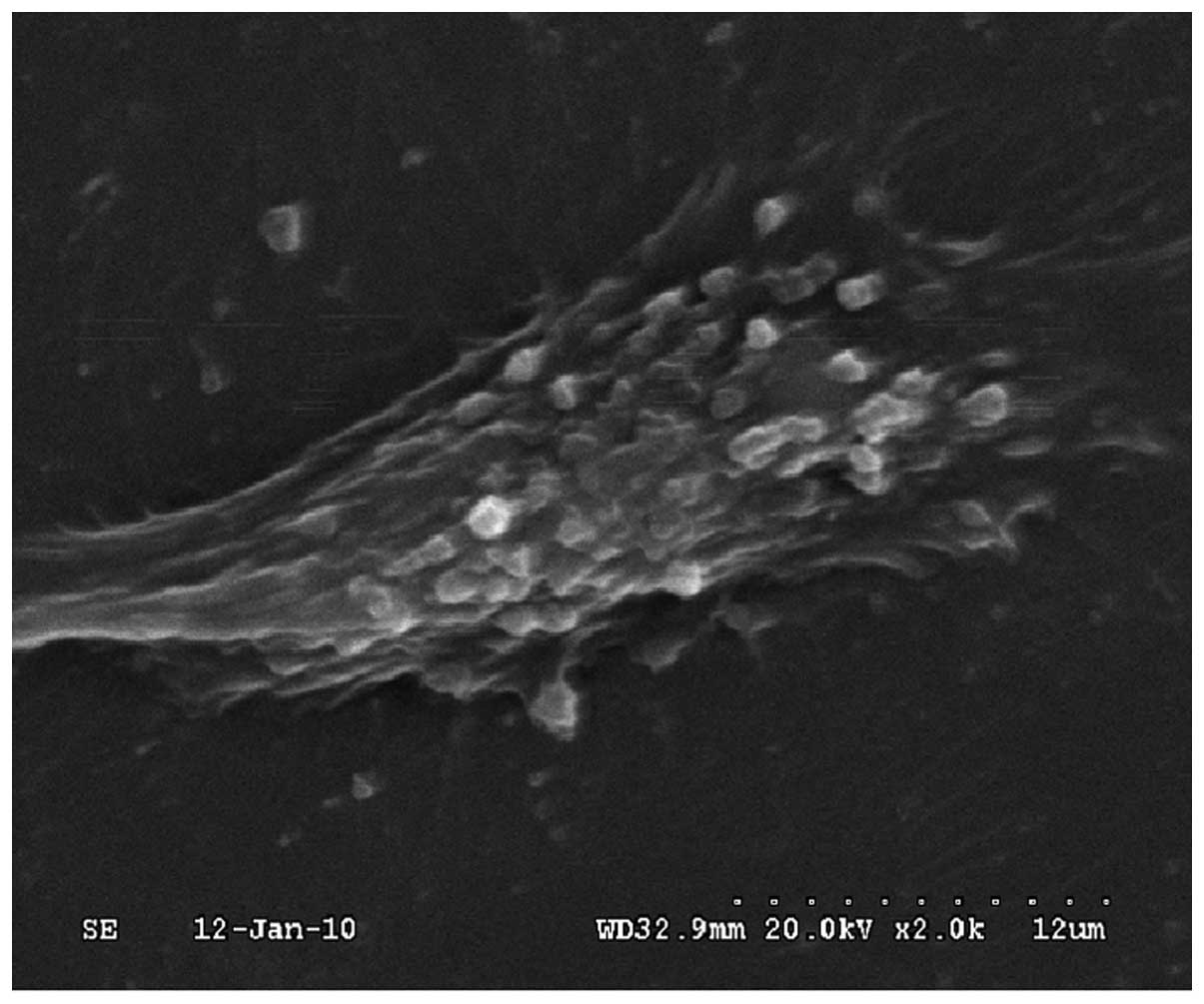
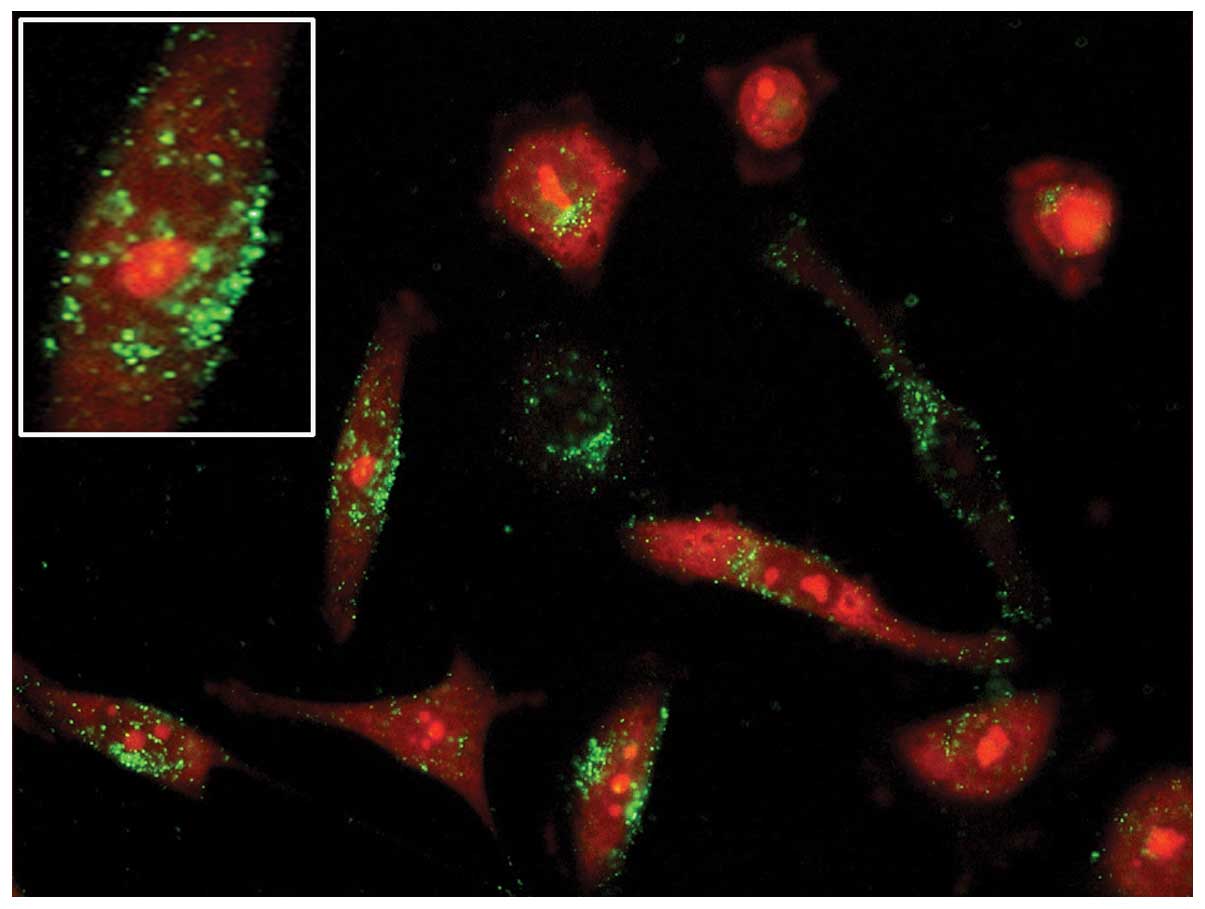
|
1
|
Spolverato G, Ejaz A, Azad N and Pawlik
TM: Surgery for colorectal liver metastases: The evolution of
determining prognosis. World J Gastrointest Oncol. 5:207–221. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alberts SR, Sargent DJ, Nair S, et al:
Effect of oxaliplatin, fluorouracil, and leucovorin with or without
cetuximab on survival among patients with resected stage III colon
cancer: a randomized trial. JAMA. 307:1383–1393. 2012. View Article : Google Scholar
|
|
3
|
Garcia-Foncillas J and Diaz-Rubio E:
Progress in metastatic colorectal cancer: growing role of cetuximab
to optimize clinical outcome. Clin Transl Oncol. 12:533–542. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wiseman LR, Adkins JC, Plosker GL, et al:
Oxaliplatin: a review of its use in the management of metastatic
colorectal cancer. Drugs Aging. 14:459–475. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Simpson D, Dunn C, Curran M and Goa KL:
Oxaliplatin: a review of its use in combination therapy for
advanced metastatic colorectal cancer. Drugs. 63:2127–2156. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang DY, Li Y, Liu JH, et al: Efficacy and
tolerance of maintenance therapy in patients with incurable
advanced colorectal cancer. J Southern Med Uni. 33:1815–1818.
2013.(In Chinese).
|
|
7
|
Brodowicz T, Ciuleanu TE, Radosavljevic D,
et al: FOLFOX4 plus cetuximab administered weekly or every second
week in the first-line treatment of patients with KRAS wild-type
metastatic colorectal cancer: a randomized phase II CECOG study.
Ann Oncol. 24:1769–1777. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Douillard JY, Oliner KS, Siena S, et al:
Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal
cancer. N Engl J Med. 369:1023–1034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Messersmith WA, Jimeno A, Jacene H, et al:
Phase I trial of oxaliplatin, infusional 5-fluorouracil, and
leucovorin (FOLFOX4) with erlotinib and bevacizumab in colorectal
cancer. Clin Colorectal Cancer. 9:297–304. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McWhinney SR, Goldberg RM and McLeod HL:
Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther. 8:10–16.
2009. View Article : Google Scholar
|
|
11
|
Ochenduszko SL and Krzemieniecki K:
Targeted therapy in advanced colorectal cancer: more data, more
questions. Anticancer Drugs. 21:737–748. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cortejoso L, Garcia MI, Garcia-Alfonso P,
et al: Differential toxicity biomarkers for irinotecan- and
oxaliplatin-containing chemotherapy in colorectal cancer. Cancer
Chemother Pharmacol. 71:1463–1472. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Di Francia R, Siesto RS, Valente D, et al:
Pharmacogenomics panel test for prevention toxicity in patient who
receive fluoropirimidine/oxaliplatin-based therapy. Eur Rev Med
Pharmacol Sci. 16:1211–1217. 2012.PubMed/NCBI
|
|
14
|
Hoff PM, Saad ED, Costa F, et al:
Literature review and practical aspects on the management of
oxaliplatin-associated toxicity. Clin Colorectal Cancer. 11:93–100.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Olszewski U and Hamilton G: A better
platinum-based anticancer drug yet to come? Anticancer Agents Med
Chem. 10:293–301. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Patil YP and Jadhav S: Novel methods for
liposome preparation. Chem Phys Lipids. 177:8–18. 2014. View Article : Google Scholar
|
|
17
|
Jain RL and Shastri JP: Study of ocular
drug delivery system using drug-loaded liposomes. Int J Pharm
Investig. 1:35–41. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suntres ZE: Liposomal antioxidants for
protection against oxidant-induced damage. J Toxicol.
2011:1524742011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pagano RE and Weinstein JN: Interactions
of liposomes with mammalian cells. Annu Rev Biophys Bioeng.
7:435–468. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yefimova SL, Kurilchenko IY, Tkacheva TN,
et al: Comparative study of dye-loaded liposome accumulation in
sensitive and resistant human breast cancer cells. Exp Oncol.
34:101–106. 2012.PubMed/NCBI
|
|
21
|
Saffari M, Shirazi HF, Oghabian MA, et al:
Preparation and in-vitro evaluation of an antisense-containing
cationic liposome against non-small cell lung cancer: a comparative
preparation study. Iran J Pharm Res. 12(Suppl): 3–10.
2013.PubMed/NCBI
|
|
22
|
Preiss MR and Bothun GD:
Stimuli-responsive liposome-nanoparticle assemblies. Expert Opin
Drug Deliv. 8:1025–1040. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rangger C, Helbok A, Sosabowski J, et al:
Tumor targeting and imaging with dual-peptide conjugated
multifunctional liposomal nanoparticles. Int J Nanomedicine.
8:4659–4671. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li X, Zhang J, Wang DK, et al: Anti-tumor
activity of folate receptor targeting docetaxel-loaded
membrane-modified liposomes. Acta Pharma Sinica. 48:1142–1147.
2013.(In Chinese).
|
|
25
|
Nag OK and Awasthi V: Surface engineering
of liposomes for stealth behavior. Pharmaceutics. 5:542–569. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Noble GT, Stefanick JF, Ashley JD, et al:
Ligand-targeted liposome design: challenges and fundamental
considerations. Trends Biotechnol. 32:32–45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Immordino ML, Dosio F and Cattel L:
Stealth liposomes: review of the basic science, rationale, and
clinical applications, existing and potential. Int J Nanomedicine.
1:297–315. 2006.PubMed/NCBI
|
|
28
|
Akbarzadeh A, Rezaei-Sadabady R, Davaran
S, et al: Liposome: classification, preparation, and applications.
Nanoscale Res Lett. 8:1022013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Allen TM and Cullis PR: Liposomal drug
delivery systems: from concept to clinical applications. Adv Drug
Deliv Rev. 65:36–48. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Samad A, Sultana Y and Aqil M: Liposomal
drug delivery systems: an update review. Curr Drug Deliv.
4:297–305. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cattel L, Ceruti M and Dosio F: From
conventional to stealth liposomes: a new frontier in cancer
chemotherapy. Tumori. 89:237–249. 2003.PubMed/NCBI
|
|
32
|
Smith-Jones PM, Vallabhajosula S, Navarro
V, et al: Radiolabeled monoclonal antibodies specific to the
extracellular domain of prostate-specific membrane antigen:
preclinical studies in nude mice bearing LNCaP human prostate
tumor. J Nucl Med. 44:610–617. 2003.
|
|
33
|
Yan Z, Zhan C, Wen Z, et al:
LyP-1-conjugated doxorubicin-loaded liposomes suppress lymphatic
metastasis by inhibiting lymph node metastases and destroying tumor
lymphatics. Nanotechnology. 22:4151032011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Brignole C, Marimpietri D, Gambini C, et
al: Development of Fab’ fragments of anti-GD(2) immunoliposomes
entrapping doxorubicin for experimental therapy of human
neuroblastoma. Cancer Lett. 197:199–204. 2003.
|
|
35
|
Yang Y, Yan Z, Wei D, et al:
Tumor-penetrating peptide functionalization enhances the
anti-glioblastoma effect of doxorubicin liposomes. Nanotechnology.
24:4051012013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yan Z, Wang F, Wen Z, et al:
LyP-1-conjugated PEGylated liposomes: a carrier system for targeted
therapy of lymphatic metastatic tumor. J Control Release.
157:118–125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ishida O and Maruyama K: Transferrin
conjugated PEG-liposomes as intracellular targeting carrier for
tumor therapy. Jpn J Clin Med. 56:657–662. 1998.(In Japanese).
|
|
38
|
Rane S and Prabhakar B: Optimization of
paclitaxel containing pH-sensitive liposomes by 3 factor, 3 level
box-behnken design. Indian J Pharm Sci. 75:420–426. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dicheva BM and Koning GA: Targeted
thermosensitive liposomes: an attractive novel approach for
increased drug delivery to solid tumors. Expert Opin Drug Deliv.
11:83–100. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Linemann T, Thomsen LB, Jardin KG, et al:
Development of a novel lipophilic, magnetic nanoparticle for in
vivo drug delivery. Pharmaceutics. 5:246–260. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Alinaghi A, Rouini MR, Johari Daha F, et
al: The influence of lipid composition and surface charge on
biodistribution of intact liposomes releasing from
hydrogel-embedded vesicles. Int J Pharm. 459:30–39. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Iversen PO: Angiogenesis and hematological
malignancies. J Norw Med Assoc. 123:3198–3200. 2003.(In
Norwegian).
|
|
43
|
Bisacchi D, Benelli R, Vanzetto C, et al:
Anti-angiogenesis and angioprevention: mechanisms, problems and
perspectives. Cancer Detect Prev. 27:229–238. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Abdollahi A and Folkman J: Evading tumor
evasion: current concepts and perspectives of anti-angiogenic
cancer therapy. Drug Resist Updat. 13:16–28. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Waite CL and Roth CM: Nanoscale drug
delivery systems for enhanced drug penetration into solid tumors:
current progress and opportunities. Crit Rev Biomed Eng. 40:21–41.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Prabhakar U, Maeda H, Jain RK, et al:
Challenges and key considerations of the enhanced permeability and
retention effect for nanomedicine drug delivery in oncology. Cancer
Res. 73:2412–2417. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Taurin S, Nehoff H and Greish K:
Anticancer nanomedicine and tumor vascular permeability; Where is
the missing link? J Control Release. 164:265–275. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Greish K: Enhanced permeability and
retention (EPR) effect for anticancer nanomedicine drug targeting.
Methods Mol Biol. 624:25–37. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Maeda H, Bharate GY and Daruwalla J:
Polymeric drugs for efficient tumor-targeted drug delivery based on
EPR-effect. Eur J Pharm Biopharm. 71:409–419. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Karn PR, Cho W and Hwang SJ: Liposomal
drug products and recent advances in the synthesis of supercritical
fluid-mediated liposomes. Nanomedicine (Lond). 8:1529–1548. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yang C, Liu HZ, Lu WD, et al:
PEG-liposomal oxaliplatin potentialization of antitumor efficiency
in a nude mouse tumor-xenograft model of colorectal carcinoma.
Oncol Rep. 25:1621–1628. 2011.PubMed/NCBI
|
|
52
|
Nakamura H, Doi Y, Abu Lila AS, et al:
Sequential treatment of oxaliplatin-containing PEGylated liposome
together with S-1 improves intratumor distribution of subsequent
doses of oxaliplatin-containing PEGylated liposome. Eur J Pharm
Biopharm. Dec 17–2013.(Epub ahead of print). View Article : Google Scholar
|
|
53
|
Rejman J, Oberle V, Zuhorn IS and Hoekstra
D: Size-dependent internalization of particles via the pathways of
clathrin- and caveolae-mediated endocytosis. Biochem J.
377:159–169. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hood RR, Shao C, Omiatek DM, et al:
Microfluidic synthesis of PEG- and folate-conjugated liposomes for
one-step formation of targeted stealth nanocarriers. Pharm Res.
30:1597–1607. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Abu Lila AS, Doi Y, Nakamura K, et al:
Sequential administration with oxaliplatin-containing PEG-coated
cationic liposomes promotes a significant delivery of subsequent
dose into murine solid tumor. J Control Release. 142:167–173.
2010.
|
|
56
|
Zalba S, Navarro I, Troconiz IF, et al:
Application of different methods to formulate PEG-liposomes of
oxaliplatin: evaluation in vitro and in vivo. Eur J Pharm Biopharm.
81:273–280. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu XP, Geng DQ, Xu HX, et al: Research on
the preparation of oxaliplatin liposome. J Wuhan Univ Technol.
30:50–53. 2008.
|
|
58
|
Yang C, Liu HZ, Fu ZX and Lu WD:
Oxaliplatin long-circulating liposomes improved therapeutic index
of colorectal carcinoma. BMC Biotechnology. 11:212011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Tippayamontri T, Kotb R, Paquette B, et
al: Cellular uptake and cytoplasm/DNA distribution of cisplatin and
oxaliplatin and their liposomal formulation in human colorectal
cancer cell HCT116. Invest New Drugs. 29:1321–1327. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Doi Y, Okada T, Matsumoto H, et al:
Combination therapy of metronomic S-1 dosing with
oxaliplatin-containing polyethylene glycol-coated liposome improves
antitumor activity in a murine colorectal tumor model. Cancer Sci.
101:2470–2475. 2010. View Article : Google Scholar
|
|
61
|
Jain A, Jain SK, Ganesh N, et al: Design
and development of ligand-appended polysaccharidic nanoparticles
for the delivery of oxaliplatin in colorectal cancer. Nanomedicine.
6:179–190. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Abu Lila AS, Matsumoto H, Doi Y, et al:
Tumor-type-dependent vascular permeability constitutes a potential
impediment to the therapeutic efficacy of liposomal oxaliplatin.
Eur J Pharm Biopharm. 81:524–531. 2012.PubMed/NCBI
|
|
63
|
Abu Lila AS, Ichihara M, Shimizu T, et al:
Ex-vivo/in-vitro anti-polyethylene glycol (PEG) immunoglobulin M
production from murine splenic B cells stimulated by PEGylated
liposome. Biol Pharm Bull. 36:1842–1848. 2013.PubMed/NCBI
|
|
64
|
Yang C, Liu HZ and Fu ZX: Effects of
PEG-liposomal oxaliplatin on apoptosis, and expression of Cyclin A
and Cyclin D1 in colorectal cancer cells. Oncol Rep. 28:1006–1012.
2012.PubMed/NCBI
|
|
65
|
Yang C, Liu HZ and Fu ZX: PEG-liposomal
oxaliplatin induces apoptosis in human colorectal cancer cells via
Fas/FasL and caspase-8. Cell Biol Int. 36:289–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wicki A, Rochlitz C, Orleth A, et al:
Targeting tumor-associated endothelial cells: anti-VEGFR2
immunoliposomes mediate tumor vessel disruption and inhibit tumor
growth. Clin Cancer Res. 18:454–464. 2012. View Article : Google Scholar : PubMed/NCBI
|