Introduction
The hippocampus is of paramount importance as it
controls a wide range of physiological processes, including
learning and memory, spatial navigation and neurological diseases,
such as Alzheimers’s disease (AD), depression, schizophrenia and
stroke, which often target the hippocampus and have profound
effects on physiology (1–4). The function of the hippocampus has
been extensively studied from a behavioral, biochemical and
neuroanatomical perspective, establishing the hippocampus as an
excellent model for studying the function of a protein. Cytoglobin
(Cygb) was identified as the fourth vertebrate heme-globin in 2002
(5,6). Cygb has, despite low sequence homology
with the canonical hemoglobin and myoglobin, retained the classical
globin fold and can reversibly bind oxygen and other diatomic
gasses (7). Cygb is expressed in a
number of tissues and is also found in neurons of the brain where
Cygb is localized in the soma, neuronal processes and nuclei
(8,9). Within the mouse brain, Cygb is
expressed in distinct areas with large regional differences in the
expression levels. The areas with pronounced Cygb expression are
the hippocampus, reticular thalamic nucleus (RT), habenula,
laterodorsal tegmental nucleus and the pedunculopontine tegmental
nucleus (8,10,11).
The Cygb expression patterns in the mouse brain were recently
determined to be identical in the rat and human brain,
demonstrating that the rodent brain can be used as a translational
model for studying Cygb in humans, at least at the anatomical level
(12,13). The function of Cygb remains largely
unknown, but several studies have linked Cygb to reactive
oxygen/nitrogen species (RNS) nitric oxide (NO) scavenging
(14–20). Furthermore, Cygb overexpression
protects against ischemic cell death in vivo (21), although not when expressed at
endogenous levels (22). In our
previous studies, Cygb-immunoreactivity (ir) was shown to be highly
co-localized with one of the enzymes producing NO, namely neuronal
nitric oxide synthase (nNOS), in the mouse brain (9) and found that the majority of the
nNOS-ir neurons of the rat hippocampus co-localized with Cygb-ir
(13). However, due to the larger
number of Cygb-ir cells in the hippocampus, the majority of Cygb-ir
cells remain uncharacterized (13).
The aim of the present study was to extend our
previous study (13) in the rat
hippocampus by providing a detailed neurochemical phenotype of the
Cygb-ir neurons in association with the subpopulation co-expressing
nNOS. Knowledge regarding the neurochemical phenotypes of neurons
expressing the protein of interest can be a valuable tool, as it
will allow the investigator to determine if the protein of interest
is primarily co-localized with one other protein or groups of
proteins associated with known specific functions or pathways. This
information can then be used to test functional hypotheses
regarding the protein of interest by investigating whether
affecting the functions/pathways of the co-expressing proteins will
also affect the protein of interest.
Materials and methods
Animals
Six male Wistar rats (250 g) from Taconic (Denmark),
were used in the experiment. All the rats were perfusion-fixed in
4% paraformaldehyde and the brains were dissected and post-fixed in
the same fixative for 24 h at 4°C. The brains were cryo-protected
with 30% sucrose in phosphate-buffered saline for five days and
stored at −80°C until required. The brains were cryo-sectioned in
40-μm coronal sections in a series of five sections. Animal care
and all the experimental procedures were conducted in accordance to
the principles of Laboratory Animal Care (Law on Animal Experiments
in Denmark, publication 1306, November 23, 2007) and approved by
the Faculty of Health, University of Copenhagen (Copenhagen,
Denmark).
Immunohistochemistry (IHC)
The IHC protocol has been described previously
(23). The primary antibodies
employed for IHC were: i) Rabbit anti-Cygb [in-house, code# 5092/6,
1:3,000 dilution and characterized previously (9,12,13)];
ii) sheep anti-nNOS [Dr Emson, University of Cambridge (Cambridge,
UK), 1:3,000 dilution and characterized previously (24,25)].
nNOS produces the gas-neurotransmitter NO, which is involved in a
number of physiological and pathological processes, including
vasodilatation and RNS-mediated damage. iii) Goat
anti-somastostatin (SOMA) [code# sc-7819, 1:1,000 dilution and
characterized previously (26,27);
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA]. SOMA is a
neuroendocrine peptide hormone and regulates a number of secondary
hormones via its G protein-coupled receptors. iv) Rabbit
anti-vasoactive intestinal peptide (VIP) [in-house, code# 291E-3,
1:15,000 dilution and characterized previously (28)]. VIP is a major regulatory peptide in
the brain and is involved in a number of processes, including
circadian and neuroendocrine control. v) Rabbit anti-parvalbumin
(PV) [code# PV25, 1:40,000 dilution and characterized previously
(29,30); Swant, Swizerland]. PV is a
calcium-binding protein involved in numerous physiological
processes. vi) Rabbit anti-heme oxygenase 1 (HO-1) [code#
ADI-SPA-895, 1:60,000 dilution and characterized previously
(10,31); Enzo Life Sciences, AH Diagnostics
AS, Aarhus, Denmark]. HO-1 is an enzyme that catalyzes the
degradation of heme groups to produce biliverdin, iron and the
gas-neurotransmitter carbon monoxide. The primary antibodies were
detected with either a donkey anti-sheep Alexa-488 or 568 (code#
A11015 and A21009, 1:800 dilution; Life Technologies, Carlsbad, CA,
USA), donkey anti-rabbit Alexa-594 or 647 (code# A21207 and A31573,
1:800 dilution; Life Technologies) and donkey anti-goat Alexa-649
(code# 705-606-147, 1:300 dilution; Jackson Immunoresearch
Laboratories, Inc., West Grove, PA, USA). When two rabbit primary
antibodies were used in continuation, a previously described
protocol (23) was used. Briefly,
following the initial block of endogen peroxide with
H2O2 treatment, the first primary antibody,
which was highly diluted, was detected with a biotin-conjugated
donkey anti-rabbit (code# 711-066-152, 1:800 dilution; Jackson
Immunoresearch Laboratories, Inc.) followed by the avidin-biotin
complex (code# PK-6100; Vector Laboratories, Peterborough, UK), the
Tyramide Signal Amplification system (code# NEL700001KT;
Perkin-Elmer, Waltham, MA, USA) and visualized with a
strepavidin-conjugated Cy2 or Cy5 antibody (code# 016-220-084 and
016-170-085, 1:800 dilution; Jackson Immunoresearch Laboratories,
Inc.). The second primary antibody was detected with a donkey
anti-rabbit Alexa-594.
Image analyses
Images were captured using an iMIC confocal
microscope (FEI Munich GmbH, Germany) equipped with the appropriate
filter settings for detecting 4′,6-diamidino-2-phenylindole and
CY2/Alexa-488, and CY3/Alexa-594 and CY5/Alexa-640. For the
overview images captured in the ×10 wide-field section of the
microscope and 9×3 images were stitched together using the
LA-stitch module of Fiji (ImageJ 1.47 64-bit, National Institutes
of Health, Bethesda, MD, USA). The image analysis in the higher
magnification, the images were captured by the spinning disk
confocal section of the microscope. The Z-stacks of ~60–70 images
were separated in the Z-level by 0.5 μm and deconvoluted in
AutoQuant X (version 3.02; Media Cybernetics, Inc., Rockville, MD,
USA) and the localization of dendritic processes and cell bodies
were further analyzed using the co-localization module of IMARIS
(version 7.6.4; Bitplane USA, South Windsor, CT, USA). Finally, the
images were corrected for brightness and contrast in Adobe
Photoshop CS5 (Adobe Systems, Mountain View, CA, USA), and extended
and mounted into plates using Adobe Illustrator CS5 (Adobe
Systems).
Results
Cygb localization and
co-localization
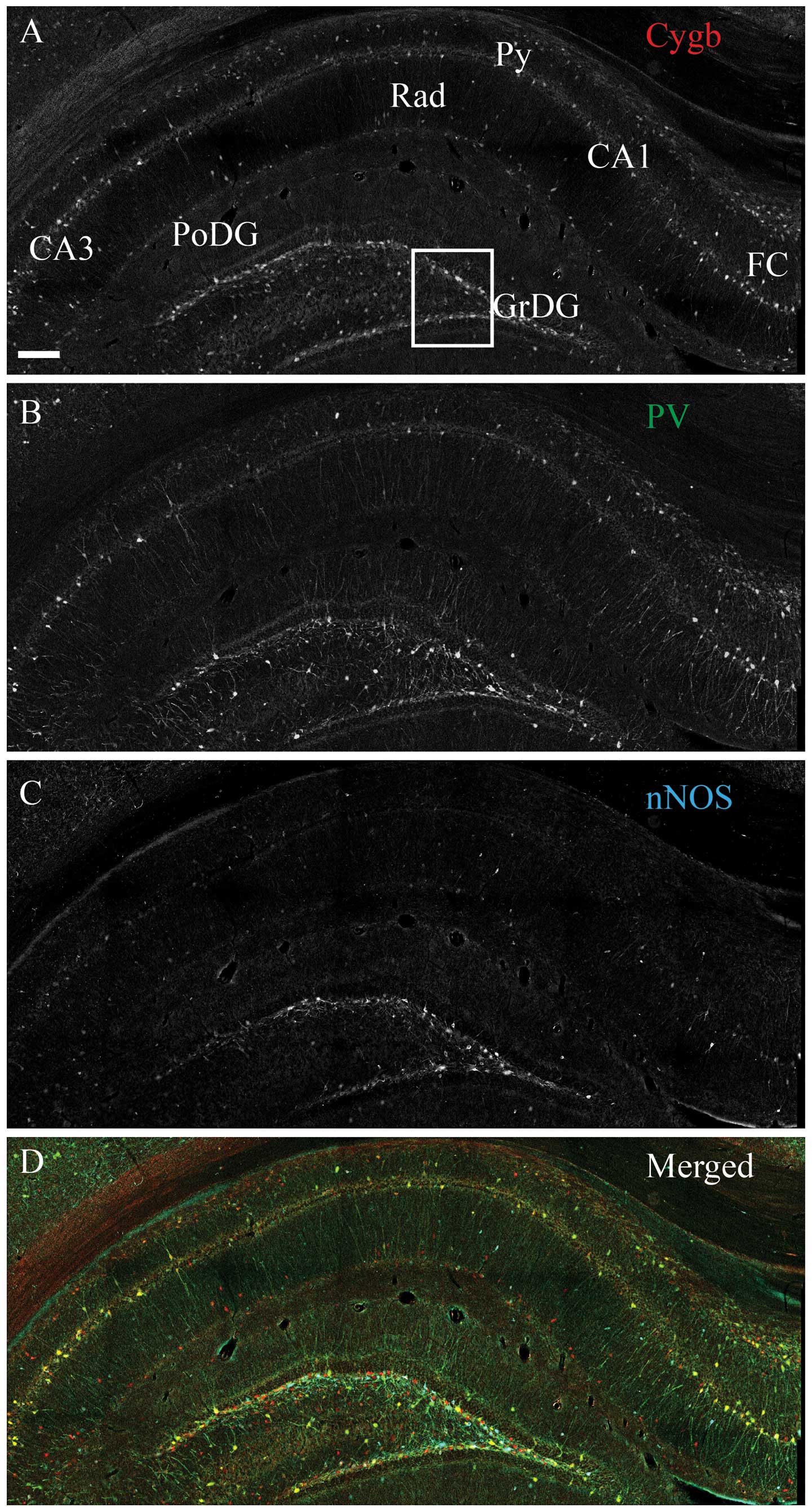
Intense Cygb-ir was observed throughout the
rostral-caudal extend and in the majority of the hippocampal
structures, with the highest density in the dentate gyrus (DG) and
hilus (Fig. 1A). In the pyramidal
cell layer (Py), in fields CA1–CA3, evident Cygb-ir was detected in
the cell soma, nuclei and processes of the pyramidal cells
(Fig. 1A). Intense nNOS-ir neurons
were observed in the DG and CA1–CA3 (Fig. 1B). PV-ir interneurons and processes
were observed in the same areas as Cygb-ir. The vast majority
co-stored Cygb-ir, and a subset also co-stored nNOS-ir (Fig. 1B and D). The majority of the stratum
radiatum (Rad) nNOS-ir cells did not contain Cygb-ir (Fig. 1D). Intense HO-1-ir cells were
observed in the DG, as well as in a few cells of the CA1–CA3
(Fig. 2B), where almost all the
cells co-stored Cygb-ir and a small subset also co-stored nNOS-ir
(Fig. 2D). A few medium-intensity
stained VIP-ir cells were observed to be scattered in DG and
CA1–CA3 (Fig. 3B), where the
majority of cells co-expressed Cygb-ir and nNOS-ir (Fig. 3D). SOMA-ir was highly expressed
throughout the hippocampus (Fig.
4B) and was found in the majority of the cells that also
expressed Cygb-ir and nNOS-ir, although a subset only co-stored
Cygb-ir (Fig. 4D). Outside the
hippocampus, such as the RT, a high degree of co-localization
between Cygb-ir and the neurochemical marker, PV, was also observed
(Fig. 5).
 | Figure 4Cytoglobin (Cygb), somastostain (SOMA)
and neuronal nitric oxide synthase (nNOS) expression in the rat
hippocampus. Immunohistochemical staining of (A) Cygb, (B) SOMA and
(C) nNOS. (D) The merged image of A–C is shown. As for vasoactive
intestinal peptide, SOMA-immunoreactivity (ir) (green) neurons
primarily belonged to the sub-population of Cygb-ir (red) neurons,
also co-localizing nNOS-ir (cyan). The area within the white box is
magnified in Fig. 6. CA1–3, corun
ammonis 1–3; FC, fasio larumcinereum; GrDG, striatum granulosum of
dentate gyrus; PoDG, polymorph layer of DG; Py, pyramidal cell
layer; Rad, stratum radiatum. Scale bar, 200 μm. |
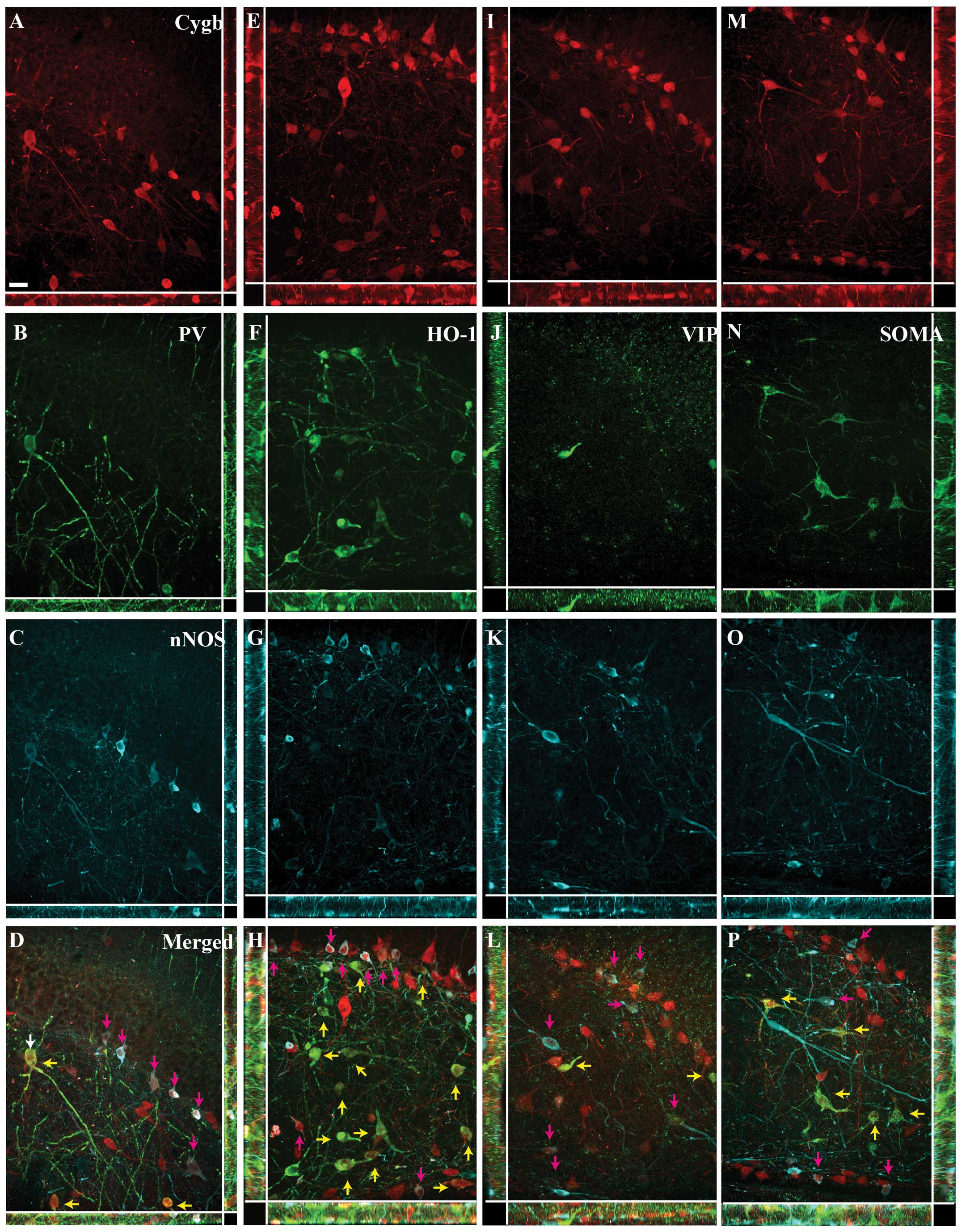
Image analysis
The Z-stack image analysis of the areas within the
white squares in Figs. 1–4 confirmed the co-localization between
Cygb-ir and the neurochemical markers, and confirmed that the
majority of the cells co-expressing Cygb-ir/PV-ir and
Cygb-ir/HO-1-ir did not express nNOS-ir (Fig. 6A–H). Similarly, the high degree of
co-localization between VIP-ir, SOMA-ir and Cygb-ir was also
confirmed (Fig. 6I–L and M–P,
respectively).
Discussion
Despite intensive research (32) over the past 12 years, the function
of Cygb remains an enigma. To the best of our knowledge, the
present study is the first detailed characterization of the
neurochemical phenotype of Cygb-ir neurons in the rat hippocampus
and can, in combination with other methods, contribute to an
improved understanding of the Cygb function. The study was
performed in the rat model as the employed antibodies produced an
optimal immunohistochemical staining, as opposed to the mouse.
Clarifying the function of a protein in the brain is
challenging due to the high complexity and the interconnections of
the brain structures. Therefore, having tools that minimize the
complexity and provide a clearer functional readout are highly
advantageous. We have previously used neurochemical phenotyping to
obtain surrogate markers of the protein of interest in knock-out
mice in order to study the fate of neurons that would have
expressed the protein of interest. This enabled the study of
whether these neurons were more prone to hypoxia-induced immediate
early gene expression and cell death (33).
The principal novel finding of the present study is
the high degree of co-localization between Cygb and the two
markers, PV and HO-1, in the hippocampus of which only a subset of
neurons also co-express nNOS-ir. The high degree of Cygb-ir/PV-ir
co-expression therefore shows for the first time that
Cygb-expressing neurons are primarily inhibitory interneurons. The
high degree of co-expression with HO-1 is notable as HO-1 is an
oxidative stress-inducible protein and enzymatic degradation of
heme leads to the production of iron, biliverdin (an antioxidant)
and CO (34). Cygb may therefore
either be a heme substrate for HO-1 or alternatively, Cygb may, in
these HO-1-expressing neurons, regulate the level of the
gas-neurontransmitter CO produced by HO-1 in a similar way to that
proposed for NO (18,19,21).
Notably, in association with the putative role of
Cygb in neuroprotection, a selective reduction of PV-ir neurons
were observed in the hippocampus of the AD patients and transgenic
animal models of AD (35–38). Of note, HO-1-ir has been shown to be
upregulated in hippocampal neurons of the AD patients (39) and to contribute to the pathology by
excessive production of free iron (40–42).
The high degree of co-expression with Cygb indicates that Cygb
could be a heme substrate for HO-1 enzymatic activity and thereby a
source for excessive iron production, which contributes to the
pathology of AD. Taken together, this indirectly indicates that
expressing Cygb alone does not provide neurons with a selective
protection against neurodegenerative cell death, which is in line
with the lack of selective sparring of Cygb-expressing neurons
following brain ischemia (22).
Cygb-ir was also co-expressed in SOMA-ir and VIP-ir
neurons to a high degree. These neurons, however, belonged
primarily to the neurons that also expressed nNOS-ir. VIP and SOMA
have a broad range of functions in the central nervous system,
however, in association with neuronal protection, a reduced number
of viable SOMA neurons were found in human AD brains (43,44),
whereas VIP have been shown to exert a level of protection in
neurodegenerative disorders (45–47).
These observations indirectly show that despite SOMA, neurons also
express Cygb and they are still not spared in neurodegenerative
disorders, thus questioning if Cygb at endogenous levels functions
in neuronal protection.
Future studies using Cygb null mice (20,48)
would be highly beneficially for studying the fate/function of
neurons expressing PV, HO-1, SOMA and VIP. These studies will show
whether a lack of Cygb affects neuronal survival or normal cell
physiology by using these four proteins as markers. Based on the
large expression of Cygb in the hippocampus, it is highly likely
that Cygb has an important function in hippocampal normal
physiology. The results in the present study will be a significant
aid for future studies in elucidating any functional roles.
Acknowledgements
The authors are most grateful to Professor Eero
Vasar and the Centre of Excellence for Translational Medicine for
providing excellent working facilities and to Dr Brent M. Witgen
for the helpful discussion of the manuscript. This work was
supported by the Estonia Research Council (PUT120) and the European
Regional Development Fund.
References
|
1
|
McEwen BS: Physiology and neurobiology of
stress and adaptation: central role of the brain. Physiol Rev.
87:873–904. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hampel H, Bürger K, Teipel SJ, Bokde AL,
Zetterberg H and Blennow K: Core candidate neurochemical and
imaging biomarkers of Alzheimer’s disease. Alzheimers Dement.
4:38–48. 2008.
|
|
3
|
Harrison PJ: The hippocampus in
schizophrenia: a review of the neuropathological evidence and its
pathophysiological implications. Psychopharmacology (Berl).
174:151–162. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Michaelis EK: The Clinical Neurobiology Of
The Hippocampus: An Integrative View. Bartsch T: Oxford University
Press; Oxford; pp. 59–70. 2012
|
|
5
|
Burmester T, Ebner B, Weich B and Hankeln
T: Cytoglobin: a novel globin type ubiquitously expressed in
vertebrate tissues. Mol Biol Evol. 19:416–421. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Trent JT III and Hargrove MS: A
ubiquitously expressed human hexacoordinate hemoglobin. J Biol
Chem. 277:19538–19545. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fago A, Hundahl C, Malte H and Weber RE:
Functional properties of neuroglobin and cytoglobin. Insights into
the ancestral physiological roles of globins. IUBMB Life.
56:689–696. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schmidt M, Gerlach F, Avivi A, et al:
Cytoglobin is a respiratory protein in connective tissue and
neurons, which is up-regulated by hypoxia. J Biol Chem.
279:8063–8069. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hundahl CA, Allen GC, Hannibal J, et al:
Anatomical characterization of cytoglobin and neuroglobin mRNA and
protein expression in the mouse brain. Brain Res. 1331:58–73. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hundahl CA, Hannibal J, Fahrenkrug J,
Dewilde S and Hay-Schmidt A: Neuroglobin expression in the rat
suprachiasmatic nucleus: colocalization, innervation, and response
to light. J Comp Neurol. 518:1556–1569. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mammen PP, Shelton JM, Ye Q, et al:
Cytoglobin is a stress-responsive hemoprotein expressed in the
developing and adult brain. J Histochem Cytochem. 54:1349–1361.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hundahl CA, Kelsen J and Hay-Schmidt A:
Neuroglobin and cytoglobin expression in the human brain. Brain
Struct Funct. 218:603–609. 2013. View Article : Google Scholar
|
|
13
|
Hundahl CA, Elfving B, Müller HK,
Hay-Schmidt A and Wegener G: A gene-environment study of cytoglobin
in the human and rat hippocampus. PLoS One. 8:e632882013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fordel E, Thijs L, Martinet W, Schrijvers
D, Moens L and Dewilde S: Anoxia or oxygen and glucose deprivation
in SH-SY5Y cells: a step closer to the unraveling of neuroglobin
and cytoglobin functions. Gene. 398:114–122. 2007. View Article : Google Scholar
|
|
15
|
Fordel E, Thijs L, Moens L and Dewilde S:
Neuroglobin and cytoglobin expression in mice. Evidence for a
correlation with reactive oxygen species scavenging. FEBS J.
274:1312–1317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li D, Chen XQ, Li WJ, Yang YH, Wang JZ and
Yu AC: Cytoglobin up-regulated by hydrogen peroxide plays a
protective role in oxidative stress. Neurochem Res. 32:1375–1380.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hodges NJ, Innocent N, Dhanda S and Graham
M: Cellular protection from oxidative DNA damage by over-expression
of the novel globin cytoglobin in vitro. Mutagenesis. 23:293–298.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gardner AM, Cook MR and Gardner PR:
Nitric-oxide dioxygenase function of human cytoglobin with cellular
reductants and in rat hepatocytes. J Biol Chem. 285:23850–23857.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Halligan KE, Jourd’heuil FL and
Jourd’heuil D: Cytoglobin is expressed in the vasculature and
regulates cell respiration and proliferation via nitric oxide
dioxygenation. J Biol Chem. 284:8539–8547. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singh S, Canseco DC, Manda SM, et al:
Cytoglobin modulates myogenic progenitor cell viability and muscle
regeneration. Proc Natl Acad Sci USA. 111:E129–E138. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tian SF, Yang HH, Xiao DP, et al:
Mechanisms of neuroprotection from hypoxia-ischemia (HI) brain
injury by up-regulation of cytoglobin (CYGB) in a neonatal rat
model. J Biol Chem. 288:15988–16003. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Raida Z, Reimets R, Hay-Schmidt A and
Hundahl CA: Effect of permanent middle cerebral artery occlusion on
Cytoglobin expression in the mouse brain. Biochem Biophys Res
Commun. 424:274–278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hundahl CA, Fahrenkrug J, Hay-Schmidt A,
Georg B, Faltoft B and Hannibal J: Circadian behaviour in
neuroglobin deficient mice. PLoS One. 7:e344622012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hao MM, Bornstein JC and Young HM:
Development of myenteric cholinergic neurons in ChAT-Cre;R26R-YFP
mice. J Comp Neurol. 521:3358–3370. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yan H and Keast JR: Neurturin regulates
postnatal differentiation of parasympathetic pelvic ganglion
neurons, initial axonal projections, and maintenance of terminal
fields in male urogenital organs. J Comp Neurol. 507:1169–1183.
2008. View Article : Google Scholar
|
|
26
|
Cox DJ and Racca C: Differential dendritic
targeting of AMPA receptor subunit mRNAs in adult rat hippocampal
principal neurons and interneurons. J Comp Neurol. 521:1954–2007.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Spiegel AM, Koh MT, Vogt NM, Rapp PR and
Gallagher M: Hilar interneuron vulnerability distinguishes aged
rats with memory impairment. J Comp Neurol. 521:3508–3523. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fahrenkrug J, Buhl T and Hannibal J:
PreproPACAP-derived peptides occur in VIP-producing tumours and
co-exist with VIP. Regul Pept. 58:89–98. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schwaller B, Dick J, Dhoot G, et al:
Prolonged contraction-relaxation cycle of fast-twitch muscles in
parvalbumin knockout mice. Am J Physiol. 276:C395–C403.
1999.PubMed/NCBI
|
|
30
|
Stephenson-Jones M, Ericsson J, Robertson
B and Grillner S: Evolution of the basal ganglia: dual-output
pathways conserved throughout vertebrate phylogeny. J Comp Neurol.
520:2957–2973. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hundahl CA, Kelsen J, Dewilde S and
Hay-Schmidt A: Neuroglobin in the rat brain (II): co-localisation
with neurotransmitters. Neuroendocrinology. 88:183–198. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Burmester T and Hankeln T: Function and
evolution of vertebrate globins. Acta Physiol (Oxf). May
8–2014.(Epub ahead of print).
|
|
33
|
Hundahl CA, Luuk H, Ilmjärv S, et al:
Neuroglobin-deficiency exacerbates Hif1A and c-FOS response, but
does not affect neuronal survival during severe hypoxia in vivo.
PLoS One. 6:e281602011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schipper HM: Heme oxygenase-1: transducer
of pathological brain iron sequestration under oxidative stress.
Ann NY Acad Sci. 1012:84–93. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Verret L, Mann EO, Hang GB, et al:
Inhibitory interneuron deficit links altered network activity and
cognitive dysfunction in Alzheimer model. Cell. 149:708–721. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Takahashi H, Brasnjevic I, Rutten BP, et
al: Hippocampal interneuron loss in an APP/PS1 double mutant mouse
and in Alzheimer’s disease. Brain Struct Funct. 214:145–160.
2010.PubMed/NCBI
|
|
37
|
Brady DR and Mufson EJ:
Parvalbumin-immunoreactive neurons in the hippocampal formation of
Alzheimer’s diseased brain. Neuroscience. 80:1113–1125.
1997.PubMed/NCBI
|
|
38
|
Popović M, Caballero-Bleda M, Kadish I and
Van Groen T: Subfield and layer-specific depletion in
calbindin-D28K, calretinin and parvalbumin immunoreactivity in the
dentate gyrus of amyloid precursor protein/presenilin 1 transgenic
mice. Neuroscience. 155:182–191. 2008.PubMed/NCBI
|
|
39
|
Schipper HM, Cissé S and Stopa EG:
Expression of heme oxygenase-1 in the senescent and
Alzheimer-diseased brain. Ann Neurol. 37:758–768. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schipper HM: Heme oxygenase-1: role in
brain aging and neurodegeneration. Exp Gerontol. 35:821–830. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schipper HM: Glial HO-1 expression, iron
deposition and oxidative stress in neurodegenerative diseases.
Neurotox Res. 1:57–70. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Beal MF: Metabolic disorders and
neurotoxicology. Curr Opin Neurol. 8:467–468. 1995. View Article : Google Scholar
|
|
43
|
Dournaud P, Cervera-Pierot P, Hirsch E, et
al: Somatostatin messenger RNA-containing neurons in Alzheimer’s
disease: an in situ hybridization study in hippocampus,
parahippocampal cortex and frontal cortex. Neuroscience.
61:755–764. 1994.PubMed/NCBI
|
|
44
|
Rossor MN, Emson PC, Mountjoy CQ, Roth M
and Iversen LL: Reduced amounts of immunoreactive somatostatin in
the temporal cortex in senile dementia of Alzheimer type. Neurosci
Lett. 20:373–377. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Delgado M, Varela N and Gonzalez-Rey E:
Vasoactive intestinal peptide protects against beta-amyloid-induced
neurodegeneration by inhibiting microglia activation at multiple
levels. Glia. 56:1091–1103. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Delgado M and Ganea D: Neuroprotective
effect of vasoactive intestinal peptide (VIP) in a mouse model of
Parkinson’s disease by blocking microglial activation. FASEB J.
17:944–946. 2003.PubMed/NCBI
|
|
47
|
Offen D, Sherki Y, Melamed E, Fridkin M,
Brenneman DE and Gozes I: Vasoactive intestinal peptide (VIP)
prevents neurotoxicity in neuronal cultures: relevance to
neuroprotection in Parkinson’s disease. Brain Res. 854:257–262.
2000.PubMed/NCBI
|
|
48
|
Thuy le TT, Morita T, Yoshida K, et al:
Promotion of liver and lung tumorigenesis in DEN-treated
cytoglobin-deficient mice. Am J Pathol. 179:1050–1060.
2011.PubMed/NCBI
|