Introduction
Enterovirus 71 (EV71) infections may cause hand,
foot and mouth disease (HFMD) and even severe neurologic
complications and/or pulmonary edema in young children. Although
understanding of EV71 infection has recently improved, EV71
pathogenesis has yet to be fully elucidated (1). The outcome of a virus infection is the
result of a complex interaction of the virus, environmental factors
and host genetics. The outcome of infectious diseases is strongly
controlled by the host immune response, while resistance to
infection in humans is, to a certain extent, genetically determined
(2). Critical host responses to
virus infection include the absolute levels of cytokines as well as
the control of cytokine secretion such as inducibility and
stability of cytokine mRNA (3).
Levels and types of cytokines and chemokines released in hosts are
mainly determined by gene polymorphisms. Numerous studies have
focused on the association between a virus and host response. The
aim of the present study was to investigate the association between
IP-10 gene polymorphism and EV71 infection in children and improve
our knowledge regarding EV71 pathogenesis.
Materials and methods
Study population
The study protocol was approved by the Ethics
Committee of the Medical School of Qingdao University (Qingdao,
China), and informed consent for the experimental use of specimens
was obtained from the participants. A total of 106 patients, aged,
2–10 years, were included in this study. The patients presented to
the Department of Pediatrics of the Affiliated Hospital of the
Medical School of Qingdao University between May, 2010 and May,
2011. Fifty eight of the 106 participants (male/female, 38/20) were
diagnosed with EV71 using reverse transcriptase-polymerase chain
reaction (RT-PCR), VPl sequencing and/or virus isolation
were included in the EV71 group. The control groups comprised 48
patients (male/female, 30/18) admitted to the Department of Surgery
with normal blood test and no infection, allergy or recent use of
antibiotics.
Reagents and instruments
Blood Genome DNA Extraction kit, Taq
Polymerase, PCR buffer (with Mg2+), dNTP mixture,
restriction enzyme XbaI and PCR primers were obtained from
Takara Biotechnology Co., Ltd. (Dalian, China); PE2400 PCR system
was purchased from Applied Biosystems (Foster City, CA, USA).
BioSpectrum 300 Imaging System was obtained from UVP and used to
capture gel images (Upland, CA, USA).
DNA extraction, amplification and
analysis
Genomic DNA extraction of peripheral blood
leukocytes was performed: 3 ml of EDTA-whole blood sample was
obtained from each participant, coded and analyzed in a blind
manner for genomic DNA extraction using the Blood Genome DNA
Extraction kit, according to the manufacturer’s instructions. The
quality of the genomic DNA was examined by measuring the optical
density (OD) at wavelengths of 260 and 280 nm. The ratio of
OD260/OD280 was between 1.70–1.80. The final DNA concentration was
10 ng/μl.
IP-10 genotypes were determined using
PCR-restriction fragment length polymorphism (PCR-RFLP) as
previously described by Humbert et al(4). The primers for IP-10 (−1595C/T) were:
F, 5′GCAGATACTGTCTCAGAACCTG GTA 3′ and R,
5′TGTCACCATCTCTCATTTTGATTGT3′ (5).
The PCR reactions were performed in 25 μl containing 10 ng
of genomic DNA, 10X PCR buffer (with Mg2+) 2.5
μl, 2.5 mM dNTPs 2.0 μl, 0.625 units TaqDNA
polymerase and 0.5 μl of each primer (10 pmol/l). Following
initial denaturation at 95°C for 5 min, the PCR reaction was
performed for 35 cycles consisting of 94°C for 30 sec, 57°C for 1
min and 72°C for 1 min, followed by a final extension step of 72°C
for 10 min. The PCR product was digested using the restriction
enzyme XbaI and then resolved on a 2% agarose gel, and
visualized by UV following ethidium bromide staining.
Statistical analysis
Statistical analysis was performed using the SPSS
software (version 17.0; SPSS, Inc., Chicago, IL, USA). The
χ2 test was used to evaluate differences in frequency
distributions of genotype and allele of the IP-10 polymorphisms
between the EV71 and control groups.
The verification of the Hardy-Weinberg equilibrium
of genotypes was performed using the χ2 test. The odds
ratios (ORs) for the risk of EV71 infection and their 95%
confidence intervals (CIs) were calculated.
Results
Characteristics of the study
subjects
The genotype frequencies of the two polymorphisms
among the controls were in agreement with the Hardy-Weinberg
equilibrium (P>0.05) (Table I),
indicating that study subjects included in the present study were
representative of the target population.
 | Table IHardy-Weinberg test of IP-10 genotype
distribution. |
Table I
Hardy-Weinberg test of IP-10 genotype
distribution.
| | IP-10 genotype, n
| | |
|---|
| Group | N | CC | CT | TT | χ2 | P-value |
|---|
| EV71 | 58 | 52 | 5 | 1 | 3.34 | 0.07 |
| Control | 48 | 34 | 13 | 1 | 0.04 | 0.85 |
Genotype distributions of IP-10
polymorphisms
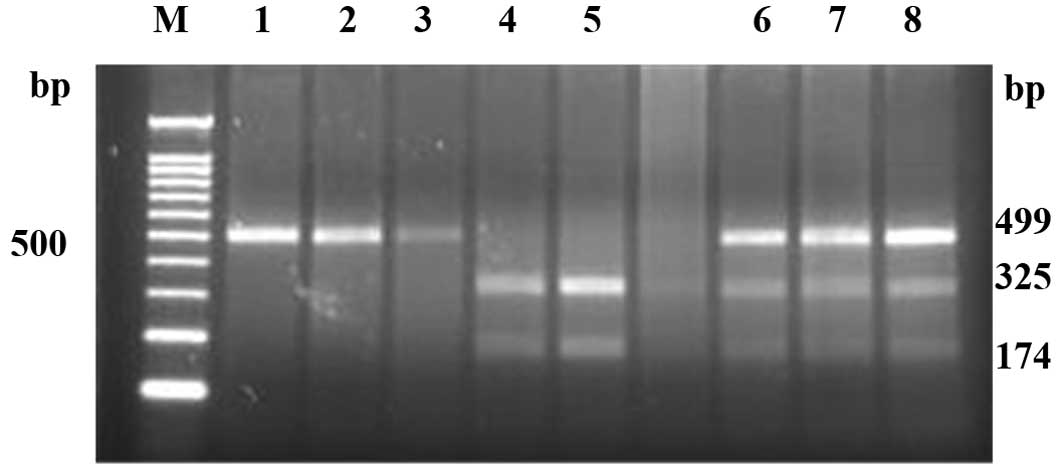
The PCR product of IP-10 (−1596C/T) was 499 bp.
Following XbaI digestion, the product included one single
band at 499 bp (CC type), two bands at 325 and 174 bp (TT type) and
three bands at 499, 325 and 174 bp (CT type) (Fig. 1). The genotype and allele
frequencies of the EV71 and control groups are shown in Table II. The percentage of CT + TT
genotype and the frequency of T allele in the EV71 group (10.3 and
6%, respectively) was significantly lower compared to the control
group (29.2 and 15.6%, respectively). Individuals with T allele had
a lower risk of EV71 infection (OR=0.35, 95% CI, 0.13–0.89).
 | Table IIGenotypes and allele frequencies of
EV71 and control groups. |
Table II
Genotypes and allele frequencies of
EV71 and control groups.
| | Genotype, n (%)
| | | Allele, n (%)
| | |
|---|
| Group | N | CC | CT + TT | OR | 95% CI | C | T | OR | 95% CI |
|---|
| EV71 | 58 | 52 (89.7) | 6 (10.3)a | 0.28 | 0.10–0.79 | 109 (94.0) | 7 (6.0)b | 0.35 | 0.13–0.89 |
| Control | 48 | 34 (70.8) | 14 (29.2) | | | 81 (84.4) | 15 (15.6) | | |
Discussion
Interferon-γ-inducible protein 10 (IP-10, also
termed CXCL10) is one of the CXC chemokines (α subunit). Luster
et al(6) first reported that
IP-10, which has a relative molecular mass of 12,378, could be
secreted by interferon (IFN)-γ-treated U937 cells. IP-10 has a
significant amino-acid homology to platelet factor-4 (PF-4)
(6). It is known to play an
important role in autoimmune disease, transplantation, infection,
allergic inflammatory disease and cardiovascular disease (7). Findings of previous studies have shown
that the two major functions of IP-10 involve the recruiting of
activated T cells into sites of tissue inflammation (8) and inhibition of angiogenesis (9). Th1 reactions depend on IP-10, which
acts as a chemoattractor for monocytes/macrophages, T and NK cells,
but not neutrophils. Numerous studies have shown that the chemokine
CXCL10/IP-10 along with CXCL9/Mig and CXCL11/I-TAC demonstrate the
ability to recruit various leukocyte subsets, the capacity to
induce the proliferation of vascular pericytes as well as powerful
antitumor effects. Thus, these chemokines have been suggested to be
potential therapeutic targets in cancer, allograft rejection,
diabetes, multiple sclerosis and autoimmune disorders of the
thyroid (10). IP-10 has also been
found to be associated with hepatic inflammation and to be a
potential marker of treatment outcome (11). Previous studies have also shown that
the CXCL10/IP-10 level is elevated in the sera and livers of
progressed HBV carriers, and that the −1596T-201A haplotype is
associated with higher CXCL10/IP-10 transcription in
IFN-γ-stimulated peripheral blood mononuclear cells (5).
In the present study, the association between IP-10
gene polymorphism and EV71 infection in children was investigated.
The results have shown that individuals with T allele have a lower
risk of EV71 infection indicating that −1596T allele for IP-10 gene
may be a beneficial factor for EV71 infection. Genetic
susceptibility may vary due to ethnicity, complexity between virus
and host as well as interaction between multiple genes and haploid
mutation. Future studies may focus on screening more candidate
polymorphic genes for EV71 susceptibility to help understand the
genetic susceptibility and pathophysiology of EV71.
References
|
1
|
Khong WX, Foo DG, Trasti SL, Tan EL and
Alonso S: Sustained high levels of interleukin-6 contribute to the
pathogenesis of enterovirus 71 in a neonate mouse model. J Virol.
85:3067–3076. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van Deventer SJ: Cytokine and cytokine
receptor polymorphisms in infectious disease. Intensive Care Med.
26(Suppl 1): S98–S102. 2000.PubMed/NCBI
|
|
3
|
Booy R, Nadel S, Hibberd M, Levin M and
Newport MJ: Genetic influence on cytokine production in
meningococcal disease. Lancet. 349:11761997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Humbert R, Adler DA, Disteche CM, Hassett
C, Omiecinski CJ and Furlong CE: The molecular basis of the human
serum paraoxonase activity polymorphism. Nat Genet. 3:73–76. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Deng G, Zhou G, Zhang R, et al: Regulatory
polymorphisms in the promoter of CXCL10 gene and disease
progression in male hepatitis B virus carriers. Gastroenterology.
134:716–726. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luster AD, Unkeless JC and Ravetch JV:
Gamma-interferon transcriptionally regulates an early-response gene
containing homology to platelet proteins. Nature. 315:672–676.
1985. View
Article : Google Scholar
|
|
7
|
Gerard C and Rollins BJ: Chemokines and
disease. Nat Immunol. 2:108–115. 2001. View
Article : Google Scholar
|
|
8
|
Dufour JH, Dziejman M, Liu MT, Leung JH,
Lane TE and Luster AD: IFN-gamma-inducible protein 10 (IP-10;
CXCL10)-deficient mice reveal a role for IP-10 in effector T cell
generation and trafficking. J Immunol. 168:3195–3204. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Angiolillo AL, Sgadari C, Taub DD, et al:
Human interferon-inducible protein 10 is a potent inhibitor of
angiogenesis in vivo. J Exp Med. 182:155–162. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lazzeri E and Romagnani P: CXCR3-binding
chemokines: novel multifunctional therapeutic targets. Curr Drug
Targets Immune Endocr Metabol Disord. 5:109–118. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zeremski M, Petrovic LM and Talal AH: The
role of chemokines as inflammatory mediators in chronic hepatitis C
virus infection. J Viral Hepat. 14:675–687. 2007.PubMed/NCBI
|