Introduction
Pertussis toxin (PTX) was first described in 1979
following the report of the PTX-induced stimulation mechanism of
insulin release from pancreatic islets in rats (1). The mechanism of PTX action is
associated with the inhibition of G protein activation, and thus
PTX inhibits the signal transmission from the activated receptor to
the effectors that are specific for the G protein-coupled receptor.
The inhibitory effect is secondary to adenoside
diphosphate-ribosylation of the α-subunit of the G protein
(2). Currently, PTX toxin is
commonly used in numerous experimental models of signaling
pathways.
Mastoparan-7 demonstrates an inhibitory mechanism of
action on G protein, mimicking the action of the active receptor
binding to its G protein. Additionally, in certain cell types
interactions with phospholipase C (PLC) have been found.
Stimulation of PLC has been described previously (3) for rat mast cells and hepatocytes and
human HL-60 leukaemia cells, whereas inhibition of PLC has been
found for SH-SY5Y human neuro-blastoma cells and human astrocytoma
cells (3). Results of recent
studies have indicated that there is a possibility of stimulation
of programmable cell death in various types of cells (4–6).
Calcium ions play a regulatory role in cell life,
but prolonged high concentrations may induce apoptosis, which leads
to cell death. Cell stimulation induces an increase in calcium
influx into the cytoplasm primarily from intracellular calcium
stores and secondarily resulting in binding to structures,
including calpain and calcineurin. Calpain belongs to the cysteine
proteases family that activates Bid and Bax and promotes their
transport to the mitochondria. An excess of Ca2+ in
mitochondria also leads to the release of proapoptotic proteins
that are located in the intracellular space; Smac/DIABLO and
cytochrome c (7,8). The results of our previous study
indicated that PTX, as a G-protein inhibitor, is not able to
inhibit contraction induced by direct stimulation of G-protein by
mastoparan-7 (9).
The aim of the present study was to evaluate the
effect of PTX on vascular smooth muscle cells that were stimulated
pharmacologically with phenylephrine (α-adrenoceptor agonist),
mastoparan-7 (direct G-protein activator) and Bay K8644 (direct
calcium channel activator).
Materials and methods
Animals
Experiments were performed on isolated and perfused
tail arteries of Wistar rats (weight, 250–270 g). The animals were
housed under a 12-h light/dark cycle and had unlimited access to
food and water. The rats were narcotized by intraperitoneal
injection of 120 mg/kg urethane and then sacrificed by stunning and
cervical dislocation. The study protocol was approved by the Local
Ethics Committee. All the studies were carried out in accordance
with the United States NIH guidelines [Guide for the Care and Use
of Laboratory Animals (1985), DHEW Publication No. (NIH) 85–23;
Office of Science and Health Reports, DRR/NIH, Bethesda, MD,
USA].
Drugs and solutions
Krebs solution contained NaCl (71.8 mM/l), KCl (4.7
mM/l), CaCl2 (1.7 mM/l) NaHCO3 (28.4 mM/l),
MgSO4 (2.4 mM/l), KH2PO4 (1.2
mM/l) and glucose (11.1 mM/l). All the reagents were purchased from
Sigma-Aldrich (St. Louis, MO, USA).
Study design and conduction
Following dissection from the surrounding tissues, a
2–3-cm long segment of a rat tail artery was cannulated and
connected to a perfusion device. The distal part was weighed with a
500 mg weight and the tail was placed in a 20-ml container filled
with oxygenated Krebs solution at 37°C (pH 7.4). The samples were
prepared in the presence of PTX (100 ng/ml) and were incubated in
oxygenated Krebs solution for 24 h. The perfusion pressure was
continuously measured. The perfusion solution flow was gradually
increased using a peristaltic pump to 1 ml/min, until the optimum
perfusion pressure of 2–4 kPa was reached (10,11).
Data analysis and statistical
procedures
The investigations were performed on a TSZ-04 system
from Experimetria Ltd. (Budapest, Hungary). The perfusion pressure
was measured on BPR-01 and BPR-02 devices, and the vascular smooth
muscle tension was measured on a FSG-01 transducer connected with a
digital recorder Graphtec GL820 midi Logger. All transducers used
in the experiments were made by Experimetria Ltd., and the
peristaltic pump was made by Zalimp (Warsaw, Poland).
Concentration-response curves (CRCs) were calculated
according to the van Rossum method. The maximum response of tissues
(Emax) was calculated as a percentage of maximal
response for phenylephrine. The half maximal effective
concentration (EC50) was estimated using classical
pharmacological methods with pD2, the negative logarithm
of the EC50. The number of the CRC and Emax
was used in all calculations to estimate the statistical
significance. Mastoparan-17 was used as a negative control.
The results were presented as mean ± standard
deviation. Statistical analysis was performed using the analysis of
variance test for multiple comparison of the means. P<0.05 was
considered to indicate a statistically significant difference.
Results
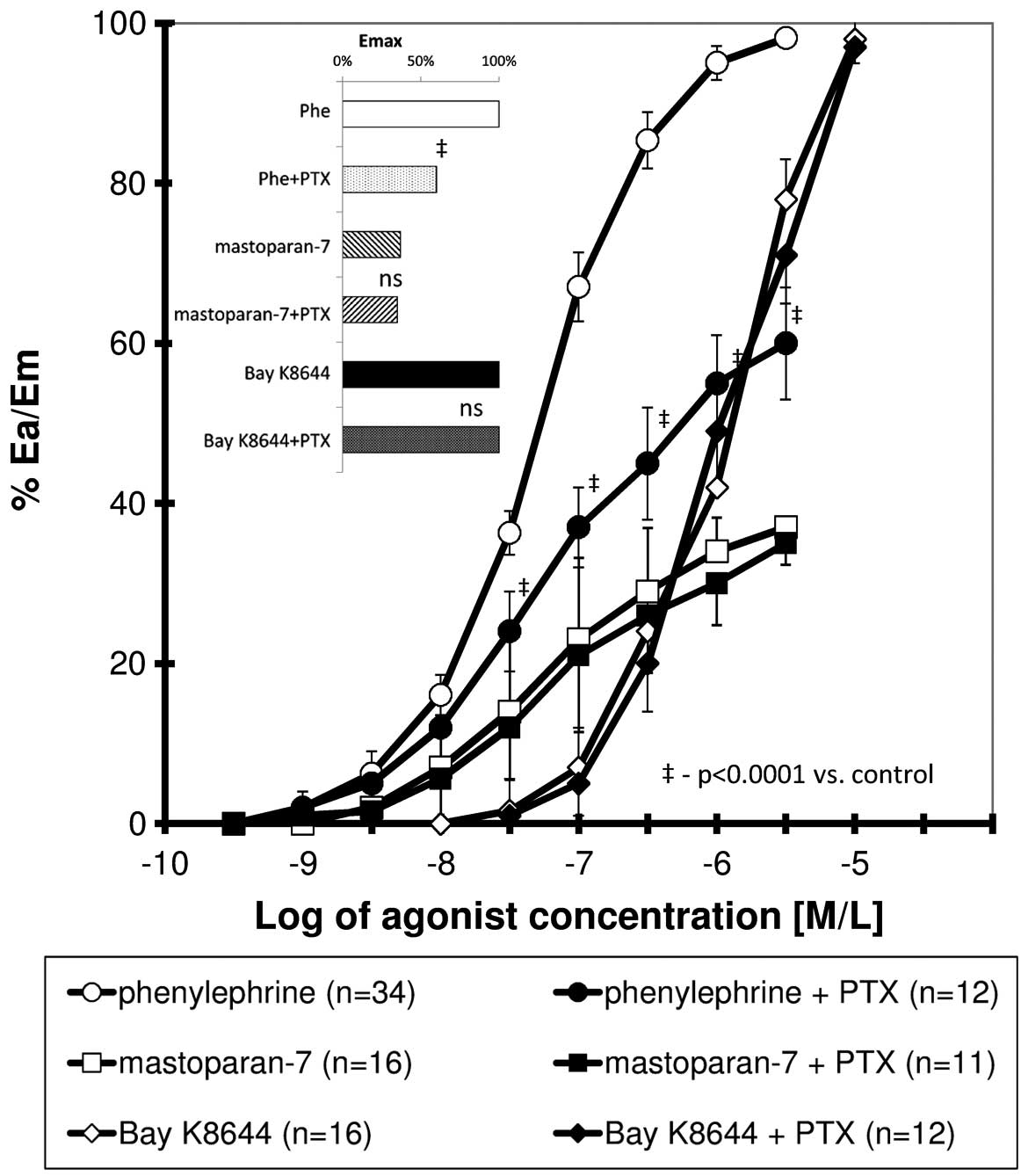
The CRC obtained for phenylephrine, mastoparan-7 and
Bay K8644 presented a sigmoidal association. The curve obtained for
phenylephrine in the presence of PTX was significantly shifted to
the right (all values of relative effect from 20 to 100%) with a
significant reduction in maximal response, whereas PTX did not
significantly modify the CRCs for mastoparan-7 and Bay K8644
(Fig. 1). Table I shows the calculated pharmacometric
date, including Emax, EC50 and
pD2.
 | Table IEC50, maximal response and
relative potency for phenylephrine, mastoparan-7 and Bay K8644 for
controls and in the presence of pertussis toxin (+PTX). |
Table I
EC50, maximal response and
relative potency for phenylephrine, mastoparan-7 and Bay K8644 for
controls and in the presence of pertussis toxin (+PTX).
| Compound | na |
Emaxb, % | EC50
(M/l) | pD2 | RPc | P-valued |
|---|
| Phenylephrine | 34 | 100 | 7.51 (±0.97)
×10−8 | 7.13±0,06 | - | - |
| Phenylephrine +
PTX | 12 | 60±7 | 1.16 (±0.85)
×10−7 | 6.93±0,05 | 0.647 | <0.0001 |
| Mastoparan-7 | 16 | 37±4 | 4.41 (±2.33)
×10−8 | 7.40±0.20 | - | - |
| Mastoparan-7 +
PTX | 11 | 35±4 | 6.12 (±3.40)
×10−8 | 7.21±0.22 | 0.721 | 0.1593 |
| Bay K8644 | 16 | 100 | 1.97 (±0.24)
×10−6 | 5,71±0,05 | - | - |
| Bay K8644 + PTX | 12 | 97±2 | 2.42 (±0.45)
×10−6 | 5.61±0,09 | 0.814 | 0.2874 |
In the presence of PTX, a significant reduction in
the calcium influx that was induced by phenyleprine from the intra-
and extracellular space was found. For the two phases of
contraction, mastoparan-7 induced a significant increase of the
perfusion pressure in comparison to its negative control,
mastoparan-17. PTX did not change the calcium influx that was
induced by mastoparan-7 and did not modify the influx from the
extracellular calcium space (phase 2 only) that was induced by Bay
K8644 (Fig. 2, Table II).
 | Table IIMaximal perfusion pressure for
phenylephrine, mastoparan-7 and Bay K8644-induced contraction
activated by calcium influx from intracellular (phase 1) and
extracellular calcium stores (phase 2), for controls and in the
presence of pertussis toxin (+PTX). |
Table II
Maximal perfusion pressure for
phenylephrine, mastoparan-7 and Bay K8644-induced contraction
activated by calcium influx from intracellular (phase 1) and
extracellular calcium stores (phase 2), for controls and in the
presence of pertussis toxin (+PTX).
| Intracellular calcium
phase 1 | Extracellular calcium
phase 2 |
|---|
|
|
|
|---|
| Compound | n | Perfusion pressure
(±SD), mmHg | n | Perfusion pressure
(±SD), mmHg |
|---|
| Phenylephrine | 30 | 57.9 (±6.2) | 30 | 93.6 (±6.1) |
| Phenylephrine +
PTX | 32 | 23.8 (±4.4)a | 32 | 43.4 (±3.8)a |
| Mastoparan-17 | 10 | 11.8 (±2.1) | 10 | 10.1 (±2.4) |
| Mastoparan-7 | 16 | 17.4 (±3.1) | 16 | 28.3 (±5.6) |
| Mastoparan-7 +
PTX | 11 | 18.2 (±4.7) | 11 | 27.4 (±8.5) |
| Bay K8644 | 16 | 15.4 (±4.0) | 16 | 75.3 (±4.5) |
| Bay K8644 + PTX | 12 | 12.4 (±6.4) | 12 | 76.2 (±6.0) |
Discussion
In the present study, the effect of PTX on arterial
contraction induced in three contraction models was compared,
including the activation of the α1-adrenoceptor with its selective
agonist phenylephrine, the direct activation of G-protein with
mastoparan-7 and the direct activation of the L-type calcium
channel with Bay K8644. Stimulation with phenylephrine or Bay K8644
resulted in a rapid increase in the perfusion pressure and the
maximal responses were achieved in seconds, whereas
mastoparan-7-induced contraction required significantly more time;
typically the maximal result was observed after 30–40 min of
incubation. Additionally, no influence was observed of PTX on
contraction induced by the adrenoceptor stimulation pathway
elements located on and below the G protein, including mastoparan-7
or Bay K8644.
PTX consists of two subunits. The first is
A-protomer, which ribosylates the α-subunits of heterotrimeric G
(i/o) proteins, thus inhibiting the possibility of binding between
the active receptor and its G protein. The second subunit,
B-oligomer, may induce an intracellular signal transduction cascade
by binding to various active proteins located on the cell surface.
This is how PTX may modulate the cell by two different, partially
independent pathways (12).
In the experiment performed on the spiral fragments
of common carotid arteries, the response to mastoparan-7 was not
altered in the presence of PTX and the phospholipase A2 inhibitor,
indomethacin. The L-type calcium channel blockade with nifedipine
inhibited contractility induced by mastoparan-7. The study
indicated that the lack of reversal by nifedypine at higher
concentrations of mastoparan-7 may suggest the activation of others
than just the G protein targets during the action of mastoparan-7
(13).
G protein is the cornerstone in the activation of
different metabotropic receptors, including α-adrenergic receptors,
vasopressin receptors (V1) or angiotensin II receptors, type 1. As
the subsequent element in G protein-coupled receptor activation the
enzyme is regulated, thereby identifying phospholipase C (PLC)
(14–16).
Mastoparan-7 penetrates through biological barriers
and binds to the G protein binding site ligand receptor and
stimulates G protein in an analogical way by activating the
receptor. Results of a previous biochemical study indicate that the
affinity of mastoparam-7 to various G-proteins differs
significantly and is higher for Gi and Gs in
comparison to Gq (17).
This is the reason for PTX not modifying the
Gq11-dependent contraction of vascular smooth muscle
cells following mastoparan-7 stimulation (11). The L-type calcium channel blockers
inhibit the calcium influx from extracellular calcium stores only
and are able to inhibit this process which is present following the
stimulation of the G protein-coupled receptor, mastoparan-7 and Bay
K8644 (10,13,18,19).
Direct stimulation of the L-type calcium channel
with Bay K8644 in the present study induced a significant smooth
muscle contraction, while the presence of PTX did not inhibit this
process. The mechanism of mastoparan-7 action is not clear.
Mastoparan-7 may also induce vascular smooth muscle contraction,
not only by G-protein activation, but also by modulation and
voltage-independent calcium channels (18), inhibition of PLC in low
concentration (<3×10−6 M/l) or activation in high
concentrations (>5×10−6 M/l) (21,22).
Contraction in the models was induced at a concentration range from
3×10−10 to 3×10−6 M/l, and thus the
additional inhibition of PLC may be confused with contraction. In
view of this, the concentrations used were not sufficiently
increased to alter, other than the G-protein elements of the
signaling pathway. Contraction in the presence of phenylephrine and
mastoparan-7 was induced by calcium influx from intra- and
extracellular calcium stores, whereas in the presence of Bay K8644
it was associated with the calcium influx from extracellular
calcium stores only. The inhibition of phenylephrine-induced
contraction and no inhibition in Bay K8644-induced contraction
appears to be clear. However, no change in mastoparan-7-induced
contractility may be the result of various binding places on the
G-protein or the activation of sites other than the G-protein
binding places.
In conclusion, the results of the study have shown
that PTX significantly inhibited the phenylephrine-induced
contraction of vascular smooth muscle cells by inhibition of the
calcium influx from intra- and extracellular calcium space. PTX did
not change the smooth muscle contraction induced by mastoparan-7
and Bay K8644. The predominant effect of mastoparan-7 may be
associated with sites other than the G-protein binding sites or PTX
binds to other sites than that of mastoparan-7.
Abbreviations:
|
CRC
|
concentration response curve
|
|
EC50
|
half maximal effect concentration
|
|
Emax
|
maximal tissue response
|
|
mas-7
|
mastoparan-7
|
|
PLC
|
phospholipase C
|
|
PTX
|
pertussis toxin
|
References
|
1
|
Katada T: The inhibitory G protein G(i)
identified as pertussis toxin-catalyzed ADP-ribosylation. Biol
Pharm Bull. 35:2103–2111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sowa NA, Street SE, Vihko P and Zylka MJ:
Prostatic acid phosphatase reduces thermal sensitivity and chronic
pain sensitization by depleting phosphatidylinositol
4,5-bisphosphate. J Neurosci. 30:10282–10293. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
King TP, Jim SY and Wittkowski KM:
Inflammatory role of two venom components of yellow jackets
(Vespula vulgaris): a mast cell degranulating peptide
mastoparan and phospholipase A1. Int Arch Allergy Immunol.
131:25–32. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hoshina MM, Santos LD, Palma MS and
Marin-Morales MA: Cytotoxic, genotoxic/antigenotoxic and
mutagenic/antimutagenic effects of the venom of the wasp Polybia
paulista. Toxicon. 72:64–70. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yordanova ZP, Woltering EJ,
Kapchina-Toteva VM and Iakimova ET: Mastoparan-induced programmed
cell death in the unicellular alga Chlamydomonas
reinhardtii. Ann Bot. 111:191–205. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin CH, Hou RF, Shyu CL, Shia WY, Lin CF
and Tu WC: In vitro activity of mastoparan-AF alone and in
combination with clinically used antibiotics against
multiple-antibiotic-resistant Escherichia coli isolates from
animals. Peptides. 36:114–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hajnóczky G, Davies E and Madesh M:
Calcium signaling and apoptosis. Biochem Biophys Res Commun.
304:445–454. 2003.
|
|
8
|
Newmeyer DD and Ferguson-Miller S:
Mitochondria: releasing power for life and unleashing the
machineries of death. Cell. 112:481–390. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grześk G, Malinowski B, Grześk E, Wiciński
M and Szadujkis-Szadurska K: Direct regulation of vascular smooth
muscle contraction by mastoparan-7. Biomed Rep. 2:34–38.
2014.PubMed/NCBI
|
|
10
|
Grześk G, Wiciński M, Malinowski B, Grześk
E, Manysiak S, Odrowąż-Sypniewska G, Darvish N and Bierwagen M:
Calcium blockers inhibits cyclosporine A-induced hyperreactivity of
vascular smooth muscle cells. Mol Med Rep. 5:1469–1474.
2012.PubMed/NCBI
|
|
11
|
Grzesk G, Kozinski M, Navarese EP,
Krzyzanowski M, Grzesk E, Kubica A, Siller-Matula JM, Castriota F
and Kubica J: Ticagrelor, but not clopidogrel and prasugrel,
prevents ADP-induced vascular smooth muscle cell contraction: a
placebo-controlled study in rats. Thromb Res. 130:65–69. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mangmool S and Kurose H: G(i/o)
protein-dependent and -independent actions of Pertussis Toxin
(PTX). Toxins (Basel). 3:884–899. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kanagy NL and Webb RC: Enhanced vascular
reactivity to mastoparan, a G protein activator, in genetically
hypertensive rats. Hypertension. 23:946–950. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Birnbaumer L: The discovery of signal
transduction by G-proteins: a personal account and an overview of
the initial findings and contributions that led to our present
understanding. Biochim Biophys Acta. 1768:756–771. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cotecchia S: The α1-adrenergic receptors:
diversity of signaling networks and regulation. J Recept Signal
Transduct Res. 30:410–419. 2010.
|
|
16
|
Bylund DB, Bond RA, Clarke DE, et al: The
IUPHAR Media Compendium of Receptor Characterization and
Classification. 2nd edition. IUPHAR Media; London: 2000
|
|
17
|
Higashijima T, Burnier J and Ross EM:
Regulation of Gi and Go by mastoparan, related amphiphilic
peptides, and hydrophobic amines. Mechanism and structural
determinants of activity. J Biol Chem. 265:14176–14186.
1990.PubMed/NCBI
|
|
18
|
Perianin A and Synderman R: Mastoparan, a
wasp venom peptide, indentifies two discrete mechanisms of
elevating cytosolic calcium and inositol triphosphates in human
polymorphonuclear leukocytes. J Immunol. 143:1669–1673. 1989.
|
|
19
|
Dostal DE, Murahashi T and Peach MJ:
Regulation of cytosolic calcium by angiotensis in vascular smooth
muscle. Hypertension. 15:815–822. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Argiolas A and Pisano JJ: facilitation of
phospholipase A2 activity by mastoparan, a new class of mast cell
degranulating peptides from wasp venom. J Biol Cchem.
258:13697–13702. 1983.PubMed/NCBI
|
|
21
|
Wallace MA and Carter HR: effects of the
wasp venom peptide, mastoparan, on a phosphoinositide-specific
phospholipase C purified from rabbit brain membranes. Biochim
Biophys Acta. 1006:311–316. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hiramatsu Y, Horn VJ, Baum BJ and Ambudkar
IS: Characterization of polyphosphoinositide-specific phospholipase
C in rat parotid gland membranes. Arch Biochem Biophys.
297:368–376. 1992. View Article : Google Scholar : PubMed/NCBI
|