Introduction
Hypertriglyceridemia (HTG) is associated with acute
pancreatitis in 12–38% of reported cases. The relationship between
these diseases and the role of hypertriglyceridemia in acute
pancreatitis pathogenesis remains to be elucidated (1). Although the pathogenesis of acute
pancreatitis depends on a number of factors, its recurrent form is
the most common complication of HTG and is often observed in type I
hyperlipoproteinemic patients with various lipoprotein lipase (LPL)
or apolipoprotein CII gene mutations (2). LPL-deficient HTG heterozygous mice
were rescued by somatic gene transfer of a mutant LPL gene. The
surviving animals exhibited LPL deficiency with high plasma
triglyceride (TG) levels during adulthood (3). Thus, this study aimed to assess the
susceptibility of LPL-deficient heterozygous mice with HTG to
pancreatitis. Furthermore, gas chromatography/mass spectrometry
(GC/MS) was utilized to compare serum metabolite levels between
normal controls and hyperlipidemic acute pancreatitis (HLP)
subjects.
The recent revival of systems biology that
integrates the pertinent components, such as genes, proteins and
metabolites, into a holistic biological network provides a
comprehensive understanding of system behavior (4,5).
Metabolomic profiling is a practical approach used to determine
system response to perturbations by measuring variations in small
molecule/endpoint metabolic products resulting from systemic
biochemical regulation (6). In this
study, we aimed to demonstrate the use of GC/MS for HLP serum
metabolic analysis, and provide potential metabolic biomarkers to
distinguish HLP subjects from normal subjects.
Materials and methods
Animals
LPL-deficient mice were rescued from neonatal death
by intramuscular injection of an adenoviral vector coding a
naturally occurring human LPL beneficial mutant, Ad-LPLS447X, as
previously described (3). Animals
injected with saline were used as the control. Genotyping was
performed by polymerase chain reaction. LPL-deficient heterozygous
mice were hybridized by female wild-type and LPL-deficient mice.
Female wild-type and LPL-deficient mice with a C57B6 background at
8 weeks weighing 13–16 g were used for the experiments. The animals
were fed a normal chow diet and had free access to water. The
‘Principles of Laboratory Animal Care’ (NIH publication no. 85-23,
revised 1996) was followed and the experimental protocol was
approved by the Animal Care Committee, Peking University Health
Science Center.
Experimental group
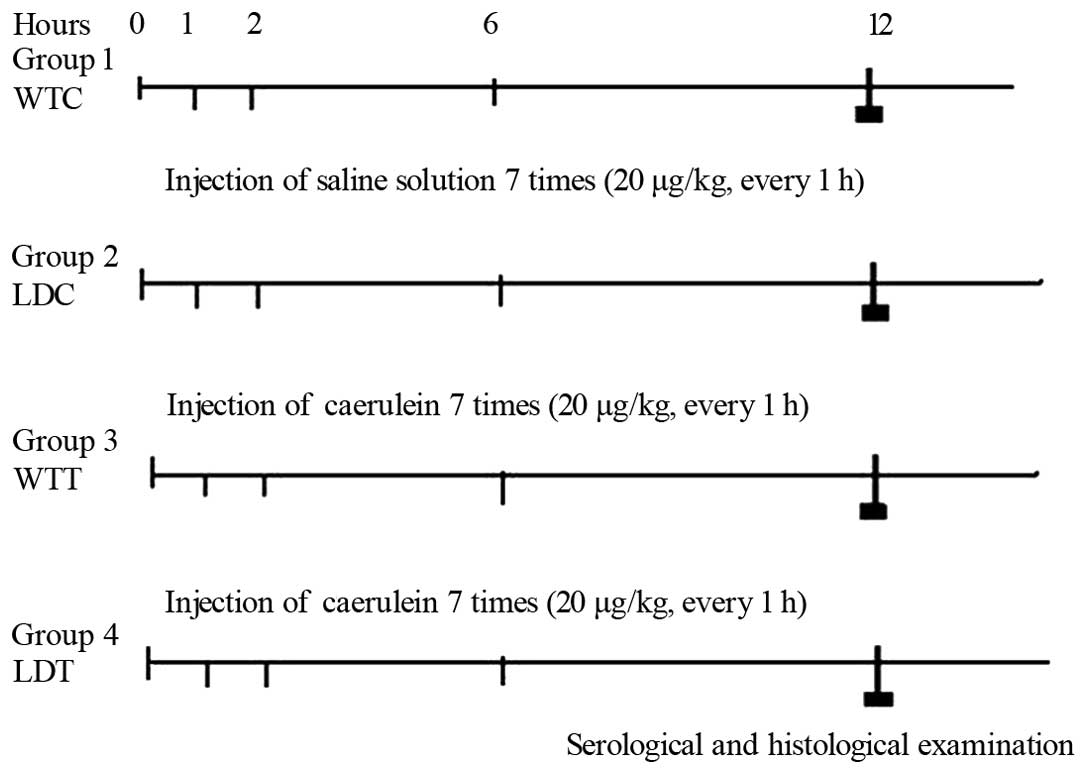
The experimental protocol is shown in Fig. 1. Four groups were prepared: group 1
[WTC, wild-type control: 0.9% sodium chloride was intraperitoneally
(i.p.) injected 7 times over 1 day], group 2 (LDC, LPL-deficient
heterozygous control), group 3 [WTT, acute pancreatitis model;
caerulein was i.p. injected 7 times over 1 day, Sigma Aldrich
Chemie GmbH (Steinheim, Germany) dosage, 20 μg/kg b.w. every
hour] and group 4 (LDT HLP model). Blood samples were collected 12
h after injection to measure amylase, triacylglycerol (TG) and
conduct GC/MS analysis. Mice were then sacrificed and pancreatic
tissues were fixed in buffered formalin and embedded in
paraffin.
Biochemical measurement and
histopathology
Serum was analyzed for amylase and TG concentrations
using commercial kits (Biosino Bio-Technology and Science, Beijing,
China). Histological samples were fixed in 10% buffered formalin,
embedded in paraffin, sectioned and stained with hematoxylin and
eosin stain (H&E).
GC/MS sample preparation, derivatization
and spectral acquisition
Serum samples collected from fasting subjects were
kept frozen at −80°C until use, at which point the ice samples were
thawed. Each 200-μl aliquot of the serum samples was added
to a 1.5 ml tube followed by the addition of 400 μl of
acetone for protein precipitation. The mixture was vortexed for 30
sec and centrifuged at 10,000 × g for 10 min. A 400-μl
supernatant was transferred to a 500 μl of glass tube and
dried under vacuum. The dried analytes were dissolved in 80
μl of methoxylamine hydrochloride (15 mg/ml, dissolved in
pyridine) for 90 min at 30°C and then silylated with 80 μl
N,O-bis(trimethylsilyl)-trifluoroacetamide (BSTFA) and
trimethylchlorosilane (at a ratio of 99:1) (Supelco, Bellefonte,
PA, USA) for 2 h at 70°C. Each 70-μl aliquot of hexane was
added to the derivatization flasks. After the sample was stirred
for 1 min and kept at room temperature for 1 h, 1-μl aliquot
of the solution was injected into a PerkinElmer GC coupled with a
TurboMass-Autosystem XL mass spectrometer (PerkinElmer, Inc., St.
Waltham, MA, USA) in the splitless mode. A DB-5MS capillary column
coated with 5% diphenyl cross-linked 95% dimethylpolysiloxane (30 m
× 250 μm i.d., 0.25-μm film thickness; Agilent
J&W Scientific, Folsom, CA, USA) was used for separation. The
injection and interface temperatures were set to 260°C and the ion
source temperature was adjusted to 200°C. Initial GC oven
temperature was set at 80°C for 2 min following injection and then
raised up to 285°C with 5°C/min and maintained at 285°C for 7 min.
Helium at a flow rate of 1 ml/min was used as the carrier gas.
Measurements were made with electron impact ionization (70 eV) in
the full scan mode (m/z 30–550) (7).
Data analysis
Biochemical measurement results were expressed as
the mean ± standard deviation (SD). Statistical evaluation was
performed with SPSS 11.0 and differences between the 2 groups were
tested by independent t-tests. P<0.05 was considered
statistically significant.
GC/MS data files were converted into NetCDF format
via DataBridge (PerkinElmer, Inc.) and pretreatment was conducted
as previously described (8). The
mean-centered and autoscaled data were then introduced into SIMCA-P
11.5 Software (Umetrics, Umeå, Sweden) for multivariate statistical
analysis. Principal component analysis (PCA) was used to obtain an
overview of variations among the different groups.
Orthogonal-projections to latent structures-discriminant analysis
(OPLS-DA), a supervised pattern recognition approach, was utilized
to construct a predictive model to identify the differential
metabolites involved in causing disease. To avoid the overfitting
of the models, the OPLS-DA model was carefully validated by the
following three steps: i) an iterative 7-round cross-validation
with one seventh of the samples being excluded from the model in
each round (9); ii) 1,000 random
permutations test (10); iii) blind
prediction test in which the data set was randomly divided into the
training set (70%) and test set (30%) and the model built on the
training set was applied to construct the classification model to
predict the class membership of the test set (11).
Results
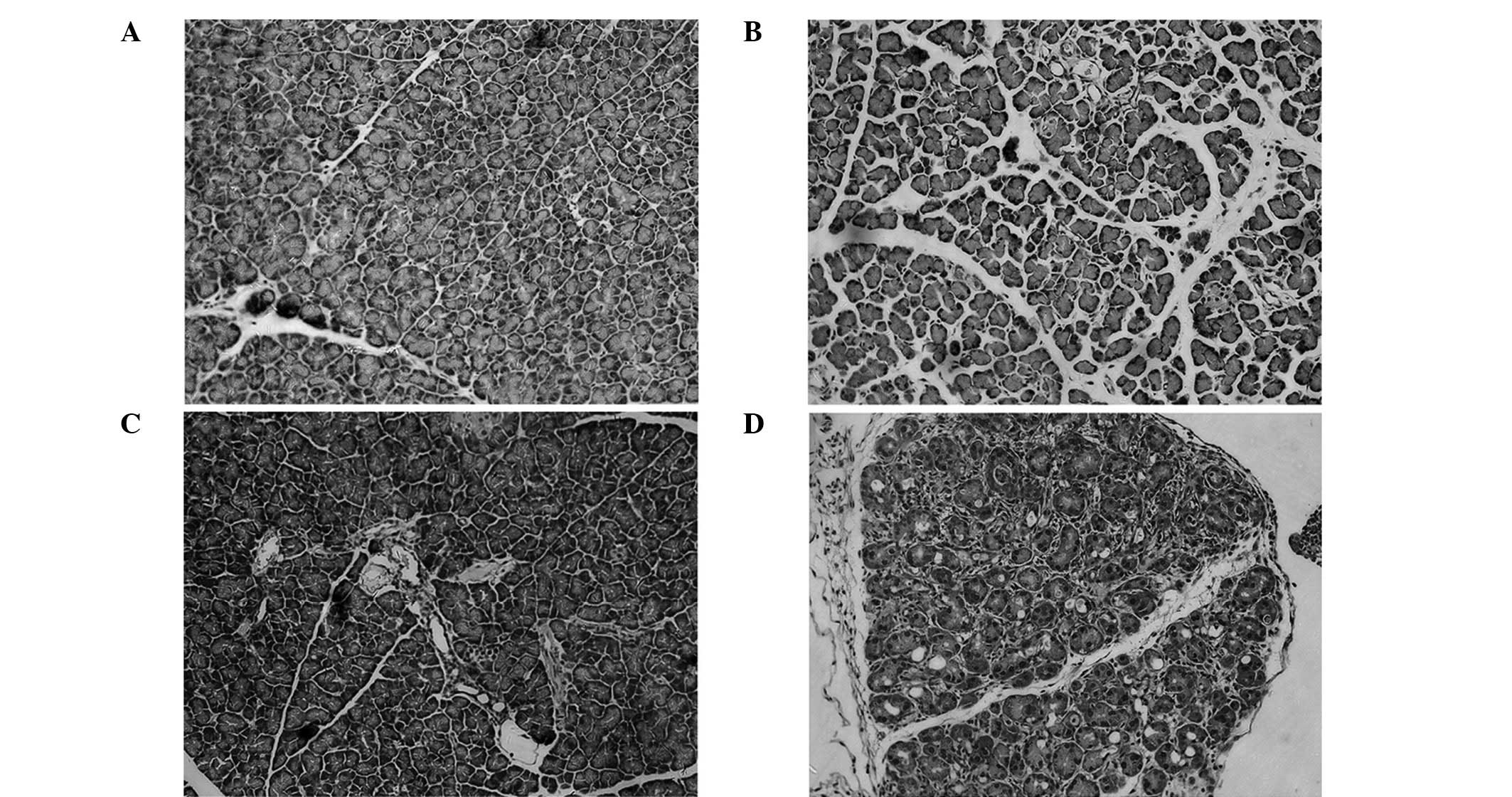
Histopathologic observation
No discernable pathological alteration in either
wild-type or LPL-deficient heterozygous mice injected relative to
the saline-injected control was observed. However, mice injected
with caerulein exhibited pancreatic injury including edema,
neutrophil infiltration, hemorrhage and parenchymal necrosis. In
LPL-deficient heterozygous mice injected with caerulein, pancreatic
injury was more severe compared to other mice (Fig. 2).
Measurement of serum amylase and plasma
triglyceride (TG) levels
Serum amylase levels increased after caerulein
injection in both wild-type and LPL-deficient mice, but were
significantly higher in LPL-deficient heterozygous mice 12 h after
injection. The difference between wild-type control and
LPL-deficient control mice was not significant. Moreover, the
plasma TG levels in LPL-deficient mice were significantly higher
than the control animals (Table I,
P<0.05).
 | Table IPlasma triglyceride (TG) and plasma
amylase levels in pancreatic injury. |
Table I
Plasma triglyceride (TG) and plasma
amylase levels in pancreatic injury.
| Wild-type
control | LPL-deficient
control | Wild-type, after
caerulein | LPL-deficient, after
caerulein |
|---|
| Plasma TG mm/l
(n=7) | 0.91±0.02 | 3.58±0.03 | 0.93±0.03 | 3.56±0.02 |
| Plasma amylase
μ/l (n=7) | 358.33±42.72 | 471.29± 22.74 |
2500.89±409.59a |
3685.06±483.98a |
GC/MS spectroscopic data analysis
Compound identification was carried out either by
comparing mass spectra and retention time with values obtained from
commercially available reference compounds or based on commercial
libraries of NIST, NBS and Wiley. We were able to identify
metabolites, primarily organic acids, amino acids and free fatty
acids (FFA). The OPLS-DA model calculated from GC/MS data of HLP
vs. wild-type subjects was employed. There were more differentially
expressed metabolites identified in HLP compared to the healthy
group (Table II and Fig. 3), including compounds such as
valine, leucine, glutamine, proline, citrate, malic acid,
tetradecanoic acid (14:0) and oleic acid (18:1).
 | Table IIStatistical analysis of differentially
expressed endogenous metabolites correlated with HLP and wild-type
mice. |
Table II
Statistical analysis of differentially
expressed endogenous metabolites correlated with HLP and wild-type
mice.
| Rt/min | Metabolites | Correlation
coefficient | KEGG pathway |
|---|
| 9.38 | Valine | 0.32 | Amino acid
metabolism |
| 10.42 | Leucine | 0.33 | Amino acid
metabolism |
| 15.21 | Citrate | −0.26 | Energy
metabolism |
| 16.24 | Malic acid | −0.3 | Energy
metabolism |
| 17.00 | Proline | 0.3 | Amino acid
metabolism |
| 17.34 | Tetradecanoic acid
(14:0) | 0.26 | Fatty acid
metabolism |
| 17.36 | Glutamine | −0.35 | Energy
metabolism |
| 31.32 | Oleic acid
(18:1) | 0.32 | Fatty acid
metabolism |
Discussion
HLP typically presents as an episode of acute
pancreatitis or recurrent acute pancreatitis (1) and has a high morbidity rate. It is
important to determine the pathogenetic factors involved as the
disease is treatable and recurrences can be prevented (12). The mechanisms by which
hyperlipidemia induces acute severe pancreatitis have yet to be
elucidated.
LPL-deficient heterozygous mice developed
pancreatitis subequent to caerulein stimulation. It appears that
pancreatic cells are more susceptible to damage by an additional
stimulus or injury in the presence of high TG plasma levels
surrounding pancreatic cells (13).
Once cells are damaged, high concentrations of pancreatic lipase,
exceeding normal blood plasma levels, may reach the
interstitium.
Although various animal models for chronic, acute or
severe pancreatitis have previously been established and
investigated (14–16), no appropriate HLP model has been
developed. In this study, by using the combination of LPL-deficient
heterozygous mice and injection with caerulein, we established
animal models for HLP and conducted further studies using these
models.
Since HLP is a metabolic disorder that disturbs the
metabolism of carbohydrates, lipids and amino acids, the
pathological process likely involves an altered expression of
downstream low molecular weight metabolites such as glucose. This
study was designed to visualize the alteration of global serum
metabolites associated with HLP pathophysiology. Compared to
healthy controls, the HLP subjects exhibited altered serum
metabolites (Table II) including
significantly increased valine, leucine, proline, tetradecanoic
acid (14:0) and oleic acid (18:1), as well as decreased citrate,
malic acid and glutamine levels. Several pathways involving the
tricarboxylic acid (TCA) cycle (citrate and malic acid) and
glutamate and glutamine biosynthesis were affected.
Citrate, malic acid and glutamine are crucial
components of the TCA cycle, which is the main pathway of glucose
degradation and primary energy supplier for most organisms. These
compounds were significantly decreased in the HLP subjects than in
the healthy control animals, suggesting that HLP may inhibit the
activity of these compounds, ultimately repressing energy
metabolism. As the metabolic enzymes of the TCA cycle are located
mainly in the mitochondria, TCA cycle disorder results in disrupted
mitochondrial function.
Compared to healthy controls, the HLP subjects
exhibited altered serum metabolites (Table II) including significantly increased
oleic acid (18:1) and tetradecanoic acid (14:0). This suggests a
hypercatabolic state in HLP subjects, which is consistent with
previously reported results (17–19). A
well-accepted mechanism initially proposed by Havel (20) states that TG hydrolysis in and
around the pancreas by pancreatic lipase seeping out of the acinar
cells, leading to the accumulation of FFA in high concentrations.
FFA may disturb microcirculation in the pancreas via detrimental
effects on the vessel endothelium (21,22). A
metabolic factor may play a key role in HLP pathogenesis.
In conclusion, LPL-deficient mice with high HTG have
enhanced susceptibility to pancreatitis. Serum metabolite profiling
using GC/MS in conjunction with modern multi variate statistical
techniques permits non-invasive, simultaneous monitoring of
numerous metabolic pathways. Significantly altered serum
metabolites were detected in HLP subjects and these changes impact
some biochemical pathways.
Abbreviations:
|
FFA
|
free fatty acid
|
|
HTG
|
hypertriglyceridemia
|
|
GC/MS
|
gas chromatography/mass
spectrometry
|
|
PCA
|
principal component analysis
|
|
OPLS-DA
|
orthogonal projections to latent
structures discriminant analysis
|
|
TG
|
triglyceride
|
|
HLP
|
hyperlipidemic acute pancreatitis
|
|
H&E
|
hematoxylin and eosin stain
|
|
LPL
|
lipoprotein lipase
|
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (grant no. 30971359).
We would like to thank the technical support of Li Cheng, Zhenjun
Gao and Kai Wu at the Department of Gastroenterology of Shanghai
Jiaotong University Affiliated First People’s Hospital.
References
|
1
|
Yadav D and Pitchumoni CS: Issues in
hyperlipidemic pancreatitis. J Clin Gastroenterol. 36:54–62. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Toskes PP: Hyperlipidemic pancreatitis.
Gastroenterol Clin North Am. 19:783–791. 1990.PubMed/NCBI
|
|
3
|
Ross CJ, Liu G, Kuivenhoven JA, et al:
Complete rescue of lipoprotein lipase-deficient mice by somatic
gene transfer of the naturally occurring LPLS447X beneficial
mutation. Arterioscler Thromb Vasc Biol. 25:2143–2150. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hood L and Perlmutter RM: The impact of
systems approaches on biological problems in drug discovery. Nat
Biotechnol. 22:1215–1217. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hwang D, Rust AG, Ramsey S, et al: A data
integration methodology for systems biology. Proc Natl Acad Sci
USA. 102:17296–17301. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nicholson JK, Holmes E and Wilson ID: Gut
microorganisms, mammalian metabolism and personalized health care.
Nat Rev Microbiol. 3:431–438. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bao Y, Zhao T, Wang X, et al: Metabonomic
variations in the drug-treated type 2 diabetes mellitus patients
and healthy volunteers. J Proteome Res. 8:1623–1630. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang X, Su M, Qiu Y, et al: Metabolic
regulatory network alterations in response to acute cold stress and
ginsenoside intervention. J Proteome Res. 6:3449–3455. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ni Y, Su M, Lin J, et al: Metabolic
profiling reveals disorder of amino acid metabolism in four brain
regions from a rat model of chronic unpredictable mild stress. FEBS
Lett. 582:2627–2636. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Westerhuis JA, Hoefsloot HCJ, Smit S, et
al: Assessment of PLSDA cross validation. Metabolomics. 4:81–89.
2008. View Article : Google Scholar
|
|
11
|
Jellum E, Bjornson I, Nesbakken R, et al:
Classification of human cancer cells by means of capillary gas
chromatography and pattern recognition analysis. J Chromatogr.
217:231–237. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Karne S and Gorelick FS: Etiopathogenesis
of acute pancreatitis. Surg Clin North Am. 79:699–710. 1999.
View Article : Google Scholar
|
|
13
|
Wang Y, Sternfeld L, Yang F, et al:
Enhanced susceptibility to pancreatitis in severe
hypertriglyceridaemic lipoprotein lipase-deficient mice and
agonist-like function of pancreatic lipase in pancreatic cells.
Gut. 58:422–430. 2009. View Article : Google Scholar
|
|
14
|
Frossard JL and Pastor CM: Experimental
acute pancreatitis: new insights into the pathophysiology. Front
Biosci. 7:d275–d287. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Esposito I, Friess H and Buchler MW:
Molecular mechanisms in chronic pancreatitis. Zentralbl Chir.
126:867–872. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gaeta L, Dionigi G and Dionigi R:
Experimental models of acute pancreatitis. Critical review. Chir
Ital. 52:469–497. 2000.(In Italian).
|
|
17
|
Chen M, Zhao L and Jia W: Metabonomic
study on the biochemical profiles of a hydrocortisone-induced
animal mode. J Proteome Res. 4:2391–2396. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen M, Su M, Zhao L, et al: Metabonomic
study of aristolochic acid-induced nephrotoxicity in rats. J
Proteome Res. 5:995–1002. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Holmes E, Nicholson JK, et al:
Metabonomic investigations in mice infected with Schistosoma
mansoni: an approach for biomarker identification. Proc Natl Acad
Sci USA. 101:12676–12681. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Havel RJ: Pathogenesis, differentiation
and management of hypertriglyceridemia. Adv Intern Med. 15:117–154.
1969.PubMed/NCBI
|
|
21
|
Hofbauer B, Saluja AK, Lerch MM, et al:
Intra-acinar cell activation of trypsinogen during
caerulein-induced pancreatitis in rats. Am J Physiol.
275:G352–G362. 1998.PubMed/NCBI
|
|
22
|
Mayer J, Rau B, Schoenberg MH, et al:
Mechanism and role of trypsinogen activation in acute pancreatitis.
Hepatogastroenterology. 46:2757–2763. 1999.PubMed/NCBI
|