Introduction
Parkinson’s disease (PD; OMIM: #168600), which is
attributed to the death of nigrostriatal dopaminergic neurons, is a
neurodegenerative movement disorder that affects ~2% of the
population aged ≥65 years. The clinical features of PD, including
tremor, rigidity and bradykinesia (1) and other severe complications (2), leading to considerable damage to human
body.
PD is a complex disease affected by genetic and
environmental factors. Environmental factors comprise of oxidative
stress (3), smoking (4) and environmental toxins (5). These environmental risk factors have
been found to play an important role in the progression of PD. In
addition, genetic predisposition plays a clear role in this complex
disease. Previous genetic studies have identified numerous genetic
markers of PD (6–8).
Oxidative stress caused by the accumulation of
oxidative species is closely associated with PD (9). The quinone intermediates that are
derived from dopamine metabolism are pivotal to the generation of
oxidative stress. NQO1, which is located on chromosome
16q22.1, encodes NAD(P)H-quinone oxidoreductase 1 (NQO1), which is
a detoxification enzyme involved in dopamine metabolism.
NQO1 has been found to be expressed in astroglial and
endothelial cells in substantia nigra pars compacta (10). Tumor necrosis factor (TNF) is
located in the human leukocyte antigen class III region on
chromosome 6p21.3 (11). TNF
encodes TNF-α, which is one of the principal cytokines involved in
promoting inflammation and oxidative stress (12).
A number of case-control studies have been carried
out previously to identify the association between PD and the
NQO1 and TNF polymorphisms (13–29).
There were four positive and four negative studies for the
association of the NQO1 C609T polymorphism and PD. There was
one positive and six negative studies for the association between
the TNF-308 polymorphism and PD, and there were two positive
and one negative study between TNF-1031 polymorphism and PD. The
inconsistent conclusions may be due to the limited power of each
study and difference in ethnicity in these genetic loci.
Meta-analyses are able to enhance the credibility of
association studies by combining data from different studies and
drawing a more comprehensive conclusion (30). In the present study, a comprehensive
meta-analysis was conducted to establish the role of these loci in
the risk of PD.
Materials and methods
Data collection
A systematic literature search was performed using
online databases [PubMed, WanFang, WeiPu and China National
Knowledge Infrastructure (CNKI)], without time and language
restriction, and by searching the following keywords: ‘Parkinson
NQO1 association or Parkinson NQO1 polymorphism’ and
‘Parkinson TNF association or Parkinson TNF
polymorphism’ to collect available studies. The studies were
involved when they met the following criteria: i) The study was an
original case-control study with assessment of the association
between PD and polymorphisms of NQO1 and TNF in
humans; ii) it contained sufficient information to infer the odd
ratios (ORs) and 95% confidence intervals (CIs); and iii) the
genotype distribution of each polymorphism in the controls met the
Hardy-Weinberg equilibrium (HWE). All the studies included in the
meta-analyses were carefully considered and selected in January
2014. As shown in previous studies (31–34),
the following information was precisely extracted or calculated
from each study: Genetic locus, first author’s name, year of
publication, country, ethnicity, the numbers of cases and controls,
control source, HWE for controls, the power of individuals and
whether the study had significant association with PD and the power
of individuals (Table I).
 | Table ICharacteristics of the case-control
studies in the present meta-analyses. |
Table I
Characteristics of the case-control
studies in the present meta-analyses.
| Genetic locus | Authors | Year | Country | Ethnicity | Cases/controls | Control source | HWE | Result | Power | (Refs.) |
|---|
| NQO1
C609T | Harada et
al | 2001 | Japan | Asians | 111/100 | Hospital | Yes | NS | 0.152 | (13) |
| Shao et
al | 2001 | China | Asians | 126/136 | Hospital | Yes | S | 0.179 | (14) |
| Jiang et
al | 2004 | China | Asians | 274/161 | Hospital | No | S | 0.195 | (15) |
| Okada et
al | 2005 | USA | Europeans | 163/269 | Hospital | Yes | NS | 0.188 | (16) |
| Shao et
al | 2005 | China | Asians | 140/144 | Population | Yes | S | 0.117 | (17) |
| Xu et
al | 2007 | China | Asians | 52/133 | Population | Yes | NS | 0.184 | (18) |
| Fong et
al | 2007 | China | Asians | 149/153 | Hospital | No | NS | 0.199 | (20) |
| Punia et
al | 2011 | Indian | Asians | 339/344 | Population | Yes | S | 0.373 | (19) |
| TNF-308 | Kruger et
al | 2000 | Germany | Europeans | 237/177 | Population | Yes | S | 0.133 | (21) |
| Ross et
al | 2004 | UK | Europeans | 90/93 | Hospital | Yes | NS | 0.102 | (22) |
| Wahner et
al | 2007 | USA | Europeans | 289/269 | Population | Yes | NS | 0.281 | (23) |
| Wu et
al | 2007 | China | Asians | 369/326 | Hospital | Yes | NS | 0.199 | (24) |
| Bialecka et
al | 2008 | Poland | Europeans | 316/300 | Population | Yes | NS | 0.106 | (25) |
| Du et
al | 2009 | China | Asians | 114/133 | Population | Yes | NS | 0.081 | (26) |
| Pascale et
al | 2010 | Italy | Europeans | 146/156 | Hospital | Yes | NS | 0.184 | (27) |
|
TNF-1031 | Nishimura et
al | 2001 | Japan | Asians | 172/157 | Hospital | Yes | S | 0.126 | (28) |
| Wu et
al | 2007 | China | Asians | 369/326 | Hospital | Yes | S | 0.180 | (24) |
| Infante et
al | 2008 | Spain | Europeans | 194/170 | Population | Yes | NS | 0.096 | (29) |
Statistical analysis
The Arlequin program was used to test HWE (35). The power of each study was
calculated by the Power and Sample Size Calculation program
(36). The statistical
heterogeneity across the studies included in the meta-analysis was
assessed by Cochran’s Q statistic and I2 tests (37) to determine the type of analysis. In
the meta-analysis, the fixed-effects model was used for the studies
with minimal to moderate heterogeneity (I2<50%) and
the random-effects model was used for the studies with significant
heterogeneity (I2≥50%). In addition to the allelic
analysis model, the meta-analyses were performed under the
dominant, recessive and additive models. Funnel plots were also
drawn to observe the potential publication bias. The statistical
analyses of the meta-analyses were carried out in Review Manager 5
(The Cochrane Collection, Copenhagen, Denmark) (38).
Results
Data selection
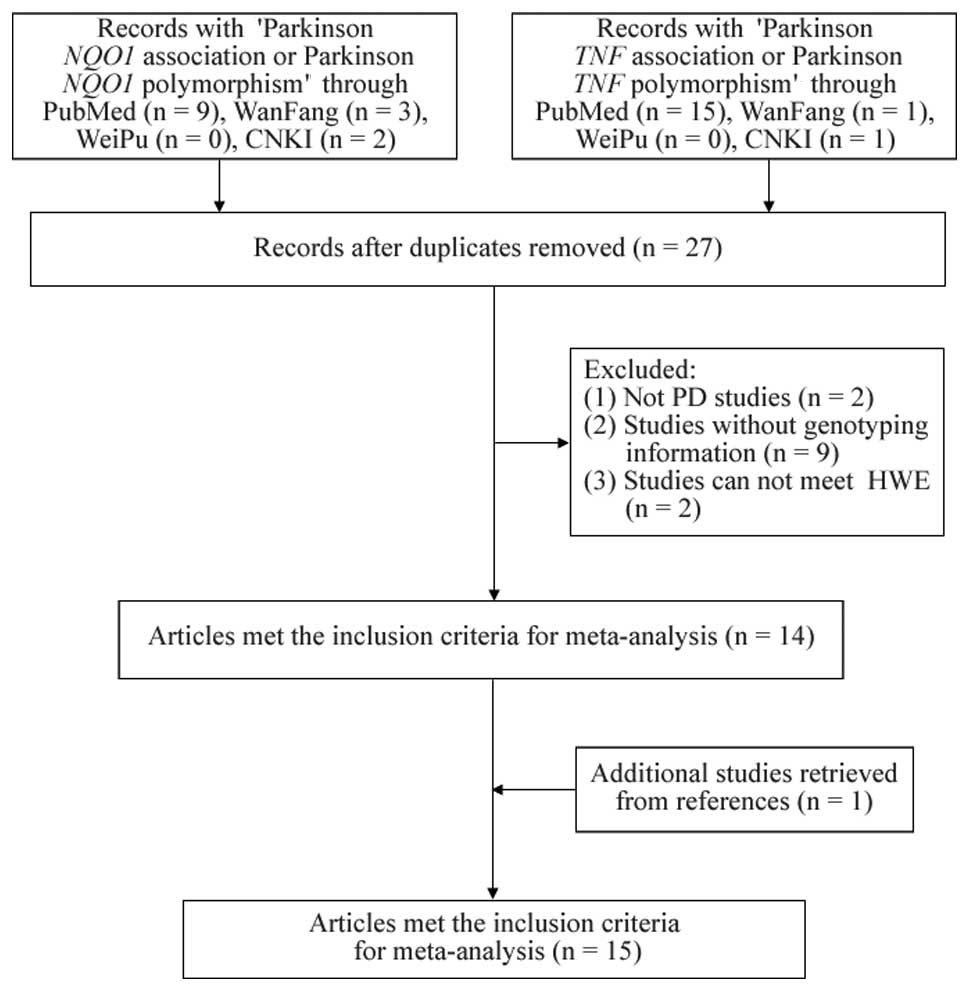
As shown in Fig. 1,
nine relevant NQO1 studies and 16 TNF studies were
selected from PubMed. In addition, three NQO1 studies and
one TNF study from the WanFang literature database, and two
NQO1 studies and one TNF study from CNKI were
included. Following the removal of the duplicates, two studies with
no PD association, nine without genotyping information and two with
significant deviation from HWE (P<0.05) in the controls were
excluded (Table I). A study was
also found from the references in the retrieved literature.
Finally, 15 eligible studies (16 stages) (13,14,16–19,21–29)
were included in the meta-analyses (Table I).
Meta-analyses of NQO1 and TNF
polymorphisms with PD
As shown in Table
II, the meta-analysis of the NQO1 C609T polymorphism
included 931 PD patients and 1,126 healthy controls among six
studies. The statistical heterogeneity was observed in the
meta-analyses of NQO1 C609T (allelic, I2=83%;
dominant, I2=88%; recessive, I2=51%; and
additive models, I2=83%). The frequency of the
NQO1 C609T-C allele in Europeans was 0.788 (HapMap-CEU),
which was much higher than that of the Asian population
(HapMap-CHB=0.478; HapMap-JPT=0.611). A further analysis showed a
difference in ethnicity of the NQO1 C609T polymorphism
between Europeans and Asians (Fst=0.103). As a different genotypic
distribution existed in the NQO1 C609T polymorphism between
Europeans and Asians, further subgroup meta-analyses were performed
by ethnicity. There was no significant association observed in
NQO1 C609T (P=0.69; OR, 1.07; 95% CI, 0.76–1.50; Table II; Fig.
2), and no significant association was found in other subgroup
meta-analyses by genotype and ethnicity (Table II).
 | Table IIMeta-analyses of the association
between NQO1 C609T, TNF-308 and TNF-1031 and
Parkinson’s disease. |
Table II
Meta-analyses of the association
between NQO1 C609T, TNF-308 and TNF-1031 and
Parkinson’s disease.
| Genetic model | Cases/Controls | Ethnicity | No. of studies | OR (95% CI) | P-value | I2
(%) | Power |
|---|
| NQO1
C609T |
| Overall (T vs.
C) | 931/1126 | Overall | 6 | 1.07
(0.76–1.50) | 0.69 | 83 | 0.791 |
| 768/852 | Asians | 5 | 1.15
(0.78–1.70) | 0.48 | 85 | 0.715 |
| 163/269 | Europeans | 1 | 0.76
(0.52–1.09) | 0.14 | NA | 0.188 |
| Dominant (TT/TC
vs. CC) | 805/990 | Overall | 5 | 1.24
(0.67–2.31) | 0.49 | 88 | 0.771 |
| 642/721 | Asians | 4 | 1.43
(0.63–3.27) | 0.39 | 90 | 0.641 |
| 163/269 | Europeans | 1 | 0.76
(0.50–1.16) | 0.20 | NA | 0.245 |
| Recessive (TT vs.
TC/CC) | 805/990 | Overall | 5 | 0.77
(0.44–1.34) | 0.36 | 51 | 0.402 |
| 642/721 | Asians | 4 | 0.83
(0.47–1.47) | 0.53 | 57 | 0.381 |
| 163/269 | Europeans | 1 | 0.23
(0.03–1.90) | 0.17 | NA | 0.077 |
| Additive (TT vs.
CC) | 429/574 | Overall | 5 | 0.92
(0.32–2.61) | 0.87 | 83 | 0.356 |
| 314/395 | Asians | 4 | 1.13
(0.36–3.53) | 0.83 | 87 | 0.320 |
| 115/179 | Europeans | 1 | 0.22
(0.03–1.78) | 0.15 | NA | 0.077 |
| TNF-308 |
| Overall (A vs.
G) | 1561/1454 | Overall | 7 | 1.08
(0.93–1.24) | 0.31 | 28 | 0.719 |
| 1078/995 | Europeans | 5 | 1.08
(0.92–1.27) | 0.35 | 5 | 0.609 |
| 483/459 | Asians | 2 | 0.79
(0.29–2.18) | 0.65 | 76 | 0.216 |
| Dominant (AA/AG
vs. GG) | 1245/1154 | Overall | 6 | 0.96
(0.69–1.31) | 0.78 | 60 | 0.793 |
| 842/752 | Europeans | 4 | 0.98
(0.66–1.44) | 0.90 | 64 | 0.638 |
| 403/402 | Asians | 2 | 0.77
(0.27–2.22) | 0.63 | 76 | 0.332 |
| Recessive (AA vs.
AG/GG) | 1254/1154 | Overall | 6 | 1.34
(0.80–2.23) | 0.26 | 15 | 0.163 |
| 762/695 | Europeans | 4 | 1.40
(0.82–2.41) | 0.22 | 32 | 0.149 |
| 483/459 | Asians | 2 | 0.88
(0.18–4.40) | 0.88 | NA | 0.062 |
| Additive (AA vs.
GG) | 960/896 | Overall | 6 | 1.31
(0.78–2.20) | 0.31 | 0 | 0.163 |
| 567/514 | Europeans | 4 | 1.36
(0.79–2.35) | 0.27 | 18 | 0.148 |
| 393/382 | Asians | 2 | 0.93
(0.19–4.64) | 0.93 | NA | 0.062 |
|
TNF-1031 |
| Overall (C vs.
T) | 735/653 | Overall | 3 | 1.24
(0.85–1.79) | 0.26 | 58 | 0.383 |
| Dominant (CC/CT
vs. TT) | 735/653 | Overall | 3 | 1.12
(0.70–1.78) | 0.64 | 62 | 0.495 |
| Recessive (CC vs.
CT/TT) | 735/653 | Overall | 3 | 3.19
(1.66–6.13) | 0.0005a | 0 | 0.101 |
| Additive (CC vs.
TT) | 587/507 | Overall | 3 | 3.15
(1.63–1.07) | 0.0006a | 0 | 0.101 |
Meta-analysis of the TNF-308 polymorphism was
involved with 3,122 PD patients and 2,908 healthy controls among
seven studies (Table II). No
significant heterogeneity was found in the meta-analysis under the
allelic model (I2=28%), and no significant association
of TNF-308 with PD was observed (P=0.31; OR, 1.08; 95% CI,
0.93–1.24; Table II; Fig. 2). Analysis of the association
between TNF-308 with PD in ethnicity and genetic models was
performed, but no positive result was found (Table II).
The meta-analysis of the TNF-1031
polymorphism was conducted with 735 PD patients and 653 healthy
controls among three studies (Table
II). The TNF-1031 polymorphism was shown to be a risk
factor of PD in the meta-analyses under the recessive (P=0.0005;
OR, 3.19; 95% CI, 1.66–6.13) and additive models (P=0.0006, OR,
3.15; 95% CI, 1.63–6.07), however, there was no significant
association in the meta-analysis under the allelic model (P=0.26,
OR, 1.24; 95% CI, 0.85–1.79; Table
II; Fig. 2). As there were only
three studies in the meta-analysis of the TNF-1031
polymorphism, the result of this strong association requires
interpreting with caution (Table
II). No publication bias was found for all the meta-analyses
(Fig. 3).
Discussion
To the best of our knowledge, the present study is
the first meta-analysis on NQO1 and TNF. The
meta-analysis involved 15 studies with 2,858 patients and 2,907
healthy controls. Allelic analysis and genetic models were
performed for the meta-analyses and subgroup analyses were also
conducted by ethnicity in NQO1 C609T and TNF-308. The
power of the study was 0.791 in NQO1 C609T and 0.719 in
TNF-308, which were much stronger than each of the original
case-control studies (power≤0.373).
There was no significant association observed
between NQO1 C609T and PD. The same conclusion was drawn
when a further subgroup study by ethnicity was conducted. The
result of NQO1 C609T was consistent with three involved
studies (13,16,18),
whereas it was inconsistent with another three involved studies
(14,17,19)
that had a stronger power. The present meta-analysis draws a more
reliable conclusion than the previous studies. Notably, as there
were five Asian studies and only one European study (Table I), the conclusion may have shown a
deviation to the Asian population, and the lower power of the
European data suggested that larger case-control studies are
required.
The meta-analysis also suggested that TNF-308
had no association for PD. This result is consistent with the
majority of previous studies (22–27),
and only one study presented an opposing conclusion (21). In comparison to the former studies,
the meta-analysis showed a stronger power and involved subgroup
analyses by genetic models and ethnicity, which allowed for a more
stable and comprehensive conclusion.
TNF-1031 was observed to significantly
increase the risk of PD in the recessive and additive models
(P=0.0005 and P=0.0006, respectively). TNF-1031 is a key
polymorphism located in the promoter of TNF that influences
the transcriptional regulation of TNF production (39). Among the three studies included in
the meta-analysis of TNF-1031, two studies showed that
TNF-1031 was associated with an increased risk of PD
(24,28). The present meta-analysis may provide
novel information for the association between TNF-1031 and
PD. However, due to the small power of TNF-1031, the
positive result of TNF-1031 should be interpreted with
caution.
Certain limitations of the meta-analysis should be
considered. Firstly, PD is a complicated disorder influenced by
numerous factors, including gender and age differences. The
aforementioned information was not provided in the original
case-control studies. Thus, a subgroup meta-analysis cannot be
performed by gender or age to establish a more credible result.
Secondly, there were a number of populations involved in the
meta-analyses of NQO1 C609T (Chinese, Japanese, Indian and
American populations), TNF-308 (German, Italian, United
Kingdom, American, Polish and Chinese populations) and
TNF-1031 (Chinese, Japanese and Spanish populations). Future
studies in other ethnic populations are required to completely
assess the contribution of these polymorphisms to PD. Thirdly, the
clinical diagnostic accuracy of PD is only 70% (40). Different diagnostic criteria among
the various case-control studies may have an impact on the results
of the meta-analyses. Fourthly, there are 1,574 polymorphisms on
the NQO1 and TNF loci, respectively. The present
meta-analyses only focused on specific polymorphisms, NQO1
(NQO1 C609T) and TNF (TNF-308 and
TNF-1031). These findings may not completely represent the
overall contribution of NQO1 and TNF to PD.
In conclusion, the results of the present study
indicated that TNF-1031 polymorphism may be a risk factor
for PD under either the recessive or additive models. However, the
meta-analyses did not support the involvement of NQO1 C609T
and TNF-308 in the risk of PD.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 31100919
and 81371469), the Natural Science Foundation of Zhejiang Province
(grant no. LR13H020003) and the K. C. Wong Magna Fund in Ningbo
University and Ningbo Social Development Research Projects (grant
no. 2012C50032).
References
|
1
|
Dauer W and Przedborski S: Parkinson’s
disease: mechanisms and models. Neuron. 39:889–909. 2003.
|
|
2
|
Arora A and Fletcher P: Problem based
review: a patient with Parkinson’s disease. Acute Med. 12:246–250.
2013.
|
|
3
|
Olanow CW and Tatton WG: Etiology and
pathogenesis of Parkinson’s disease. Annu Rev Neurosci. 22:123–144.
1999.
|
|
4
|
Wirdefeldt K, Adami HO, Cole P,
Trichopoulos D and Mandel J: Epidemiology and etiology of
Parkinson’s disease: a review of the evidence. Eur J Epidemiol.
26(Suppl 1): S1–S58. 2011.
|
|
5
|
Vaglini F, Viaggi C, Piro V, et al:
Acetaldehyde and parkinsonism: role of CYP450 2E1. Front Behav
Neurosci. 7:712013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lill CM, Roehr JT, McQueen MB, et al:
Comprehensive research synopsis and systematic meta-analyses in
Parkinson’s disease genetics: The PDGene database. PLoS Genet.
8:e10025482012.PubMed/NCBI
|
|
7
|
McInerney-Leo A, Hadley DW, Gwinn-Hardy K
and Hardy J: Genetic testing in Parkinson’s disease. Mov Disord.
20:1–10. 2005.
|
|
8
|
Bekris LM, Mata IF and Zabetian CP: The
genetics of Parkinson disease. J Geriatr Psychiatry Neurol.
23:228–242. 2010. View Article : Google Scholar
|
|
9
|
Dias V, Junn E and Mouradian MM: The role
of oxidative stress in Parkinson’s disease. J Parkinsons Dis.
3:461–491. 2013.
|
|
10
|
van Muiswinkel FL, de Vos RA, Bol JG, et
al: Expression of NAD(P)H:quinone oxidoreductase in the normal and
Parkinsonian substantia nigra. Neurobiol Aging. 25:1253–1262.
2004.PubMed/NCBI
|
|
11
|
Kamoun M, Chelbi H, Houman MH, Lacheb J
and Hamzaoui K: Tumor necrosis factor gene polymorphisms in
Tunisian patients with Behcet’s disease. Hum Immunol. 68:201–205.
2007.PubMed/NCBI
|
|
12
|
Dobbs RJ, Charlett A, Purkiss AG, Dobbs
SM, Weller C and Peterson DW: Association of circulating TNF-alpha
and IL-6 with ageing and parkinsonism. Acta Neurol Scand.
100:34–41. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Harada S, Fujii C, Hayashi A and Ohkoshi
N: An association between idiopathic Parkinson’s disease and
polymorphisms of phase II detoxification enzymes: glutathione
S-transferase M1 and quinone oxidoreductase 1 and 2. Biochem
Biophys Res Commun. 288:887–892. 2001.
|
|
14
|
Shao M, Liu Z, Tao E and Chen B:
Polymorphism of MAO-B gene and NAD(P)H: quinone oxidoreductase gene
in Parkinson’s disease. Zhonghua Yi Xue Yi Chuan Xue Za Zhi.
18:122–124. 2001.(In Chinese).
|
|
15
|
Jiang XH, Yang H, Yang JF, Wang HT, Xu QY
and Chen B: A study on the relationship between polymorphism of
human NAD(P)H: quinone oxidoreductase and Parkinson’s disease in
Chinese. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 21:120–123. 2004.(In
Chinese).
|
|
16
|
Okada S, Farin FM, Stapleton P, et al: No
associations between Parkinson’s disease and polymorphisms of the
quinone oxidoreductase (NQO1, NQO2) genes. Neurosci Lett.
375:178–180. 2005.
|
|
17
|
Shao M, Liu ZL, Tao EX and Chen B:
Correlation between the genetic polymorphism of dopamine metabolic
enzymes and the genetic susceptibility of Parkinson’s disease. Chin
J Gerontol. 25:743–745. 2005.
|
|
18
|
Xu H, Du W, Sun Z, et al: Genetic analysis
of P2X7 C489T and NQO1 C609T polymorphisms and susceptibility of
primary Parkinson’s disease. Chin J Exp Surg. 24:1310–1312.
2007.
|
|
19
|
Punia S, Das M, Behari M, et al: Leads
from xenobiotic metabolism genes for Parkinson’s disease among
north Indians. Pharmacogenet Genomics. 21:790–797. 2011.
|
|
20
|
Fong CS, Wu RM, Shieh JC, et al: Pesticide
exposure on southwestern Taiwanese with MnSOD and NQO1
polymorphisms is associated with increased risk of Parkinson’s
disease. Clin Chim Acta. 378:136–141. 2007.PubMed/NCBI
|
|
21
|
Krüger R, Hardt C, Tschentscher F, et al:
Genetic analysis of immunomodulating factors in sporadic
Parkinson’s disease. J Neural Transm. 107:553–562. 2000.PubMed/NCBI
|
|
22
|
Ross OA, O’Neill C, Rea IM, et al:
Functional promoter region polymorphism of the proinflammatory
chemokine IL-8 gene associates with Parkinson’s disease in the
Irish. Hum Immunol. 65:340–346. 2004.PubMed/NCBI
|
|
23
|
Wahner AD, Sinsheimer JS, Bronstein JM and
Ritz B: Inflammatory cytokine gene polymorphisms and increased risk
of Parkinson disease. Arch Neurol. 64:836–840. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu YR, Feng IH, Lyu RK, et al: Tumor
necrosis factor-alpha promoter polymorphism is associated with the
risk of Parkinson’s disease. Am J Med Genet B Neuropsychiatr Genet.
144B:300–304. 2007.
|
|
25
|
Bialecka M, Klodowska-Duda G, Kurzawski M,
et al: Interleukin-10 (IL10) and tumor necrosis factor alpha (TNF)
gene polymorphisms in Parkinson’s disease patients. Parkinsonism
Relat Disord. 14:636–640. 2008.
|
|
26
|
Du WD, Tang XF, Tang HY, et al: Tumor
necrosis factor alpha (TNFα) rs1800629 and lymphotoxin alpha (LTA)
rs909253 might not be potential susceptibility locus leading to
Chinese sporadic Parkinson’s disease. Chin J Dis Control Prev.
13:33–38. 2009.
|
|
27
|
Pascale E, Passarelli E, Purcaro C, et al:
Lack of association between IL-1β, TNF-α, and IL-10 gene
polymorphisms and sporadic Parkinson’s disease in an Italian
cohort. Acta Neurol Scand. 124:176–181. 2011.
|
|
28
|
Nishimura M, Mizuta I, Mizuta E, et al:
Tumor necrosis factor gene polymorphisms in patients with sporadic
Parkinson’s disease. Neurosci Lett. 311:1–4. 2001.
|
|
29
|
Infante J, García-Gorostiaga I,
Sánchez-Juan P, et al: Inflammation-related genes and the risk of
Parkinson’s disease: a multilocus approach. Eur J Neurol.
15:431–433. 2008.PubMed/NCBI
|
|
30
|
Zintzaras E and Lau J: Trends in
meta-analysis of genetic association studies. J Hum Genet. 53:1–9.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu X, Wang Y, Wang L, et al: Meta-analyses
of 8 polymorphisms associated with the risk of the Alzheimer’s
disease. PloS One. 8:e731292013.
|
|
32
|
Yu X, Huang Y, Li C, Yang H, Lu C and Duan
S: Positive association between lymphotoxin-alpha variation
rs909253 and cancer risk: a meta-analysis based on 36 case-control
studies. Tumour Biol. 35:1973–1983. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ye H, Li X, Wang L, et al: Genetic
associations with coronary heart disease: meta-analyses of 12
candidate genetic variants. Gene. 531:71–77. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tang L, Wang L, Liao Q, et al: Genetic
associations with diabetes: meta-analyses of 10 candidate
polymorphisms. PloS One. 8:e703012013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Excoffier L, Laval G and Schneider S:
Arlequin (version 3.0): an integrated software package for
population genetics data analysis. Evol Bioinform Online. 1:47–50.
2007.
|
|
36
|
Gibson E, Fenster A and Ward AD: The
impact of registration accuracy on imaging validation study design:
A novel statistical power calculation. Med Image Anal. 17:805–815.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Coory MD: Comment on: Heterogeneity in
meta-analysis should be expected and appropriately quantified. Int
J Epidemiol. 39:932–933. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kawalec P, Mikrut A, Wiśniewska N and Pilc
A: The effectiveness of tofacitinib, a novel Janus kinase
inhibitor, in the treatment of rheumatoid arthritis: a systematic
review and meta-analysis. Clin Rheumatol. 32:1415–1424. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Higuchi T, Seki N, Kamizono S, et al:
Polymorphism of the 5′-flanking region of the human tumor necrosis
factor (TNF)-alpha gene in Japanese. Tissue Antigens. 51:605–612.
1998.
|
|
40
|
Hughes AJ, Daniel SE, Kilford L and Lees
AJ: Accuracy of clinical diagnosis of idiopathic Parkinson’s
disease: a clinico-pathological study of 100 cases. J Neurol
Neurosurg Psychiatry. 55:181–184. 1992.
|