receptors expressed on
endothelial cells (26). Tumor
cells upregulate the expression of widely distributed chemokine
receptors (CXCR4, in particular) and indicate an unexpected,
tissue-of-origin unrelated, chemokine receptor repertoire, which
possibly supports tumor cell survival and invasion. The most common
receptor is CXCR4, expressed by different tumor types of
epithelial, hematological and mesenchymal origin (14). The expression of chemokine receptors
influences diverse aspects of cancer cell behaviour, with
chemokines serving as cues for the secondary localization of tumors
(27). Malignant cells bearing
chemokine receptors on the cell surface are capable of responding
to the chemokine gradient and selectively migrating to specific
organs where the chemokine is present, meaning that chemokines were
able to direct tumor cell migration in vivo. In addition to
affecting tumor cell proliferation, angiogenesis and metastasis,
chemokines also seem to regulate senescence and cell survival.
Thus, the chemokine system is a valuable target for the development
of innovative therapeutic strategies (27).
Atypical chemokine receptors
Numerous studies have reported findings on the
atypical action of chemokine receptor proteins. These receptor
proteins are known as ‘decoy’ or ‘scavenger proteins’ due to the
fact that the binding of these proteins to the respective ligands
does not lead to a typical signalling pathway, but intercepts the
respective pathway and neutralizes chemokine action (4,28–30).
Therefore, these chemokine receptor proteins are also regarded as
‘intercepting receptors’ and are able to bind a broader spectrum of
chemokines. The ability of these receptors to couple to signal
transduction has been principally analysed by examining
intracellular calcium ion mobilization and chemotaxis in
transfected cells following ligand binding, where they appeared to
be functionally ‘silent’. In addition, they are predominantly
expressed on non-leukocytic cell types and are unlikely to be
directly involved in leukocyte migration. It has been proposed that
atypical receptors may act in several ways: i) competing for ligand
binding and, thus, inhibiting the migration of cells bearing
typical receptors; ii) internalizing and degrading ligands and,
therefore, depleting bioavailable chemokine levels in a particular
microenvironment in order to reduce the cell recruitment to that
site; iii) in the transcytosis of chemokines in order to then
transfer ligands across certain barriers; or iv) retaining or
presenting chemokines (2). Four
endogenous proteins are reportedly included in this group:
pro-inflammatory CC chemokine receptors (D6 and DARC), the
homeostatic CC chemokine receptor (CCX-CKR) and CXCR7, a second
receptor for CXCL11 and CXCL12, with critical roles in development
and tumori genesis. These proteins are crucial in inflammation and
chemokine-associated diseases, such as cancer, since some of them
act to trap the chemokine, internalize it as well as direct it
towards degradation and transport the bound chemokine across the
plasma membrane. The ability of these proteins to block the
function of chemokines in cancer cells has been widely described
(31).
D6
D6 is an atypical receptor that acts as a decoy and
scavenger receptor protein for most inflammatory CC chemokines,
including CCL2 (9). In humans, D6
is abundant on lymphatic endothelial cells (LECs) of the vessels
draining the skin, the gut and the lungs (32) and may be found on trophoblasts,
leukocytes (27), tissue mast
cells, macrophages and also dendritic cells (31). Previous studies have shown that D6
was found to be expressed by malignant vascular tumors, T-cell
large granular lymphocyte leukemia cells, choriocarcinomas
(32–34) and also human breast cancer cells
(28). D6 overexpression in human
breast cancer cells was reported to downregulate CCL2 levels and to
subsequently inhibit the proliferation and metastasis of breast
cancer cells in vivo and in vitro (4,27,28,32).
D6-deficient mice have demonstrated increased susceptibility to
skin carcinogenesis (35) and
colitis-associated cancer, the latter being representative of a
clinical paradigm of the inflammation-cancer connection (36).
D6 has been shown to be able to undergo
ligand-independent constitutive internalization. When D6 binds
chemokines, they rapidly enter the cell through endosomal
compartments, dissociate from the receptor and internalized
chemokines remain trapped in the cell and are targeted for
degradation. Simultaneously, D6 recycles back to the cell surface
for further chemokine sequestration. Trafficking is unaffected by
chemokine exposure, chemokine-induced signalling is not required,
and the receptor recycles without causing a reduction in the
cell-surface D6 levels (37).
Through repeated rounds of chemokine internalization, D6 is capable
of removing and destroying large quantities of free extracellular
proinflammatory chemokines (38).
As a result, D6 constitutive internalization and recycling regulate
continuous chemokine sequestration (39).
DARC
DARC is a promiscuous and non-signalling chemokine
receptor (40). In humans, this
receptor is expressed in red blood cells, endothelial cells as well
as neuronal cells (41) and it has
been observed to bind to CC and CXC chemokines (4,30).
DARC is involved in the transcytosis or neutralization of
chemokines at endothelial barriers (42) and on erythrocytes, and it may act to
regulate plasma chemokine concentrations (43). An additional noteworthy feature of
DARC is that it binds angiogenic (ELR+) CXC chemokines
and certain CC chemokines, but not angiostatic (ELR–)
CXC chemokines. Within the ELR+ CXC chemokines, DARC was
found to bind the angiogenic ELR+ CXC chemokines CXCL1,
CXCL5 and CXCL8 (44,45).
Research data have indicated that DARC expression in
tumor or endothelial cells plays a negative role in tumor
progression, through the control of angiogenic and inflammatory
chemokines, or transmitting a senescence signal to tumor cells
through interaction with tumor tetraspanin KAI1/CD82 (30). In a DARC-deficient mouse model with
spontaneous prostate cancer, larger and more aggressive tumors were
developed (46). This finding was
of particular interest since African-American men, of whom 70%
lacked erythrocyte DARC, had exhibited increased occurrence and
mortality from prostate cancer (47). This has been associated with
increased intra-tumor levels of angiogenic ELR+ CXC
chemokines and blood vessel density, supporting the hypothesis that
DARC acts as a decoy receptor that sequesters angiogenic
chemokines, thereby, inhibiting tumor growth. Therefore, DARC
expression on erythrocytes may normally negatively regulate the
levels of angiogenic plasma chemokines that promote prostate cancer
progression. This receptor is also important in regulating breast
cancer growth and metastasis. The expression of CCL2 has been
reported to be significantly correlated with the progression of
tumor and microvessel density in human breast cancer cells
(27,28,48).
Wang et al (30) have
demonstrated that tumor cell lines expressing high levels of
ectopic DARC are less able to grow and metastasize compared to
wild-type tumor cells. This finding has been associated with a
decrease in tumor angiogenesis and lower levels of CCL2 within the
tumor. Chemokines, such as CCL2, have been found to accelerate
tumor growth and metastasis of cancer cells upon binding with
typical specific receptors. The overexpression of DARC in human
breast cancer cells has been reported to downregulate CCL2 levels
and to subsequently inhibit the proliferation and metastasis of
breast cancer cells in vivo and in vitro (4,27,30,34).
DARC has been previously demonstrated to regulate
the biological effects of chemokines in three different ways:
through scavenging, retention or transportation (2). Erythrocyte DARC may act as a chemokine
buffer, sequestering chemokines present at high levels in the
serum, while maintaining a residual homeostatic level as their
presence subsides. Since plasma chemokines likely desensitize
circulating leukocytes, buffering by DARC potentially controls
leukocyte sensitivity to pro-inflammatory chemokines, limiting
under- or over-responsiveness (2).
These different ways that DARC manipulation may affect chemokine
biology and cell migration should be considered when DARC is
therapeutically targeted.
CCX-CKR
CCX-CKR, also known as CCR11 or CCRL1, is a
heptahelical surface protein. It has been demonstrated that
CCX-CKR, when expressed in HEK293 cells, is unable to mediate
Ca(2+) fluxes upon ligand binding (49). It cannot couple to typical chemokine
receptor signalling pathways or mediate chemotaxis. As an atypical
chemokine receptor, it is less well-characterized compared to D6
and DARC. Similar to DARC, CCX-CKR binds chemokines of CC and CXC
subfamilies. However, unlike DARC and D6, it binds the homeostatic
chemokines such as CCL19, CCL21, CCL25 and CXCL13 with high
affinity. These chemokines participate in the axis role of
CCR7/CCL19 (CCL21), CCR9/CCL25 and CXCR5/CXCL13 in leukocytes and
cancer cell migration (2,49) through interaction with the receptors
CCR7, CCR9 or CXCR5, respectively. CCR7 and CXCR5 control lymph
node organogenesis (50), while
CCR7 and CCR9 control thymocyte localization during T-cell
development (51–53). CCR7 also regulates the recruitment
of dendritic cells, naive T-cells and some memory T-cell subsets
into T-cell compartments in secondary lymphoid organs, and
contributes to B-cell extravasation. Therefore, CCX-CKR has been
predicted to regulate homoeostatic lymphocyte and dendritic cell
trafficking, which constitute key migratory events in acquired
immune responses directed by CCX-CKR-binding chemokines (39). CCX-CKR, which is expressed
exclusively by stromal cells, and not hematopoietic cells,
regulates homeostatic leukocyte migration through the control of
chemokine availability in the extracellular space (54).
By contrast, previous investigations have indicated
that CCR7/CCL19 (CCL21), CCR9/CCL25 and CXCR5/CXCL13 axes are able
to promote the growth and metastasis of various tumors, including
breast cancer. The CCR7/CCL19 (CCL21) axis was able to promote the
pathogenesis and progression of breast cancer, melanoma, non-small
cell lung cancer, head and neck, gastrointestinal and hematologic
cancer. The CCR9/CCL25 axis has been involved in breast carcinoma,
ovarian and prostate cancer as well as cutaneous melanoma, while
the CXCR5/CXCL13 axis has been previously demonstrated in various
cancers including non-Hodgkin’s lymphomas, primary intraocular
lymphoma, metastatic neuroblastoma and breast cancer (55). CCX-CKR overexpression in breast
cancer cells inhibited proliferation and invasion in vitro,
as well as tumor growth, lung and lymph node metastasis in
vivo. It has been shown to be a negative regulator of growth
and metastasis in breast cancer mainly through the sequestration of
homeostatic chemokines as well as the subsequent inhibition of
intratumoral neovascularity. CCX-CKR as a scavenger receptor for
chemokines, binds and clears expressed chemokines, including the CC
chemokine ligands CCL19, CCL21, CCL25 and CXCL13. CCX-CKR
expressing cells sequestered and degraded these cognate chemokines
with notable efficiency and also negatively regulated cancer
development and progression. CCX-CKR has been demonstrated to be
associated with longer patient survival and has been identified as
an independent prognostic factor for disease-free survival in
breast cancer patients.
CXCR7
CXCR7 (RDC1), a heptahelical receptor with strong
phylogenetic similarity to chemokine receptors or GPCRs (56), has recently been described as a
second receptor for CXCL12 after CXCR4. CXCL12, a chemokine also
known as stromal-derived factor 1 (SDF1) employs CXCR4 and CXCR7
receptors and modulates homeostatic and pathologic processes, such
as the development of primary epithelial tumors, where it is known
to regulate organogenesis, leukocyte homeostasis, proliferation as
well as survival of tumor cells, tumor angiogenesis, and metastasis
(57). Besides playing a role in
fetal endothelial biology, cardiac development and B-cell
localization, CXCR7 potentially modulates CXCR4 functions. It
dimerizes with CXCR4, a coreceptor for CXCL12 and studies have
suggested the modulation of CXCR4-signalling through
heterodimerization with CXCR7. CXCR7 is coexpressed with CXCR4 in
primary human T lymphocytes and is known to be involved in the
regulation of CXCL12-promoted cell migration (58). CXCR7 expression has also been proven
to induce conformational rearrangements within preassembled CXCR4-G
protein complexes. This phenomenon may explain findings
demonstrating that CXCR7 impairs CXCR4-mediated G protein
activation and calcium responses (57).
Binding of CXCR7 to CXCL12 and CXCL11 has recently
been reported without triggering chemotaxis or calcium mobilization
in response to ligation. It has been shown to bind CXCL12 and
CXCL11 with high affinity, while it does not induce typical
chemokine receptor-mediated cell responses, such as migration and
associated intracellular signal transduction (59,60).
However, CXCR7 expression increases cell survival and adhesive
properties of transfected cell lines, a fact suggesting that
alternative signals originate from this receptor (59). Another study provided contradictory
evidence, demonstrating chemotaxis and calcium mobilization in
CXCR7 transfectants (58). The
function of CXCR7 is controversial with certain studies (61,62)
suggesting CXCR7 to possess signalling activity in mammalian cells
and zebrafish embryos, while other studies (63) have demonstrated that CXCR7 possesses
decoy activity in fish. Further studies are needed in order for
these discrepancies to be clarified prior to the inclusion of CXCR7
in the atypical chemokine receptor family.
These receptors therefore exhibit the following
critical features: high-affinity binding to chemokines, lack of
chemotactic signalling and a marked ability to continuously
internalize their ligands. In vivo, these receptors function
either by regulating the levels of bioavailable chemoattractants,
competing with signalling receptors, or mediating transcytosis of
chemoattractants across endothelial and epithelial barriers.
Moreover, there appear to be some structural similarities between
the above-mentioned receptors. Residues important for initiating
signal transduction in typical receptors are often either altered
or absent. Thus, generating these proteins in a significant amount
in order to investigate their roles in various contexts is
crucial.
Conclusion and future perspectives
Breast cancer is one of the most frequently
diagnosed life-threatening types of cancer in women. Most breast
cancer patients succumb to the disease due to cancer invasion and
metastasis. Treatment of breast cancer invasion and metastasis is
difficult and the survival rate for patients with invasive and
metastatic cancer is low. Apart from their role in regulating
leukocyte trafficking, chemokines have been shown to be involved in
cancer growth and metastasis. The complex network of chemokines and
the respective receptors in the tumor microenvironment constitutes
the object of intensive investigation aimed at targeting these
molecules for therapeutic interventions. Chemokine (C-C motif)
ligand 2 (CCL2), also referred to as monocyte chemotactic protein-1
(MCP-1), is primarily secreted by monocytes, macrophages and
dendritic cells. An elevated serum level of CCL2 has been found to
be associated with breast cancer invasion and metastasis. D6 or
DARC overexpression in breast cancer cells was detected to
intercept the signalling pathway of CCL2, thereby decreasing the
production level of this chemokine. Single administration of either
D6 or DARC has been demonstrated to be able to neutralize the
action of CCL2 in vitro and suppress invasion on MDA-MB-231
cells (30). However, the
combinatorial effects of D6 and DARC on MDA-MB-231 cell invasion
has yet to be demonstrated. A combination of D6 and DARC may yield
better effects on invasive breast cancer cells compared to the
single administration of either D6 or DARC. Neutralization of
protein ligands, such as chemokines, are likely to lead to the
validation of signalling pathways under physiological or
pathophysiological conditions, and in certain cases, to the
development of novel therapeutic molecules, such as chemokine
receptors, to be used in disease treatment in the future.
Although attention has been focused on the
expression of chemokines in human and experimental tumors, the
expression of chemokine receptors has been investigated to a much
lesser extent. Since chemokine decoy receptor proteins have been
reported to have inhibitory effects on invasive and metastatic
human breast cancer cells, the attempt of generating functional
recombinant clones in research laboratories is highly attractive in
order for future studies to clarify the role of these proteins in
cancer studies, and particularly in breast cancer in vitro.
An understanding of the function and mechanism of chemokine
receptors underlying chemokine action in cancer requires the
availability of a significant amount of highly purified and
biologically active chemokine receptors. Subsequently, sufficient
quantities of D6 and DARC are to be generated from MDA-MB-231 cell
RNA in future studies compared to the commonly reported blood serum
receptors. As such, clones are to be constructed and the
recombinant chemokine decoy receptor proteins of D6 and DARC are to
be expressed using the Pichia pastoris (P. pastoris)
expression system. The recombinant proteins produced are to be
applied in various combinations (compared to single-compound
administration) to a highly invasive breast cancer cell line in
order to investigate their effects on the tumorigenesis and
metastatic potential of breast cancer cells in vitro.
P. pastoris is a non-pathogenic,
methylotrophic yeast. It is a versatile microorganism capable of
efficient secretion (64) and
possesses the ability to produce soluble and correctly folded
recombinant proteins (65). It also
offers numerous benefits such as the strong expression promoter
(AOX1) and the ability to stably integrate expression plasmids at a
specific location (66–68). Compared to Escherichia coli
(E. coli), P. pastoris shares some advantages of this
bacterial expression system, such as the simplicity of genetic
manipulation, its cost effectiveness and the high heterologous
protein expression efficiency (69). Moreover, P. pastoris does not
produce high levels of intrinsic proteins, thus making the
expressed foreign proteins easy to be isolated. Furthermore, unlike
the E. coli expression system, P. pastoris can
produce foreign proteins intracellulary or extracellulary,
depending on the signal sequence used in the system (70). The ability to be cultured at a high
cell density, as well as the folding, processing of proteins,
reduced heterologous proteins degradation and a much easier
heterologous protein purification procedure compared to other
bacterial expression systems, render P. pastoris more
attractive as an excellent alternative to the E. coli
expression system (71). Moreover,
heterologous proteins expressed by the P. pastoris strain
were able to be post-translationally modified as observed in
mammalian expression systems without the use of expensive culture
media and cell culture equipment, which always significantly raise
the cost of the mammalian cell expression systems (67,68,72).
Besides, P. pastoris-expressed proteins are free from
endotoxins or pyrogens, a problem always faced in bacterial-derived
proteins (64), as well as viral
inclusions, a problem always faced in mammalian cell-derived
proteins (73). This constitutes
the expressed proteins safe for human use and suitable for clinical
and therapeutic applications.
This study provides useful information on the
effects of D6 and DARC on the tumorigenesis and metastatic
potential of breast cancer cells and may lead to novel therapeutic
strategies against breast cancer. Notably, in future studies,
recombinant chemokine decoy receptor proteins are expected to be
produced rather than using commercially available proteins, which
are are considered to be expensive, in order to facilitate the
basic requirement of studies such as this one. The use of the P.
pastoris expression system in producing D6 and DARC is
considered to constitute an attractive option for scale-up
production in the future, since it is cost-effective and highly
efficient in protein expression. The ability to generate the
proteins of interest at increased concentration levels would allow
significant improvements in study approaches and related
applications. Integrating in-house protein production and
translational research constitute potential challenges in
facilitating research in low-resource settings in developing and
under-developed countries. In the future, similar protocols may be
utilized to produce other recombinant proteins in order to
understand their roles in cancer biology as part of the ongoing
investigation for novel therapeutic interventions.
Acknowledgements
This study was funded by the
Exploratory Research Grant Scheme (ERGS) from the Ministry of
Higher Education Malaysia (203/CIPPM/6730059).
References
|
1
|
Comerford I, Nibbs RJ, Litchfield W,
Bunting M, Harata Lee Y, Haylock Jacobs S, Forrow S, Korner H and
McColl SR: The atypical chemokine receptor CCX-CKR scavenges
homeostatic chemokines in circulation and tissues and suppresses
Th17 responses. Blood. 116:4130–4140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Comerford I, Litchfield W, Harata-Lee Y,
Nibbs RJ and McColl SR: Regulation of hemotactic networks by
‘atypical’ receptors. Bioessays. 29:237–247. 2007.
|
|
3
|
Savarin-Vuaillat C and Ransohoff RM:
Chemokines and chemokine receptors in neurological disease: raise,
retain, or reduce? Neurotherapeutics. 4:590–601. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Galzi JL, Hachet-Haas M, Bonnet D, Daubeuf
F, Lecat S, Hibert M, Haiech J and Frossard N: Neutralizing
endogenous chemokines with small molecules: principles and
potential therapeutic applications. Pharmacol Ther. 126:39–55.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fernandez EJ and Lolis E: Structure,
function, and inhibition of chemokines. Annu Rev Pharmacol Toxicol.
42:469–499. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hansell CA, Hurson CE and Nibbs RJ: DARC
and D6: silent partners in chemokine regulation? Immunol Cell Biol.
89:197–206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thelen M: Dancing to the tune of
chemokines. Nat Immunol. 2:129–134. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Graham GJ: D6 and the atypical chemokine
receptor family: novel regulators of immune and inflammatory
processes. Eur J Immunol. 39:342–351. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mantovani A, Bonecchi R and Locati M:
Tuning inflammation and immunity by chemokine sequestration: decoys
and more. Nat Rev Immunol. 6:907–918. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Müller A, Homey B, Soto H, Ge N, Catron D,
Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL,
Mohar A, Verástegui E and Zlotnik A: Involvement of chemokine
receptors in breast cancer metastasis. Nature. 410:50–56.
2001.PubMed/NCBI
|
|
11
|
Rollins BJ: Inflammatory chemokines in
cancer growth and progression. Eur J Cancer. 42:760–767. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ben-Baruch A: Organ selectivity in
metastasis: regulation by chemokines and their receptors. Clin Exp
Metastasis. 25:345–356. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ali S and Lazennec G: Chemokines: novel
targets for breast cancer metastasis. Cancer Metastasis Rev.
26:401–420. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Balkwill F: Cancer and the chemokine
network. Nat Rev Cancer. 4:540–550. 2004. View Article : Google Scholar
|
|
15
|
Bottazzi B, Polentarutti N, Acero R,
Balsari A, Boraschi D, Ghezzi P, Salmona M and Mantovani A:
Regulation of the macrophage content of neoplasms by
chemoattractants. Science. 220:210–212. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
de Visser KE, Eichten A and Coussens LM:
Paradoxical roles of the immune system during cancer development.
Nat Rev Cancer. 6:24–37. 2006.
|
|
17
|
Balkwill F, Charles KA and Mantovani A:
Smoldering and polarized inflammation in the initiation and
promotion of malignant disease. Cancer Cell. 7:211–217. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Strieter RM, Belperio JA, Burdick MD,
Sharma S, Dubinett SM and Keane MP: CXC chemokine: angiogenesis,
immunoangiostasis, and metastases in lung cancer. Ann N Y Acad Sci.
1028:351–360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vandercappellen J, Van Damme J and Struyf
S: The role of CXC chemokines and their receptors in cancer. Cancer
Lett. 267:226–244. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Allavena P, Sica A, Solinas G, Porta C and
Mantovani A: The inflammatory micro-environment in tumor
progression: the role of tumor-associated macrophages. Crit Rev
Oncol Hematol. 66:1–9. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Allavena P, Marchesi F and Mantovani A:
The role of chemokines and their receptors in tumor progression and
invasion: potential new targets of biological therapy. Curr Cancer
Ther Rev. 1:81–92. 2005. View Article : Google Scholar
|
|
22
|
Mantovani A, Sozzani S, Locati M, Allavena
P and Sica A: Macrophage polarization: tumor-associated macrophages
as a paradigm for polarized M2 mononuclear phagocytes. Trends
Immunol. 23:549–555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bingle L, Brown NJ and Lewis CE: The role
of tumour associated macrophages in tumour progression:
implications for new anticancer therapies. J Pathol. 196:254–265.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mehrad B, Keane MP and Strieter RM:
Chemokines as mediators of angiogenesis. Thromb Haemost.
97:755–762. 2007.PubMed/NCBI
|
|
25
|
Strieter RM, Polverini PJ, Kunkel SL, et
al: The functional role of the ‘ELR’ motif in CXC
chemokine-mediated angiogenesis. J Biol Chem. 270:27348–27357.
1995.
|
|
26
|
Strieter RM, Burdick MD, Mestas J,
Gomperts B, Keane MP and Belperio JA: Cancer CXC chemokine networks
and tumour angiogenesis. Eur J Cancer. 42:768–778. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mantovani A, Savino B, Locati M, Zammataro
L, Allavena P and Bonecchi R: The chemokine system in cancer
biology and therapy. Cytokine Growth Factor Rev. 21:27–39. 2010.
View Article : Google Scholar
|
|
28
|
Wu FY, Ou ZL, Feng LY, Luo JM, Wang LP,
Shen ZZ and Shao ZM: Chemokine decoy receptor D6 plays a negative
role in human breast cancer. Mol Cancer Res. 6:1276–1288. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang JM, Deng X, Gong W and Su S:
Chemokines and their role in tumor growth and metastasis. J Immunol
Methods. 220:1–17. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang J, Ou ZL, Hou YF, Luo JM, Shen ZZ,
Ding J and Shao ZM: Enhanced expression of Duffy antigen receptor
for chemokines by breast cancer cells attenuates growth and
metastasis potential. Oncogene. 25:7201–7211. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Graham GJ and McKimmie CS: Chemokine
scavenging by D6: a movable feast? Trends Immunol. 27:381–386.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nibbs RJ, Kriehuber E, Ponath PD, Parent
D, Qin S, Campbell JD, Henderson A, Kerjaschki D, Maurer D, Graham
GJ and Rot A: The beta-chemokine receptor D6 is expressed by
lymphatic endothelium and a subset of vascular tumors. Am J Pathol.
158:867–877. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Martinez de la Torre Y, Buracchi C,
Borroni EM, Dupor J, Bonecchi R, Nebuloni M, Pasqualini F, Doni A,
Lauri E, Agostinis C, Bulla R, Cook DN, Haribabu B, Meroni P,
Rukavina D, Vago L, Tedesco F, Vecchi A, Lira SA, Locati M and
Mantovani A: Protection against inflammation-and
autoantibody-caused fetal loss by the chemokine decoy receptor D6.
Proc Natl Acad Sci USA. 104:2319–2324. 2007.PubMed/NCBI
|
|
34
|
Zeng XH, Ou ZL, Yu KD, Feng LY, Yin WJ, Li
J, Shen ZZ and Shao ZM: Coexpression of atypical chemokine binders
(ACBs) in breast cancer predicts better outcomes. Breast Cancer Res
Treat. 125:715–727. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nibbs RJ, Gilchrist DS, King V, Ferra A,
Forrow S, Hunter KD and Graham GJ: The atypical chemokine receptor
D6 suppresses the development of chemically induced skin tumors. J
Clin Invest. 117:1884–1892. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vetrano S, Borroni EM, Sarukhan A, Savino
B, Bonecchi R, Correale C, Arena V, Fantini M, Roncalli M, Malesci
A, Mantovani A, Locati M and Danese S: The lymphatic system
controls intestinal inflammation and inflammation associated colon
cancer through the chemokine decoy receptor D6. Gut. 59:197–206.
2010. View Article : Google Scholar
|
|
37
|
Weber M, Blair E, Simpson CV, O’Hara M,
Blackburn PE, Rot A, Graham GJ and Nibbs RJ: The chemokine receptor
D6 constitutively traffics to and from the cell surface to
internalize and degrade chemokines. Mol Biol Cell. 15:2492–2508.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bonecchi R, Locati M, Galliera E, Vulcano
M, Sironi M, Fra AM, Gobbi M, Vecchi A, Sozzani S, Haribabu B, Van
Damme J and Mantovani A: Differential recognition and scavenging of
native and truncated macrophage-derived chemokine (macrophage
derived chemokine/CC chemokine ligand 22) by the D6 decoy receptor.
J Immunol. 172:4972–4976. 2004. View Article : Google Scholar
|
|
39
|
Hansell CAH, Simpson CV and Nibbs RJ:
Chemokine sequestration by atypical chemokine receptors. Biochem
Soc Trans. 34:1009–1013. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Addison CL, Belperio JA, Burdick MD and
Strieter RM: Overexpression of the duffy antigen receptor for
chemokines (DARC) by NSCLC tumor cells results in increased tumor
necrosis. BMC Cancer. 4:282004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Peiper SC, Wang Z, Neote K, Martin AW,
Showell HJ, Conklyn MJ, Ogborne K, Hadley TJ, Lu ZH, Hesselgesser J
and Horuk R: The Duffy antigen/receptor for chemokines (DARC) is
expressed in endothelial cells of Duffy negative individuals who
lack the erythrocyte receptor. J Exp Med. 181:1311–1317. 1995.
View Article : Google Scholar
|
|
42
|
Lee JS, Frevert CW, Wurfel MM, Peiper SC,
Wong VA, Ballman KK, Ruzinski JT, Rhim JS, Martin TR and Goodman
RB: Duffy antigen facilitates movement of chemokine across the
endothelium in vitro and promotes neutrophil trans¬migration in
vitro and in vivo. J Immunol. 170:5244–5251. 2003.PubMed/NCBI
|
|
43
|
Darbonne WC, Rice GC, Mohler MA, Apple T,
Hebert CA, Valente AJ and Baker JB: Red blood cells are a sink for
inter-leukin 8, a leukocyte chemotaxin. J Clin Invest.
88:1362–1369. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Neote K, Darbonne W, Ogez J, Horuk R and
Schall TJ: Identification of a promiscuous inflammatory peptide
receptor on the surface of red blood cells. J Biol Chem.
268:12247–12249. 1993.PubMed/NCBI
|
|
45
|
Neote K, Mak JY, Kolakowski LF and Schall
TJ: Functional and biochemical analysis of the cloned Duffy
antigen: identity with the red blood cell chemokine receptor.
Blood. 84:44–52. 1994.PubMed/NCBI
|
|
46
|
Shen H, Schuster R, Stringer KF, Waltz S
and Lentsch A: The Duffy antigen/receptor for chemokines (DARC)
regulates prostate tumor growth. Faseb J. 20:59–64. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lentsch AB: The Duffy antigen/receptor for
chemokines (DARC) and prostate cancer. A role as clear as black and
white? FASEB J. 16:1093–1095. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Saji H, Koike M, Yamori T, Saji S, Seiki
M, Matsushima K and Toi M: Significant correlation of monocyte
chemoattractant protein 1 expression with neovascularization and
progression of breast carcinoma. Cancer. 92:1085–1091. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Townson JR and Nibbs RJ: Characterization
of mouse CCX-CKR, a receptor for the lymphocyte-attracting
chemokines TECK/ mCCL25, SLC/mCCL21 and MIP-3beta/mCCL19:
comparison to human CCX-CKR. Eur J Immunol. 32:1230–1241. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Muller G, Hopken UE and Lipp M: The impact
of CCR7 and CXCR5 on lymphoid organ development and systemic
immunity. Immunol Rev. 195:117–135. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Misslitz A, Pabst O, Hintzen G, Ohl L,
Kremmer E, Petrie HT and Förster R: Thymic T cell development and
progenitor local¬ization depend on CCR7. J Exp Med. 200:481–491.
2004.
|
|
52
|
Uehara S, Grinberg A, Farber JM and Love
PE: A role for CCR9 in T lymphocyte development and migration. J
Immunol. 168:2811–2819. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ueno T, Saito F, Gray DH, Kuse S, Hieshima
K, Nakano H, Kakiuchi T, Lipp M, Boyd RL and Takahama Y: CCR7
signals are essential for cortex–medulla migration of developing
thymocytes. J Exp Med. 200:493–505. 2004.
|
|
54
|
Heinzel K, Benz C and Bleul CC: A silent
chemokine receptor regulates steady-state leukocyte homing in vivo.
Proc Natl Acad Sci USA. 104:8421–8426. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Feng LY, Ou ZL, Wu FY, Shen ZZ and Shao
ZM: Involvement of a novel chemokine decoy receptor CCX-CKR in
breast cancer growth, metastasis and patient survival. Clin Cancer
Res. 15:2962–2970. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Fredriksson R, Lagerstrom MC, Lundin LG
and Schioth HB: The G-protein coupled receptors in the human genome
form five main families. Phylogenetic analysis, paralogon groups,
and fingerprints. Mol Pharmacol. 63:1256–1272. 2003. View Article : Google Scholar
|
|
57
|
Levoye A, Balabanian K, Baleux F,
Bachelerie F and Lagane B: CXCR7 heterodimerizes with CXCR4 and
regulates CXCL12 mediated G protein signalling. Blood.
113:6085–6093. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Balabanian K, Lagane B, Infantino S, Chow
KYC, Harriague J, Moepps B, Arenzana-Seisdedos F, Thelen M and
Bachelerie F: The chemokine SDF-1/CXCL12 binds to and signals
through the orphan receptor RDC1 in T lymphocytes. J Biol Chem.
280:35760–35766. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Burns JM, Summers BC, Wang Y, Melikian A,
Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ,
Wei K, McMaster BE, Wright K, Howard MC and Schall TJ: A novel
chemokine receptor for SDF-1 and I-TAC involved in cell survival,
cell adhesion, and tumor development. J Exp Med. 203:2201–2213.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Thelen M and Thelen S: CXCR7, CXCR4 and
CXCL12: an eccentric trio? J Neuroimmunol. 198:9–13. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang J, Shiozawa Y, Wang J, Wang Y, Jung
Y, et al: The role of CXCR7/RDC1 as a chemokine receptor for
CXCL12/SDF-1 in prostate cancer. J Biol Chem. 283:4283–4294. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Valentin G, Haas P and Gilmour D: The
chemokine SDF1a coordinates tissue migration through the spatially
restricted activation of Cxcr7 and Cxcr4b. Curr Biol. 17:1026–1031.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Boldajipour B, Mahabaleshwar H, Kardash E,
Reichman-Fried M, Blaser H, Minina S, Wilson D, Xu Q and Raz E:
Control of chemokine-guided cell migration by ligand sequestration.
Cell. 132:463–473. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Salunkhe S, Soorapaneni S, Prasad KS,
Raiker VA and Padmanabhan S: Strategies to maximize expression of
rightly processed human interferon alpha2b in Pichia
pastoris. Protein Expr Purif. 71:139–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Gurkan C and Ellar DJ: Recombinant
production of bacterial toxins and their derivatives in the
methylotrophic yeast Pichia pastoris. Microb Cell Fact.
4:332005. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cregg J, Cereghino J, Shi J and Higgins D:
Recombinant protein expression in Pichia pastoris. Mol
Biotechnol. 16:23–52. 2000. View Article : Google Scholar
|
|
67
|
Cereghino GPL, Cereghino JL, Ilgen C and
Cregg JM: Production of recombinant proteins in fermenter cultures
of the yeast Pichia pastoris. Curr Opin Biotechnol.
13:329–332. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Romanos M: Advances in the use of
Pichia pastoris for high level gene expression. Curr Opin
Biotechnol. 6:527–533. 1995.
|
|
69
|
Verma R, Boleti E and George AJT: Antibody
engineering: comparison of bacterial, yeast, insect and mammalian
expression systems. J Immunol Methods. 216:165–181. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Cregg JM, Vedvick TS and Raschke WC:
Recent advances in the expression of foreign genes in Pichia
pastoris. Biotechnology (NY). 11:905–910. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wysocka-Kapcinska M, Campos-Sandoval JA,
Pal A and Findlay JBC: Expression and characterization of
recombinant human retinol-binding protein in Pichia
pastoris. Protein Expr Purif. 71:28–32. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Bretthauer RK and Castellino FJ:
Glycosylation of Pichia pastoris-derived proteins.
Biotechnol Appl Biochem. 30:193–200. 1999.
|
|
73
|
Batra G, Gurramkonda C, Nemani SK, Jain
SK, Swaminathan S and Khanna N: Optimization of conditions for
secretion of dengue virus type 2 envelope domain III using
Pichia pastoris. J Biosci Bioeng. 110:408–414. 2010.
View Article : Google Scholar : PubMed/NCBI
|