Introduction
Lung cancer is the leading cause of cancer-related
mortality worldwide (1,2). Adenocarcinoma (AC) is the most common
histological subtype of non-small-cell lung cancer (NSCLC)
accounting for approximately half of all lung cancers (3). A previous study reported that >80%
of lung ACs are diagnosed as mixed AC subtype according to the 2004
World Health Organization (WHO) classification (4). Therefore, comprehensive histological
profiling is required through semi-quantitative evaluation of the
percentages of the various histological components. To address such
issues, a new classification based on a multidisciplinary approach
to the diagnosis of lung ACs was established by the International
Association for the Study of Lung Cancer, the American Thoracic
Society and the European Respiratory Society in 2011 (5). In the new nomenclature system,
invasive ACs are classified according to the predominant
histological pattern into specific subtypes, such as lepidic
[formerly the majority of mixed subtype tumors with non-mucinous
bronchioloalveolar carcinoma (BAC)], mucinous (formerly mucinous
BAC), acinar, papillary, solid and micropapillary ACs. Although
widely divergent clinical, radiological, molecular and pathological
data have been collected on lung AC, there remains some confusion
(5). Despite significant advances
regarding the understanding of this type of tumor over the last few
decades, there remains a need for universally accepted criteria for
the classification of AC subtypes (6,7). As
significant resources are invested on trials investigating the
molecular and therapeutic aspects of lung AC, the development of
standardized criteria is crucial and may lead to improvements in
patient care and prognosis. The various subtypes of AC may be
associated with a different prognosis and the underlying mechanisms
have not been clearly determined.
Mutations in exons 18 through 21 of the epidermal
growth factor receptor gene (EGFR) are associated with
sensitivity to tyrosine kinase inhibitors (TKIs); therefore, it is
crucial to elucidate the nature of these mutations. EGFR
mutations are mainly classified as ‘classical’ activating mutations
(del19 and L858R) and as variants of unknown function; thus,
further analyses are required accordingly (8). Persistent EGFR signaling
activation underlies tumor development in human lung ACs. Treatment
with gefitinib or erlotinib achieves specific inhibition of the
EGFR signaling pathway, leading to apoptosis of cancer cells
(9,10).
Echinoderm microtubule-associated protein-like 4
(EML4)-anaplastic lymphoma kinase (ALK), a
transforming fusion gene resulting from ALK rearrangement,
is a potent oncogenic driver and a promising therapeutic target in
ACs via the administration of crizotinib (11–13).
The method for the detection of ALK rearrangement includes
fluorescent in situ hybridization (FISH),
immunohistochemistry (IHC) and reverse transcription-polymerase
chain reaction (RT-PCR). FISH is currently the only approved
diagnostic test for ALK rearrangement for the detection of
break-apart signals. However, there are certain disadvantages to
FISH, particularly that apparatuses are not always readily
available in routine diagnostic laboratories and subtle
intrachromosomal rearrangements may be difficult to interpret
(14,15); therefore, false-negative outcomes
are inevitable. IHC has been considered as an alternative to FISH,
as it is able to detect ALK rearrangements.
It was previously reported that the CRKL is
selectively upregulated in a number of malignant tumors, including
49, 55, 67, 50, 50 and 63% of breast, lung, skin, ovarian and colon
cancers, respectively (16).
CRKL is a member of the human Crk adapter protein family and
is amplified in lung cancer cells with enhanced expression. In
addition, knockdown of CRKL in lung cancer cell lines is
associated with a significant decrease in the proliferation,
progression, survival, motility and invasiveness of lung cancer
cells. These findings suggest that the overexpression of CRKL may
contribute to the oncogenic phenotype in lung cancer (17). Although evidence favors CRKL
gene amplification in several human malignancies, including lung
cancer, the correlation between CRKL and EGFR and the
clinicopathological characteristics in lung ACs has not been
clearly determined.
AXL, a receptor tyrosine kinase, is
associated with the development of several tumors. Elevated AXL
expression and interaction with its ligand, growth arrest-specific
6 (Gas6), have been associated with cell survival, proliferation
and migration in solid tumors (18,19).
AXL was shown to be increasingly upregulated during multistep
esophageal carcinogenesis and is an adverse prognostic marker in
esophageal AC (20). A recent study
identified AXL activation as a novel mechanism of acquired
resistance to EGFR inhibitors in NSCLC (21). The overexpression of AXL is
consistently manifested in prostate cancer cell lines and human
prostate tumors, whereas blockade of AXL expression strongly
inhibits proliferation, migration, invasion and tumor growth
(22).
The primary aim of this study was to analyze the
original expression levels of CRKL and AXL proteins prior to TKI
therapy and determine the correlation of CRKL and AXL expression
with the status of ALK and EGFR among the different
histological subtypes of lung AC. We hypothesized that the
characteristics of CRKL and AXL expression are correlated with
multiple clinicopathological factors. Owing to the presence of lung
AC subtypes and targeted therapy with gefitinib and crizotinib, it
is crucial to evaluate the expression of CRKL and AXL in ACs by
different EGFR and ALK status.
Materials and methods
Tumor samples
A total of 212 primary lung AC samples from patients
who had undergone surgery at the Beijing Chest Hospital (Beijing,
China) between 2006 and 2012 were analyzed. All the sections were
reviewed by 2 reference pathologists (Yi-Ran Cai and Yu-Jie Dong)
to confirm the diagnosis and predominance (>70%) of tumor
tissues. The ACs were reviewed following the new classification
(5) and the 2 observers were
blinded to the outcomes. The clinicopathological characteristics,
including age, gender and histological subtype were recorded.
DNA extraction, PCR amplification and
direct sequencing for EGFR mutations
Genomic DNA was extracted from 50–100 mg tumor
tissue scraped off formalin-fixed and paraffin-embedded blocks
according to the previously described protocol (23). Briefly, PCR for exons 18 through 21
was performed with 100 ng template DNA in a 50-μl volume containing
0.75 U HotStarTaq DNA polymerase (Fermentas International Inc.,
Ontario, Canada), 5 μl PCR buffer, 0.8 μM deoxyribonucleotide
triphosphate, 0.5 μM of each primer and different concentrations of
MgCl2, depending on the various markers. The nucleic
acid used for mutations was based on NM_005228.3. The primers were
designed as follows: exon 18, forward 5′-CAACCAAGCTCTCTTGAGGATC-3′
and reverse 5′-CCCAGCCCAGAGGCCTGT-3′; exon 19, forward 5′-GCA
GCATGTGGCACCATCTC-3′ and reverse 5′-AGAGCCATG GACCCCCACAC-3′; exon
20, forward 5′-CACACTGAC GTGCCTCTCC-3′ and reverse
5′-AGCAGGTACTGGGAG CCAAT-3′; and exon 21, forward
5′-TCTGTCCCTCACAGC AGGGTCT-3′ and reverse 5′-GCTGGCTGACCTAAAGCC
ACC-3′. Amplification and sequencing of the exon fragments were
performed as previously described (23). The PCR products were sequenced in
the sense and antisense directions. Only specimens with an
identified mutation in both rounds were recorded as
mutation-positive.
Construction of tissue microarrays
(TMAs)
In cases with varied histological patterns, the most
representative area was selected for TMA construction. Three cores
(2 mm in diameter) from each patient were drilled out of individual
paraffin-embedded blocks (donor blocks) and placed into new
recipient TMA paraffin blocks (24). Serial sections were cut and IHC was
performed.
IHC for the CRKL and AXL genes and ALK
rearrangement
Tissue sections (4 μm) prepared from TMA blocks were
deparaffinized using xylene and rehydrated through an ethanol
series to water. The slides were incubated with rabbit anti-CRKL
(ab151791; Abcam, Cambridge, UK) and goat anti-AXL (AF154; R&D
Systems, Minneapolis, MN, USA) polyclonal antibodies, using a
modification of the avidin-biotin-peroxidase method provided by the
manufacturer. The slides were incubated with the primary antibody
overnight at 4°C and at a 1:200 dilution. MaxVision™ HRP-Polymer
system (kit 5030 and 5108; Maixin Bio, Fuzhou, China) was used for
immunohistochemical analysis following the manufacturer’s
instructions. Detection was accomplished using
3,3′-diaminobenzidine staining (DAB; ImmunoCruz™ staining system;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Subsequently,
the slides were counterstained with hematoxylin and the stained
tumor cells (≥1,000 cells) were scored by 2 independent observers
(Yi-Ran Cai and Yu-Jie Dong). Cytoplasmic staining was considered
positive for CRKL and AXL. The immunoreactivity of carcinoma
samples was semi-quantitatively evaluated via 2 aspects, the
percentage of positive cells and staining intensity, which was
scored as follows: 0, no staining; 1, light-yellow staining; 2,
yellow staining; and 3, brown staining. The final scores, ranging
from 0 to 300, were the product of the percentage and staining
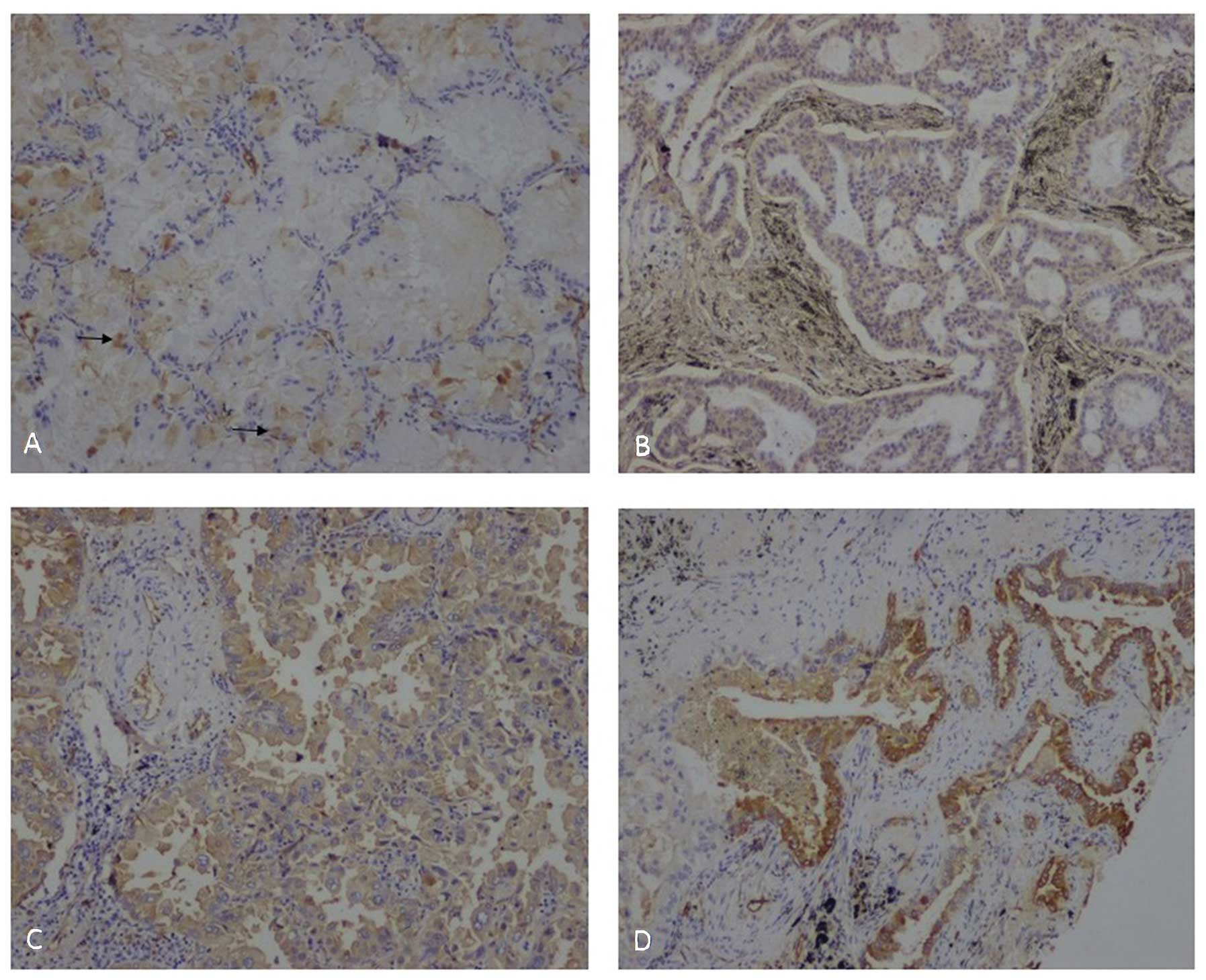
intensity of the positive cells for statistical analysis (Fig. 1).
IHC analysis for ALK rearrangement was
performed using the Ventana method on a BenchMark XT™ autostainer
(Ventana Medical Systems, Inc., Tucson, AZ, USA) with a
ready-to-use primary anti-ALK rabbit monoclonal antibody (D5F3;
Ventana Medical Systems, Inc.). The staining procedure followed the
Ventana ALK test protocol using an OptiView Amplification
kit and an OptiView DAB IHC detection kit (Ventana Medical Systems,
Inc.). The presence or absence of ALK rearrangement was
evaluated by adopting a binary judgment system following the
manufacturer’s protocol. Neoplastic cells with diffuse brown
cytoplasmic staining were defined as ALK
rearrangement-positive, otherwise cells were defined as negative
(Fig. 2D).
Statistical analysis
Non-parametric analysis of variance (ANOVA) was used
to analyze the non-normal distribution data of CRKL and AXL
expression. The correlation of CRKL and AXL expression with the
clinicopathological characteristics were analyzed by the cross
tabulation χ2 test or Fisher’s exact test. The mutual
correlation between the expression of CRKL and AXL was assayed by
Spearman’s rho. Factorial ANOVA was used for mutual comparisons to
evaluate the expression of CRKL and AXL in different AC subtypes.
All the statistical tests were two-sided and P=0.05 was considered
to indicate a statistically significant difference. All the
statistical analyses were performed on a SAS system, version 9.2
(SAS Institute, Inc., Cary, NC, USA) for Windows.
Results
Patient and histopathological
characteristics
The clinicopathological characteristics are listed
in Table I. Of the 212 patients
with lung ACs, 118 were men (55.7%). The median age was 63 years
(range, 32–79 years) and 55 years (range, 23–74 years) in the male
and female patients, respectively. All the samples were diagnosed
as invasive AC and classified into 6 subtypes as follows: 69 acinar
ACs (32.5%), 17 lepidic predominant ACs (LPAs) (8%), 63 papillary
(29.7%), 14 mucinous (6.6%), 17 micropapillary (8%) and 32 solid
ACs (15.1%). Of the 212 cases, 128 (60.4%) had no history of
smoking and 113 ACs (53.3%) had positive local lymph nodes. A total
of 92 cases (43.4%) were diagnosed with early-stage lung cancer
(stages I and II), 72 (34%) were diagnosed at stage IIIA and 48
(22.6%) were diagnosed with late-stage lung cancer (stages IIIB and
IV).
 | Table INon-parametric test (Kruskal-Wallis
test) for the correlation of CRKL and AXL staining scores with the
pathological characteristics of lung ACs. |
Table I
Non-parametric test (Kruskal-Wallis
test) for the correlation of CRKL and AXL staining scores with the
pathological characteristics of lung ACs.
| Wilcoxon scores
(average score) |
|---|
|
|
|---|
|
Characteristics | CRKL | P | AXL | P |
|---|
| Gender | | 0.56 | | 0.52 |
| Male (n=118) | 104 | | 104 | |
| Female (n=94) | 109 | | 110 | |
| AC subtypes | | 0.01 | | 0.002 |
| Acinar (n=69) | 109 | | 116 | |
| Lepidic
predominant (n=17) | 71 | | 58 | |
| Micropapillary
(n=17) | 99 | | 112 | |
| Papillary
(n=63) | 125 | | 119 | |
| Solid (n=32) | 98 | | 96 | |
| Mucinous
(n=14) | 79 | | 79 | |
| EGFR
statusa | | 0.39 | | <0.001 |
| Mutation
(n=101) | 107 | | 119 | |
| Non-mutation
(n=104) | 99 | | 88 | |
| ALK
statusb | | 0.56 | | 0.43 |
| Fusion gene
(n=23) | 112 | | 96 | |
| Non-fusion gene
(n=186) | 104 | | 106 | |
| Smoking status | | 0.08 | | 0.75 |
| Non-smoker
(n=128) | 112 | | 108 | |
| Smoker (n=84) | 97 | | 105 | |
| Clinical stage | | 0.28 | | 0.53 |
| I (n=73) | 115 | | 106 | |
| II (n=19) | 87 | | 108 | |
| IIIA (n=72) | 108 | | 113 | |
| IIIB+IV
(n=48) | 100 | | 96 | |
EGFR mutation and ALK rearrangement in
lung ACs
Mutations of the EGFR kinase domain (exons 18
through 21)were successfully screened in 205 lung ACs and a total
of 101 cases (49.3%) were found to harbor EGFR mutations
(Table I). The distribution of
EGFR mutations were as follows: G719A/S was present in 3
acinar (42.9%) and 4 papillary ACs (57.1%); delK745-S753 was
identified in 13 acinar ACs (24.4%), 3 LPAs (5.7%), 4
micropapillary (7.6%), 2 mucinous (3.8%), 26 papillary (49.1%) and
5 solid ACs (9.4%); and L858R was identified in 14 acinar (37.8%),
3 LPAs (8.1%), 3 micropapillary (8.1%), 11 papillary (29.8%) and 6
solid ACs (16.2%). Composite mutations were detected in 2 cases: 1
case was papillary AC with G719S and S768I mutations; and the other
case was acinar AC with delE746-A750 and L858R mutations. The most
frequent mutations were nucleotide deletions in the K745-S753
region of exon 19 (54.1%) and point mutations of L858R in exon 21
(37%). G719A or G719S mutations were found in 7 cases (6.9%). No
T790M mutation in exon 20 was detected in our cohort.
ALK rearrangement was analyzed by Ventana
IHC. A total of 23 ACs (11%) exhibited ALK translocation,
which was increased to 22.1% in the subgroup without EGFR
mutations. The ACs exhibiting ALK rearrangement included 8
acinar (34.8%), 4 micropapillary (17.4%), 4 mucinous (17.4%), 1
papillary (4.4%) and 6 solid ACs (26.1%). There were no LPAs with
ALK rearrangement. Notably, 4 out of 5 ACs with cribriform
structure among acinar ACs were found to be positive for ALK
rearrangement (Fig. 2). No tumor
was found harboring both EGFR mutation and ALK
rearrangement.
Expression of CRKL and AXL in lung ACs
and association with EGFR and ALK status
The staining scores of CRKL and AXL were compared by
non-parametric ANOVA. The average Wilcoxon scores were used to
compare the staining scores of CRKL and AXL with the
clinicopathological characteristics (Table I). No statistical difference was
observed between CRKL expression and clinical parameters, such as
gender (P=0.56), smoking status (P=0.08), clinical stage (P=0.28),
EGFR status (P=0.39) and ALK status (P=0.56). CRKL
and AXL were expressed at different levels among the AC subtypes
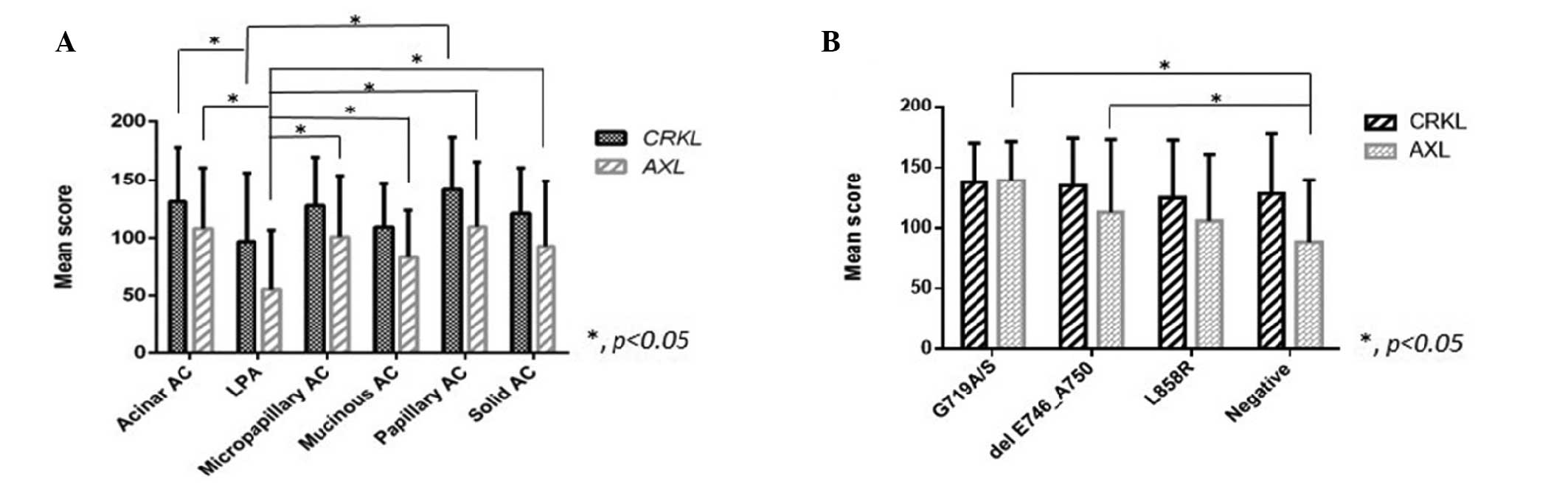
(P=0.01 and P=0.002, respectively). The AC types with the highest
and lowest CRKL expression levels were papillary ACs (141±45.5) and
LPAs (97±59.3), respectively. Mutual comparisons were used to
disclose the differences among histological subtypes (Fig. 2). The expression level of CRKL in
LPAs was significantly lower compared to that in acinar and
papillary ACs. Papillary ACs exhibited a higher CRKL expression
compared to solid, mucinous ACs and LPAs (P<0.05). Furthermore,
papillary ACs expressed the highest level of CRKL, regardless of
the EGFR status. AXL expression in LPAs was significantly
lower compared to that in any of the other 4 subtypes (P<0.05)
(Fig. 3A). The expression level of
AXL was higher only in acinar ACs with lymph node invasion,
compared to that in AC subtypes without metastasis (33.5 vs. 22.9;
P=0.02).
The levels of CRKL and AXL expression were also
analyzed among AC subtypes stratified by EGFR and ALK
status (Tables II and III). ACs with the EGFR wild-type
expressed the lowest level of AXL compared to those with G719A/S
and delE746_A750 mutations (Fig.
3B). The AXL expression levels were higher among ACs with
EGFR mutations compared to those without mutations
(P<0.001). Among the ACs with ALK rearrangement, mucinous
and micropapillary ACs exhibited the lowest and highest levels of
CRKL expression, respectively; however, there was no difference in
AXL expression. A positive correlation between CRKL and AXL
expression was detected among the 11 solid ACs with EGFR
mutations (r=0.73; P=0.01). In addition, we observed a significant
difference in AXL expression among the AC subtypes; a lower
AXL expression was found in LPAs compared to that in acinar,
micropapillary, papillary and solid ACs (P<0.05), whereas LPAs
exhibited the lowest expression level of CRKL and AXL
compared to the other subtypes (Fig.
2). Among the ACs with ALK translocation, mucinous ACs
exhibited a significantly lower CRKL level (73±27.1)
compared to acinar, micropapillary and solid ACs (P<0.05).
 | Table IICorrelation of CRKL and AXL
expression with ALK status in lung ACs. |
Table II
Correlation of CRKL and AXL
expression with ALK status in lung ACs.
| Median staining
score of CRKL | Median staining
score of AXL |
|---|
|
|
|
|---|
| AC subtypes | ALK
rearrangement (mean ± SD) | P<0.05 | ALK without
rearrangement (mean ± SD) | P<0.05 | ALK
rearrangement (mean ± SD) | P<0.05 | ALK without
rearrangement (mean ± SD) | P<0.05 |
|---|
| Acinar | 151 | a | 124 | vs. LPA | 101 | | 101 | b |
| (N1=58, N2=8) | (154±35.6) | | (131.3±45.5) | | (98±37) | | (107.5±49.4) | |
| LPA | N/A | | 100 | | N/A | | 50 | |
| (N1=17, N2=0) | | | (97±59.3) | | | | (55.3±51.4) | |
| Micropapillary | 171.7 | a | 100 | | 109 | | 107 | b |
| (N1=13, N2=4) | (166±53.3) | | (116±32) | | (94±72) | | (103±49) | |
| Papillary | 130 (130) | | 145 | vs. LPA | 100 | | 112 | b |
| (N1=62, N2=1) | | | (141±45.5) | | (100) | | (110±56.3) | |
| Solid | 121.7 | a | 118 | vs. papillary | 88 | | 92 | b |
| (N1=26, N2=6) | (129±41.3) | | (119±40) | AC | (87.5±14.7) | | (94±62) | |
| Mucinous | 77.5 | | 125 | | 81.7 | | 85 | |
| (N1=10, N2=4) | (73±27.1) | | (124±30) | | (100±56.7) | | (76±34) | |
 | Table IIIMutual comparisons of CRKL and AXL
expression with EGFR status among lung AC subtypes. |
Table III
Mutual comparisons of CRKL and AXL
expression with EGFR status among lung AC subtypes.
| Median staining
score of CRKL | Median staining
score of AXL |
|---|
|
|
|
|---|
| AC subtypes | EGFR
mutation (mean ± SD) | P<0.05 | EGFR
non-mutation (mean ± SD) | P<0.05 | EGFR
mutation (mean ± SD) | P<0.05 | EGFR
non-mutation (mean ± SD) | P<0.05 |
|---|
| Acinar | 122.9 | | 131.7 | b | 115.8 | | 100 | |
| (N1=33, N2=32) | (130.4±34.7) | | (138.9±53) | | (120.9±51.7) | | (102.5±48.4) | |
| LPA | 95 | a | 101.7 | a | 31.7 | a,d,e | 41.7 | a,d |
| (N1=10, N2=6) | (99.7±75.3) | | (100.3±52.9) | | (53.2±67.2) | | (54±46) | |
| Micropapillary | 100 | | 110 | | 141.7 | | 80 | |
| (N1=10, N2=7) | (133.3±46.2) | | (123.8±41) | | (127.6±22.6) | | (82.3±61) | |
| Papillary | 140 | | 151 | c | 116.7 | | 98.3 | |
| (N1=18, N2=43) | | | | | (114.5±54.9) | | (98.2±60.1) | |
| Solid | 120 | | 115 | | 106.7 | | 83.3 | |
| (N1=21, N2=11) | | | | | (108.8±75.4) | | (84.8±42.8) | |
| Mucinous | 125 | | 100 | | 97.5 | | 84.2 | |
| (N1=12, N2=2) | | | | | (97.5±17.7) | | (80.3±43.6) | |
Discussion
Approximately 80% of lung ACs are classified as
mixed subtype, according to the 2004 WHO classification system;
however, it has been proposed that a semi-quantitative assessment
of the percentage of the various histological components of ACs,
including acinar, papillary, micropapillary, lepidic and solid ACs,
may enable the classification of tumors according to their
predominant histological subtype (25). As 70–90% of the surgically resected
lung tumors are diagnosed as invasive ACs, it is crucial to adopt a
practical method to address tumors that comprise a complex
heterogeneous mixture of histological subtypes. Over the last few
years, multiple independent research groups have intentionally
classified lung ACs according to the predominant subtypes (25–29).
Prominent heterogeneous structures in ACs have attracted increasing
attention from pathologists following the establishment of the new
classification system. The present study was commenced once the
tissue sections were reviewed and diagnosed based on the new
classification system. We observed that, in the 2004 WHO
classification, the majority of the LPAs were merged among a
heterogeneous group of tumors that included predominantly invasive
ACs. However, LPA, according to the new classification, is defined
as a non-mucinous AC with its predominant component exhibiting
lepidic growth along the surface of the alveolar walls; thus, LPA
is now independent from invasive mucinous AC. Acinar predominant AC
exhibits a predominant glandular component (6). Cribriform arrangements are considered
to be a pattern of acinar AC (30).
Papillary predominant AC includes a major component of glandular
cells spreading along central fibrovascular cores. Solid
predominant AC exhibits recognizable patterns lacking acinar,
papillary, micropapillary or lepidic growth and intracellular
mucin. Micropapillary AC, a new subtype, exhibits tumor cells
growing in papillary tufts, which lack fibrovascular cores
(31). Micropapillary ACs were
previously reported in patients with early-stage lung cancer with a
poor prognosis (27,32) and their poor prognosis is similar to
that of ACs with a predominant solid subtype (33).
Recent studies reported that the overexpression or
amplification of CRKL and the activation of AXL are
associated with resistance to TKIs (9,11,21,34).
However, the number of available studies on the correlation between
the expression of these biomarkers and the histological subtypes is
currently limited. The correlation of CRKL and AXL expression with
the EGFR or ALK status remains to be elucidated when
stratified by histological subtype. The highest frequency of
EGFR mutations is found in the Asian population, non-smokers
and individuals with non-mucinous tumors (35). These mutations are point mutations
in exons 18 (G719A/C), 20 (T790M), 21 (L858R and L861Q) and an
in-frame deletion in exon 19 from codon 746–750 (E746-A750
deletion) (9,36). The most common mutations are L858R
and in-frame deletions in exon 19. In the present study, 101 ACs
(49.3%) were found to harbor EGFR mutations and >90% of
the EGFR mutations were L858R and delE746_A750. These
findings were consistent with several studies that investigated the
prevalence and specificity of EGFR alterations in lung ACs.
The EGFR mutation status was shown to be significantly
associated with LPA, papillary and micropapillary AC subtypes
(5). Previous clinical outcomes
suggest that accurate histological subtyping and EGFR
mutation testing are crucial and should be included to guide the
initial therapy algorithm for lung AC. A total of 54.1 and 37% of
mutant ACs in this study were found to harbor delK745-S753 and
L858R mutations, respectively. The most common subtypes carrying
exon 19 in-frame deletions were papillary (49.1%) and acinar ACs
(24.4%). It was previously reported that EGFR delE746_A750
and L858R mutants confer ligand-independent activation and
prolonged receptor kinase activity following ligand stimulation.
The activity of the L858R mutant was shown to be ~20-fold higher
compared to that of the wild-type kinase domain (37). In our study, histological subtypes
with L858R exhibited relatively lower expression levels of CRKL and
AXL compared to other mutations. Papillary ACs expressed CRKL to
the highest level, regardless of the EGFR status, suggesting
that the activation of CRKL is more common in papillary ACs in
comparison to other histological subtypes. Within the histological
groups harboring EGFR mutations, the highest expression of
AXL was observed in micropapillary ACs, suggesting that the
activation of AXL is one of the factors associated with the
poor prognosis of this histological entity.
ALK rearrangement results in a fusion gene,
EML4-ALK, which is located on chromosome 2p and is formed by
a chromosomal inversion. This fusion gene defines a distinct
molecular subset of ACs, which benefits from treatment with
ALK-inhibitors. Robust and reliable laboratory tests for
predictive biomarkers are critical in order to select appropriate
patients for targeted therapy. IHC using specific antibodies
corresponds well to EML4-ALK translocation and may be a
useful screening method (14,15,38,39).
IHC has been considered an alternative to FISH. In our cohort, 23
ACs (11%) were positive for ALK rearrangement and 22.1% of
these ACs did not harbor an EGFR mutation. The incidence of
ALK rearrangement was markedly increased in our cases
compared to that reported in young men and light or non-smokers
(5%) in Western countries (14,40,41). A
total of 13 men (56.5%) and 15 light or non-smokers (65.2%) were
found to be EML4-ALK positive. The histological subtypes
with ALK translocation included 8 acinar (34.8%), 4
micropapillary (17.4%), 4 mucinous (17.4%), 1 papillary (4.4%) and
6 solid ACs (26.1%). A variety of histological characteristics are
associated with EML4-ALK rearrangement, including acinar,
papillary, cribriform and signet-ring patterns, as well as intra-
and extracytoplasmic mucin production (14,40,41).
Notably, in our study, 4 of the 5 ACs with cribriform structure
were ALK rearrangement-positive (Fig. 1), suggesting that ACs with such
architecture may be associated with a higher ratio of ALK
translocation.
The amplification of CRKL has been implicated
in various types of human cancers and its expression is associated
with enhanced cancer cell proliferation and invasion (16,17).
However, CRKL protein expression and its correlation with
clinicopathological factors has not yet been elucidated in ACs. In
our study, CRKL protein levels were not found to be significantly
correlated with clinical characteristics, such as gender, smoking
history, clinical stage, EGFR and ALK status.
However, the expression of CRKL was significantly different among
the AC subtypes. Papillary ACs and LPAs conferred the highest and
lowest levels of CRKL protein, respectively (Table I). CRKL activation was
significantly lower in LPAs compared to that in acinar and
papillary ACs. Among the histological subtypes with ALK
translocation, mucinous ACs exhibited a lower expression level of
CRKL, in contrast to acinar, micropapillary and solid ACs.
AXL is a member of the Tyro3, AXL and
Mertk subfamily (42,43). Activation of AXL protects
cells against apoptosis and increases migration, aggregation and
growth via multiple downstream pathways. Upregulation of the
Gas6/AXL pathway is more evident under pathological compared
to normal physiological conditions. EGFR-mutant lung cancer
models in vitro and in vivo exhibited increased
activation of AXL with acquired resistance to erlotinib
without T790M alteration, suggesting that AXL may be a
promising therapeutic target whose inhibition may prevent or
overcome acquired resistance to EGFR TKIs in individuals
with EGFR-mutant lung cancer (21). In our study, a higher level of
AXL expression was observed in the 101 ACs with EGFR
mutations compared to that in ACs without EGFR mutations.
ACs without EGFR mutations expressed the lowest level of AXL
compared to those with G719A/S and delE746_A750 mutations. Higher
levels of AXL expression were observed in micropapillary ACs
compared to those in other subtypes harboring EGFR mutations
and in acinar ACs with lymph node invasion. These data suggest that
AXL activation may contribute to the invasiveness of acinar
and micropapillary ACs together with EGFR activity. The
activation of CRKL and AXL, together with EGFR
mutations, may affect the biological behavior of solid ACs. By
contrast, LPAs, which are known to present with mild invasiveness,
also exhibited lower CRKL and AXL expression levels, regardless of
the EGFR status. Previous studies reported lepidic growth to
be associated with a more favorable survival outcome in small
solitary resected lung ACs with an invasive component (44–46),
suggesting that LPA is relatively indolent among AC subtypes. As
regards the roles of CRKL and AXL in lung ACs
according to the EGFR and ALK status, the exact
histological types should be considered in order to evaluate the
biological behaviors and prognosis of these ACs, since there are
significant differences in the expression of CRKL and AXL among
various lung AC subtypes.
Acknowledgements
We would like to thank Xue-Jing Chen, Li Zhang and
Chen Zhang for their support in sample collection. This study was
supported by the Beijing Foundation for Distinguished Scientists
(grant no. 2009D003013000001) awarded by the Beijing Board of
Health.
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2
|
Boyle P and Levin B: World Cancer Report
2008. IARC Scientific Publications; Lyon: 2008
|
|
3
|
Curado MP, Edwards B, Shin HR, Storm H,
Ferlay J, Heanue M and Boyle P: Cancer Incidence in Five
Continents. IX. IARC Scientific Publications; Lyon: 2007
|
|
4
|
Terasaki H, Niki T, Matsuno Y, et al: Lung
adenocarcinoma with mixed bronchioloalveolar and invasive
components: clinicopathological features, subclassification by
extent of invasive foci, and immunohistochemical characterization.
Am J Surg Pathol. 27:937–951. 2003. View Article : Google Scholar
|
|
5
|
Travis WD, Brambilla E, Noguchi M, et al:
International Association for the Study of Lung Cancer/American
Thoracic Society/European Respiratory Society international
multidisciplinary classification of lung adenocarcinoma. J Thorac
Oncol. 6:244–285. 2011. View Article : Google Scholar
|
|
6
|
Travis WD, Brambilla E, Muller-Hermelink
HK and Harris CC: Pathology and Genetics of Tumours of the Lung,
Pleura, Thymus and Heart. IARC Press; Lyon: 2004
|
|
7
|
Travis WD, Colby TV, Corrin B, Shimosato Y
and Brambilla E: Histological Typing of Lung and Pleural Tumors.
3rd edition. Springer; Berlin: 1999, View Article : Google Scholar
|
|
8
|
Lynch TJ, Bell DW, Sordella R, et al:
Activating mutations in the epidermal growth factor receptor
underlying responsiveness of non-small-cell lung cancer to
gefitinib. New Engl J Med. 350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Riely GJ, Politi KA, Miller VA and Pao W:
Update on epidermal growth factor receptor mutations in non-small
cell lung cancer. Clin Cancer Res. 12:7232–7241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sakai K, Arao T, Shimoyama T, et al:
Dimerization and the signal transduction pathway of a small
in-frame deletion in the epidermal growth factor receptor. FASEB J.
20:311–313. 2006.PubMed/NCBI
|
|
11
|
Soda M, Choi YL, Enomoto M, et al:
Identification of the transforming EML4-ALK fusion gene in
non-small-cell lung cancer. Nature. 448:561–566. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kwak EL, Bang YJ, Camidge DR, et al:
Anaplastic lymphoma kinase inhibition in non-small-cell lung
cancer. New Engl J Med. 363:1693–1703. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gandhi L and Janne PA: Crizotinib for
ALK-rearranged non-small cell lung cancer: a new targeted therapy
for a new target. Clin Cancer Res. 18:3737–3742. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rodig SJ, Mino-Kenudson M, Dacic S, et al:
Unique clinicopathologic features characterize ALK-rearranged lung
adenocarcinoma in the western population. Clin Cancer Res.
15:5216–5223. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mino-Kenudson M, Chirieac LR, Law K, et
al: A novel, highly sensitive antibody allows for the routine
detection of ALK-rearranged lung adenocarcinomas by standard
immunohistochemistry. Clin Cancer Res. 16:1561–1571. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
ten Hoeve J, Kaartinen V, Fioretos T, et
al: Cellular interactions of CRKL and SH2-SH3 adaptor protein.
Cancer Res. 54:2563–2567. 1994.PubMed/NCBI
|
|
17
|
Senechal K, Halpern J and Sawyers CL: The
CRKL adaptor protein transforms fibroblasts and functions in
transformation by the BCR-ABL oncogene. J Biol Chem.
271:23255–23261. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hafizi S and Dahlback B: Signalling and
functional diversity within the Axl subfamily of receptor tyrosine
kinases. Cytokine Growth Factor Rev. 17:295–304. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goruppi S, Ruaro E, Varnum B and Schneider
C: Requirement of phosphatidylinositol 3-kinase-dependent pathway
and Src for Gas6-Axl mitogenic and survival activities in NIH 3T3
fibroblasts. Mol Cell Biol. 17:4442–4453. 1997.PubMed/NCBI
|
|
20
|
Hector A, Montgomery EA, Karikari C, et
al: The Axl receptor tyrosine kinase is an adverse prognostic
factor and a therapeutic target in esophageal adenocarcinoma.
Cancer Biol Ther. 10:1009–1018. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Z, Lee JC, Lin L, et al: Activation
of the AXL kinase causes resistance to EGFR-targeted therapy in
lung cancer. Nat Genet. 44:852–860. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Paccez JD, Vasques GJ, Correa RG, et al:
The receptor tyrosine kinase Axl is an essential regulator of
prostate cancer proliferation and tumor growth and represents a new
therapeutic target. Oncogene. 32:689–698. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cai YR, Zhang HQ, Qu Y, et al: Expression
of MET and SOX2 genes in non-small cell lung carcinoma with EGFR
mutation. Oncol Rep. 26:877–885. 2011.PubMed/NCBI
|
|
24
|
Lee HJ, Xu X, Choe G, et al: Protein
overexpression and gene amplification of epidermal growth factor
receptor in nonsmall cell lung carcinomas: Comparison of four
commercially available antibodies by immunohistochemistry and
fluorescence in situ hybridization study. Lung Cancer. 68:375–382.
2010. View Article : Google Scholar
|
|
25
|
Motoi N, Szoke J, Riely GJ, et al: Lung
adenocarcinoma: modification of the 2004 WHO mixed subtype to
include the major histologic subtype suggests correlations between
papillary and micropapillary adenocarcinoma subtypes, EGFR
mutations and gene expression analysis. Am J Surg Pathol.
32:810–827. 2008. View Article : Google Scholar
|
|
26
|
Sica G, Yoshizawa A, Sima CS, et al: A
grading system of lung adenocarcinomas based on histologic pattern
is predictive of disease recurrence in stage I tumors. Am J Surg
Pathol. 34:1155–1162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
De Oliveira Duarte Achcar R, Nikiforova MN
and Yousem SA: Micropapillary lung adenocarcinoma: EGFR, K-ras and
BRAF mutational profile. Am J Clin Pathol. 131:694–700.
2009.PubMed/NCBI
|
|
28
|
Kim YH, Ishii G, Goto K, et al: Dominant
papillary subtype is a significant predictor of the response to
gefitinib in adenocarcinoma of the lung. Clin Cancer Res.
10:7311–7317. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ding L, Getz G, Wheeler DA, et al: Somatic
mutations affect key pathways in lung adenocarcinoma. Nature.
455:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Okudela K, Woo T, Mitsui H, et al:
Proposal of an improved histological sub-typing system for lung
adenocarcinoma - significant prognostic values for stage I disease.
Int J Clin Exp Pathol. 3:348–366. 2010.
|
|
31
|
Amin MB, Tamboli P, Merchant SH, et al:
Micropapillary component in lung adenocarcinoma: a distinctive
histologic feature with possible prognostic significance. Am J Surg
Pathol. 26:358–364. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tsutsumida H, Nomoto M, Goto M, et al: A
micropapillary pattern is predictive of a poor prognosis in lung
adenocarcinoma and reduced surfactant apoprotein A expression in
the micropapillary pattern is an excellent indicator of a poor
prognosis. Mod Pathol. 20:638–647. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yoshizawa A, Motoi N, Riely GJ, et al:
Impact of proposed IASLC/ATS/ERS classification of lung
adenocarcinoma: prognostic subgroups and implications for further
revision of staging based on analysis of 514 stage I cases. Mod
Pathol. 24:653–664. 2011. View Article : Google Scholar
|
|
34
|
Cheung HW, Du J, Boehm JS, et al:
Amplification of CRKL induces transformation and epidermal growth
factor receptor inhibitor resistance in human non-small cell lung
cancers. Cancer Discov. 1:608–625. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Finberg KE, Sequist LV, Joshi VA, et al:
Mucinous differentiation correlates with absence of EGFR mutation
and presence of KRAS mutation in lung adenocarcinomas with
bronchioloalveolar features. J Mol Diagn. 9:320–326. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Suda K, Murakami I, Katayama T, et al:
Reciprocal and complementary role of MET amplification and EGFR
T790M mutation in acquired resistance to kinase inhibitors in lung
cancer. Clin Cancer Res. 16:5489–5498. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang X, Gureasko J, Shen K, Cole PA and
Kuriyan J: An allosteric mechanism for activation of the kinase
domain of epidermal growth factor receptor. Cell. 125:1137–1149.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Takeuchi K, Choi YL, Togashi Y, et al:
KIF5B-ALK, a novel fusion oncokinase identified by an
immunohistochemistry-based diagnostic system for ALK-positive lung
cancer. Clin Cancer Res. 15:3143–3149. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Boland JM, Erdogan S, Vasmatzis G, et al:
Anaplastic lymphoma kinase immunoreactivity correlates with ALK
gene rearrangement and transcriptional up-regulation in non-small
cell lung carcinomas. Hum Pathol. 40:1152–1158. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shaw AT, Yeap BY, Mino-Kenudson M, et al:
Clinical features and outcome of patients with non-small-cell lung
cancer who harbor EML4-ALK. J Clin Oncol. 27:4247–4253. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Takahashi T, Sonobe M, Kobayashi M, et al:
Clinicopathologic features of non-small-cell lung cancer with
EML4-ALK fusion gene. Ann Surg Oncol. 17:889–897. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
O’Bryan JP, Frye RA, Cogswell PC, et al:
Axl, a transforming gene isolated from primary human myeloid
leukemia cells, encodes a novel receptor tyrosine kinase. Mol Cell
Biol. 11:5016–5031. 1991.PubMed/NCBI
|
|
43
|
Neubauer A, O’Bryan JP, Fiebeler A,
Schmidt C, Huhn D and Liu ET: Axl, a novel receptor tyrosine kinase
isolated from chronic myelogenous leukemia. Semin Hematol. 30(3
Suppl 3)34:1993
|
|
44
|
Lee HY, Han J, Lee KS, et al: Lung
adenocarcinoma as a solitary pulmonary nodule: prognostic
determinants of CT, PET, and histopathologic findings. Lung Cancer.
66:379–385. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yokose T, Suzuki K, Nagai K, Nishiwaki Y,
Sasaki S and Ochiai A: Favorable and unfavorable morphological
prognostic factors in peripheral adenocarcinoma of the lung 3 cm or
less in diameter. Lung Cancer. 29:179–188. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lin DM, Ma Y, Zheng S, Liu XY, Zou SM and
Wei WQ: Prognostic value of bronchioloalveolar carcinoma component
in lung adenocarcinoma. Histol Histopathol. 21:627–632.
2006.PubMed/NCBI
|