Introduction
Electric field pulse may improve the proliferation
and differentiation of osteoblasts and the rehabilitation of
injured bones. In the treatment of various bone injuries,
electrical stimuli promote callusogenesis and play an important
role in the regulation of the adhesion, proliferation and
differentiation of osteoblasts (1–3). Even
very small electrical stimuli may trigger DNA synthesis and cell
division, which is attributed to the bioelectrical nature of cells
(4).
Bone repair materials have been found to interact
with osteoblasts and promote cell proliferation and osteogenic
differentiation (5–7). Currently, there is increasing interest
in conducting polymers with unique chemical and physical
properties. Several conducting polymers have been used to create
biosensors, neural probes, drug release regulators, initiators and
auto-oxidants (8–10). Conducting polymers are also
preferred for biomedical applications, due to their beneficial
bone-healing properties (11–13).
Wong et al(11) confirmed
that conducting polymers effectively control the shape and growth
of mammalian cells. Polyanilines (PAs) are a class of conducting
polymers which appear promising, due to their stability and easy
synthesis. Aniline oligomer composited with aliphatic polyester
possesses conductive and biodegradable-biocompatible properties.
The use of aniline oligomer/aliphatic polyester composites appears
promising in the enhancement of the effect of electric pulse on the
expedition of injured bone healing by multiple mechanisms.
In the present study, external electric pulse was
imposed on osteoblasts, which were cultured on nanocomposites
created from aniline oligomers and poly(lactic-co-glycolic acid)
(PLGA) blends. We investigated the adhesion, proliferation and
differentiation of osteoblasts under the action of conducting
polymers in combination with electrical stimuli.
Materials and methods
Preparation and characterization of
AP/PLGA nanocomposites
Preparations of aniline pentamer (AP) and its
chemical structures have been previously described (12,13).
AP and PLGA were dissolved in chloroform to form a 0.06 g/ml
solution. First they were mixed together and then stirred and
sonicated. Solid nanocomposites were obtained by precipitation in
anhydrous alcohol and were dried in vacuum for 48 h. The
nanocomposites were characterized as previously described (12,13).
Cell seeding
The nanocomposites and other materials were
dissolved in chloroform (0.1 g/ml) to form a 10 wt% solution. The
solution (0.5 ml) was dropped onto a 15-mm Fisherbrand coverslip
(treated with 2% dimethyldichlorosilane/chloroform solution, then
dried at 180°C for 4 h prior to use) and evaporated in vacuum for
48 h at room temperature to form a thin film. Prior to use, the
coverslips were sterilized by UV illumination for 40 min.
Rat osteoblasts were freshly isolated in the
laboratory as previously described (2) and seeded with a density of
5.0×104 cells/well (1 ml medium per well) in 6-well
plates (Corninng Costar) containing the coated coverslips. Cells
were cultured in DMEM (Gibco, Carlsbad, CA, USA) supplemented with
10% fetal bovine serum (Gibco), 1.0×105 U/l penicillin
(Sigma-Aldrich, St. Louis, MO, USA) and 100 mg/l streptomycin
(Sigma-Aldrich), at 37°C, in a humidified incubator with 5%
CO2.
Electrical stimuli
The electrical stimuli were initiated 24 h after
cell seeding. Each well of the 6-well plate had a pair of platinum
electrodes (anode and cathode) placed in the cover. Function signal
generator (TFG6030 DDS Function Signal Generator, China) supplied
the electrical stimuli with a voltage of 2 V and a frequency of 100
Hz for 1 h per day. The stimulus parameters were detected by a
Rigol DS1022C Digital Oscilloscope (Rigol, Guangdong, China). The
experiments included five groups: AP/PLGA-stimulated (0.1, 1 and 5%
AP), AP/PLGA-unstimulated and PLGA-stimulated.
Detections
Following electrical stimulation, the methyl
thiazolyl tetrazolium (MTT; Sigma-Aldrich) method was used to
assess cell viability (14). The
absorbance was measured at 492 nm with a fully-automatic Microplate
Reader (Biotek, Seattle, WA, USA).
The osteogenesis-associated genes, including
collagen I, bone morphogenetic protein (BMP)-2 and osteonectin,
were detected by quantitative real-time polymerase chain reaction
(qPCR) (15). The RNA extraction
kit and reverse transcription reagents were purchased from Promega
(Madison, WI, USA); the qPCR kit was purchased from Stratagene (La
Jolla, CA, USA). Primers were designed based on GenBank reference
sequences and were verified by BLAST. Primer sequences are listed
in Table I. The qPCR amplification
program included predenaturation at 95°C for 2 min, denaturation at
95°C for 10 sec, annealing at 54°C for 20 sec and extension at 72°C
for 10 sec. Data analysis was performed using MxPro software
equipped with Real-Time PCR Amplifier (Stratagene). GAPDH was used
as the internal control.
 | Table IGene primer sequences. |
Table I
Gene primer sequences.
| Gene | Primer sequences |
|---|
| BMP-2 | F:
5′-GCAAGGTGTCTCCA-3′ |
| R:
5′-CGCTGTTTGTGTTTC-3′ |
| Collagen I | F:
5′-TCGCTCACCACCTTCTC-3′ |
| R:
5′-TAACCACTGCTCCACTCT-3′ |
| Osteonectin | F:
5′-CGAAGAGGAAGTAGTG-3′ |
| R:
5′-GAAGTGGCAGGAGGA-3′ |
| GAPDH | F:
5′-GATGGTGAAGGTCGGA-3′ |
| R:
5′-GTGGAGGTCAATGAAT-3′ |
Cells were lysed in ice-cold
radioimmunoprecipitation assay lysis buffer (Beyotime, Nanjing,
China) for 15 min. Proteins were separated on 10% SDS-PAGE gels and
electroblotted onto a polyvinylidene fluoride (PDVF) film. The PDVF
film was incubated overnight at 4°C with anti-BMP-2, anti-Smad4,
anti-collagen I, anti-osteonectin and anti-GAPDH monoclonal
antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA),
diluted 5,000 times with phosphate-buffered saline (PBS) containing
1% bovine serum albumin and rinsed 4 times by 0.01 M PBS, for 5 min
each time. Color was developed using the Western Blotting DAB
Testing kit (Beyotime). X-ray film exposure was performed, followed
by scanning and analyzing.
Origin 7.5 software was used for data processing and
statistical analysis. Comparisons between groups were conducted
with one-way ANOVA and Dunn’s ad hoc test. Pearson’s correlation
coefficient was used to analyze the correlation between AP content
and cell viability. P<0.05 was considered to indicate a
statistically significant difference.
Results
Preparation and characterization
UV-visible spectroscopy and cyclic voltammetry
verified that the AP/PLGA nano-composites were successfully
prepared and conductive. The conductivity of AP/PLGA nanocomposites
was significantly decreased compared to pure AP (data not
shown).
Cell viability
All AP/PLGA nanocomposites in combination with
electrical stimuli resulted in significantly higher cell viability
compared to 1% AP/PLGA alone. The stimuli were effective in
improving cell proliferation. Under electrical stimulation, 5 and
1% AP/PLGA nanocomposites resulted in significantly higher cell
viability compared to 0.5% AP/PLGA and PLGA. The ability of the
stimuli to improve cell proliferation increased in proportion to
the content of AP; however, there was no statistically
significantly correlation (Pearson’s correlation coefficients:
0.79, 0.93 and 0.89; Sig.: 0.22, 0.06 and 0.11, respectively)
(Table II).
 | Table IICell viability of osteoblasts cultured
on different materials (mean ± standard deviation, n=6). |
Table II
Cell viability of osteoblasts cultured
on different materials (mean ± standard deviation, n=6).
| Cell viability (%)
|
|---|
| Group | Day 1 | Day 3 | Day 7 |
|---|
| 5% AP/PLGA with
stimuli | 15.34a±1.33 | 57.51a±4.57 | 79.63a±5.17 |
| 1% AP/PLGA with
stimuli | 14.60a±0.85 | 50.14b±8.37 | 75.63b±6.17 |
| 0.5% AP/PLGA with
stimuli | 11.25b±1.14 | 43.21c±3.71 | 71.25c±6.73 |
| PLGA with
stimuli | 10.15b,c±0.53 | 42.14c, d±5.43 | 68.28c±5.27 |
| 1% AP/PLGA alone | 9.45c±0.64 |
41.67d±4.37b |
63.77d±6.23 |
Molecular expressions
Fig. 1 shows the
expression of BMP-2, collagen I and osteonectin. The mRNA levels
under electrical stimulation were significantly higher than those
without stimulation. Under stimulation, the mRNA levels increased
in proportion to the content of AP.
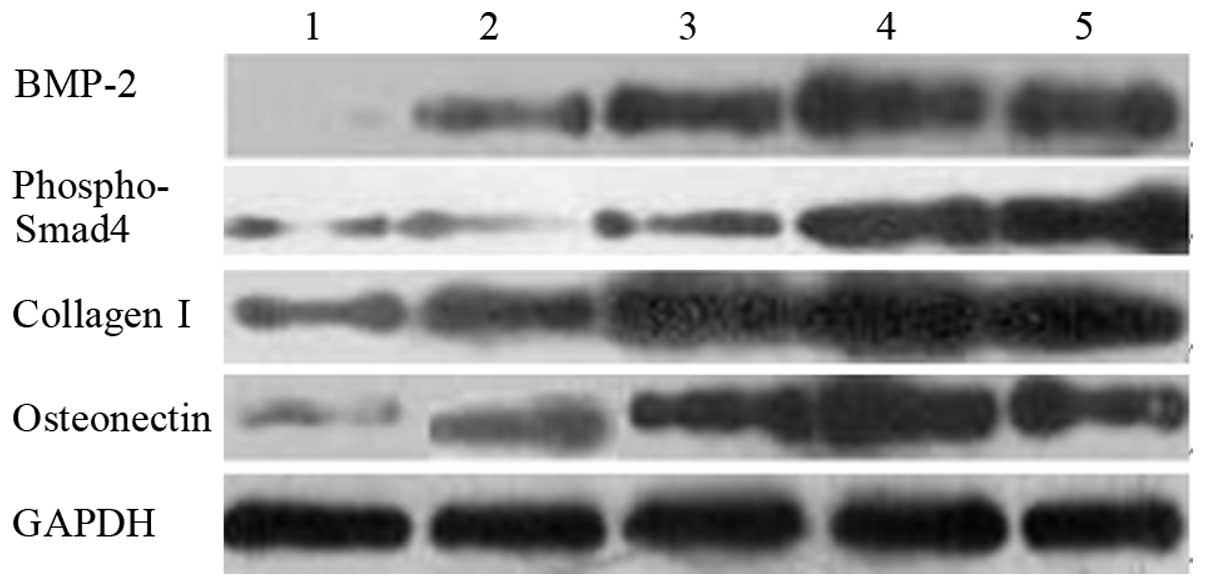
Fig. 2 shows the
results of the western blot analysis of BMP-2, phospho-Smad4,
collagen I and osteonectin. Their grayscales are listed in Table III. The protein levels under
electrical stimulation were higher than those without stimulation.
Under stimulation, the protein levels increased in proportion to
the content of AP.
 | Table IIIGrayscales vs. GAPDH as detected by
western blot analysis in 7 days (mean ± standard deviation,
n=3). |
Table III
Grayscales vs. GAPDH as detected by
western blot analysis in 7 days (mean ± standard deviation,
n=3).
| 1% AP/PLGA alone | PLGA with
stimuli | 0.5% AP/PLGA with
stimuli | 1% AP/PLGA with
stimuli | 5% AP/PLGA with
stimuli |
|---|
| BMP-2 | 9.83±0.87 | 39.31±3.17 | 54.46±3.22 | 66.57±4.37 | 71.35±2.14 |
| Phospho-Smad4 | 14.25±3.27 | 21.13±1.42 | 30.27±2.17 | 87.35±3.24 | 93.28±3.11 |
| Collagen I | 35.69±1.17 | 64.21±3.75 | 86.69±4.57 | 91.57±3.61 | 95.45±4.51 |
| Osteonectin | 25.89±4.79 | 53.74±3.57 | 79.00±3.97 | 94.23±3.19 | 92.36±3.26 |
| GAPDH | 100.1±6.31 | 99.25±3.29 | 101.1±3.47 | 100.4±3.11 | 100.3±2.59 |
Discussion
The electric field and APs alone may improve the
proliferation and osteogenic differentiation of osteoblasts. In the
present study, rat-derived osteoblasts were cultured on AP/PLGA
nano-composite films, simultaneously exposed to electrical stimuli.
The electrical stimulation combined with the AP implants
significantly improved the proliferation and osteogenic
differentiation of osteoblasts, compared to when each agent was
used individually.
Various cell functions involve complicated signal
transduction (16). The chemical
signals are the main form of information transmission between
cells; however, the intraand intercellular electrical signal
transduction is an important contributing factor to cell behavior.
Electric fields may directly promote the proliferation of
osteoblasts. Two mechanisms may be involved in electric fields
acting on osteoblasts. A previous study by de Barros Filho et
al(2) reported that magnetic
fields may directly promote DNA and protein synthesis in rat cells.
Blank and Goodman (16) reported
that pulsed electric fields may act directly on osteoblast DNA,
affecting gene expression, thereby promoting the proliferation of
osteoblasts. The electrical stimulation of osteoblasts may also be
mediated by calcium ions or cAMP. There is evidence supporting this
hypothesis. Normally, the gap junctional intercellular
communication (GJIC), which is responsible for the transport of
calcium ions or cAMP, is inhibited during normal tissue
regeneration and cell proliferation; electric fields may inhibit
the function of GJIC, thereby promoting cell proliferation
(18).
Only cells that adhere to materials are able to
migrate, differentiate and proliferate and the presence of a
scaffold is necessary for tissue engineering and osteogenesis. APs
exhibit higher solubility and conductivity. Therefore, in the
present study, AP and PLGA were composited to produce electroactive
biodegradable polymer nanocomposites, which appear promising for
research or medical applications, including tissue engineering and
regeneration of bone, cartilage or other tissues. As a result,
electrical stimuli combined with AP/PLGA nanocomposite implants led
to more positive outcomes compared to when used alone.
BMPs, particularly BMP-2, may induce mesenchymal
stem cell differentiation into cartilage and bone, namely promote
bone formation, by inducing formation and promoting proliferation
of osteoblasts (19). Smad4 is a
key signal involved in BMP expression, which promotes
osteo-differentiation and -formation. Osteonectin, a
non-collagenous matrix protein abundantly found in bone matrix,
plays an important role in the process of mineralization by
promoting mineral deposition in collagen (20). Osteonectin may promote extracellular
matrix synthesis and increase the rate of bone matrix deposition
(21). Collagen I is the scaffold
for calcium deposition and cell adhesion. Collagen fibers comprise
∼95% of the organic matter of the bone matrix and collagen I in
particular comprises ∼90%. Collagen I expression is initiated in
the cell proliferation phase and reaches its peak in the matrix
synthesis phase. In the proliferation phase, osteoblasts increase
in number to form multilayers of cells, merge and secrete collagen
I in order to mineralize and achieve the formation of bone nodules.
Thereupon, BMP-2, Smad4, collagen I and osteonectin play important
roles in the osteogenic process (22).
In the present study, qPCR and western blot analysis
demonstrated that the expression of BMP-2, Smad4, collagen I and
osteonectin was more upregulated by the combination of electrical
stimuli and AP/PLGA, compared to each agent used alone, indicating
that the combination may integrate and amplify the effects of the
individual constituents.
In conclusion, AP and PLGA were successfully blended
to create electroactive biodegradable nanocomposite AP/PLGA. The
electrical stimuli, combined with the nanocomposites, may
upregulate the expression levels of BMP-2, Smad4, collagen I and
osteonectin and promote proliferation of rat-derived osteoblasts,
compared to when each agent was used individually. The
electroactive biodegradable nanocomposite AP/PLGA bears the
potential for bone tissue engineering under electrical
stimulation.
References
|
1
|
Ungethum M: Osteogenesis and bone growth.
Modification by electrical and electromagnetic effects. MMW Munch
Med Wochenschr. 124:621–622. 1982.(In German).
|
|
2
|
de Barros Filho TE, Rossi JD, Lage Lde A,
Rodrigues CJ, de Oliveira AS, Pinto FC, dos Reis GM and Rodrigues
Júnior AJ: Effect of electromagnetic fields on osteogenesis: an
experimental study on rats. Rev Hosp Clin Fac Med Sao Paulo.
47:128–130. 1992.(In Portuguese).
|
|
3
|
Bekhite MM, Finkensieper A, Abou-Zaid FA,
El-Shourbagy IK, Omar KM, Figulla HR, Sauer H and Wartenberg M:
Static electromagnetic fields induce vasculogenesis and
chondro-osteogenesis of mouse embryonic stem cells by reactive
oxygen species-mediated up-regulation of vascular endothelial
growth factor. Stem Cells Dev. 19:731–743. 2010. View Article : Google Scholar
|
|
4
|
Pietak AM, Reid JW, Stott MJ and Sayer M:
Silicon substitution in the calcium phosphate bioceramics.
Biomaterials. 28:4023–4032. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang L, Fan H, Zhang ZY, Lou AJ, Pei GX,
Jiang S, Mu TW, Qin JJ, Chen SY and Jin D: Osteogenesis and
angiogenesis of tissue-engineered bone constructed by
prevascularized β-tricalcium phosphate scaffold and mesenchymal
stem cells. Biomaterials. 31:9452–9461. 2010.PubMed/NCBI
|
|
6
|
Gurkan UA, Kishore V, Condon KW, Bellido
TM and Akkus O: A scaffold-free multicellular three-dimensional in
vitro model of osteogenesis. Calcif Tissue Int. 88:388–401. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao J, Han W, Chen H, Tu M, Huan S, Miao
G, Zeng R, Wu H, Cha Z and Zhou C: Fabrication and in vivo
osteogenesis of biomimetic poly(propylene carbonate) scaffold with
nanofibrous chitosan network in macropores for bone tissue
engineering. J Mater Sci Mater Med. 23:517–525. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu F, Chen S and Wang C, Yuan R, Xiang Y
and Wang C: Multi-wall carbon nanotube-polyaniline biosensor based
on lectin-carbohydrate affinity for ultrasensitive detection of Con
A. Biosens Bioelectron. 34:202–207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Plonska-Brzezinska ME, Mazurczyk J, Palys
B, Breczko J, Lapinski A, Dubis AT and Echegoyen L: Preparation and
characterization of composites that contain small carbon
nano-onions and conducting polyaniline. Chemistry. 18:2600–2608.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang Y and Luan J: Synthesis, property
characterization and photocatalytic activity of the novel composite
polymer poly-aniline/Bi2SnTiO7. Molecules.
17:2752–2772. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wong JY, Langer R and Ingber DE:
Electrically conducting polymers can noninvasively control the
shape and growth of mammalian cells. Proc Natl Acad Sci USA.
91:3201–3204. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang L, Hu J, Lang L, Wang X, Zhang P,
Jing X, Wang X, Chen X, Lelkes PI, Macdiarmid AG and Wei Y:
Synthesis and characterization of electroactive and biodegradable
ABA block copolymer of polylactide and aniline pentamer.
Biomaterials. 28:1741–1751. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang J, Li Q, Wang Y, Wang Y, Dong L, Xie
H and Xiong C: Self-suspended polyaniline doped with a protonic
acid containing a polyethylene glycol segment. Chem Asian J.
6:2920–2924. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Feng W and Jia S: Rapamycin inhibits the
invasive ability of thyroid cancer cells by down-regulating the
expression of VEGF-C in vitro. Cell Biochem Funct. 30:487–491.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Galassi F, Kaman WE, Anssari Moin D, van
der Horst J, Wismeijer D, Crielaard W, Laine ML, Veerman EC, Bikker
FJ and Loos BG: Comparing culture, real-time PCR and fluorescence
resonance energy transfer technology for detection of
Porphyromonas gingivalis in patients with or without
peri-implant infections. J Periodontal Res. 47:616–625. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Blank M and Goodman R: Do electromagnetic
fields interact directly with DNA? Bioelectromagnetics. 18:111–115.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang L, Zhuang X, Hu J, Lang L, Zhang P,
Wang Y, Chen X, Wei Y and Jing X: Synthesis of biodegradable and
electroactive multiblock polylactide and aniline pentamer copolymer
for tissue engineering applications. Biomacromolecules. 9:850–858.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li CM, Chiang H, Fu YD, Lu DQ and Shao J:
Exposure to 50-Hz electromagnetic fields: effects of time and field
strength on GAP junctional intercellular communications.
Electromagn Biol Med. 18:249–256. 1999. View Article : Google Scholar
|
|
19
|
Kim SS, Park MS, Jeon O, Choi CY and Kim
BS: Poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds
for bone tissue engineering. Biomaterials. 27:1399–1409. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Termine JD, Kleinman HK, Whitson SW, Conn
KM, McGarvey ML and Martin GR: Osteonectin, a bone-specific protein
linking mineral to collagen. Cell. 26:99–105. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ribeiro N, Sousa SR and Monteiro FJ:
Influence of crystallite size of nanophased hydroxyapatite on
fibronectin and osteonectin adsorption and on MC3T3-E1 osteoblast
adhesion and morphology. J Colloid Interface Sci. 351:398–406.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lopez JM, Balemans W, Piters E, van Hul W
and González G: Genetic analysis and effect of triiodothyronine and
prednisone trial on bone turnover in a patient with craniotubular
hyperostosis. Bone. 43:405–409. 2008. View Article : Google Scholar : PubMed/NCBI
|