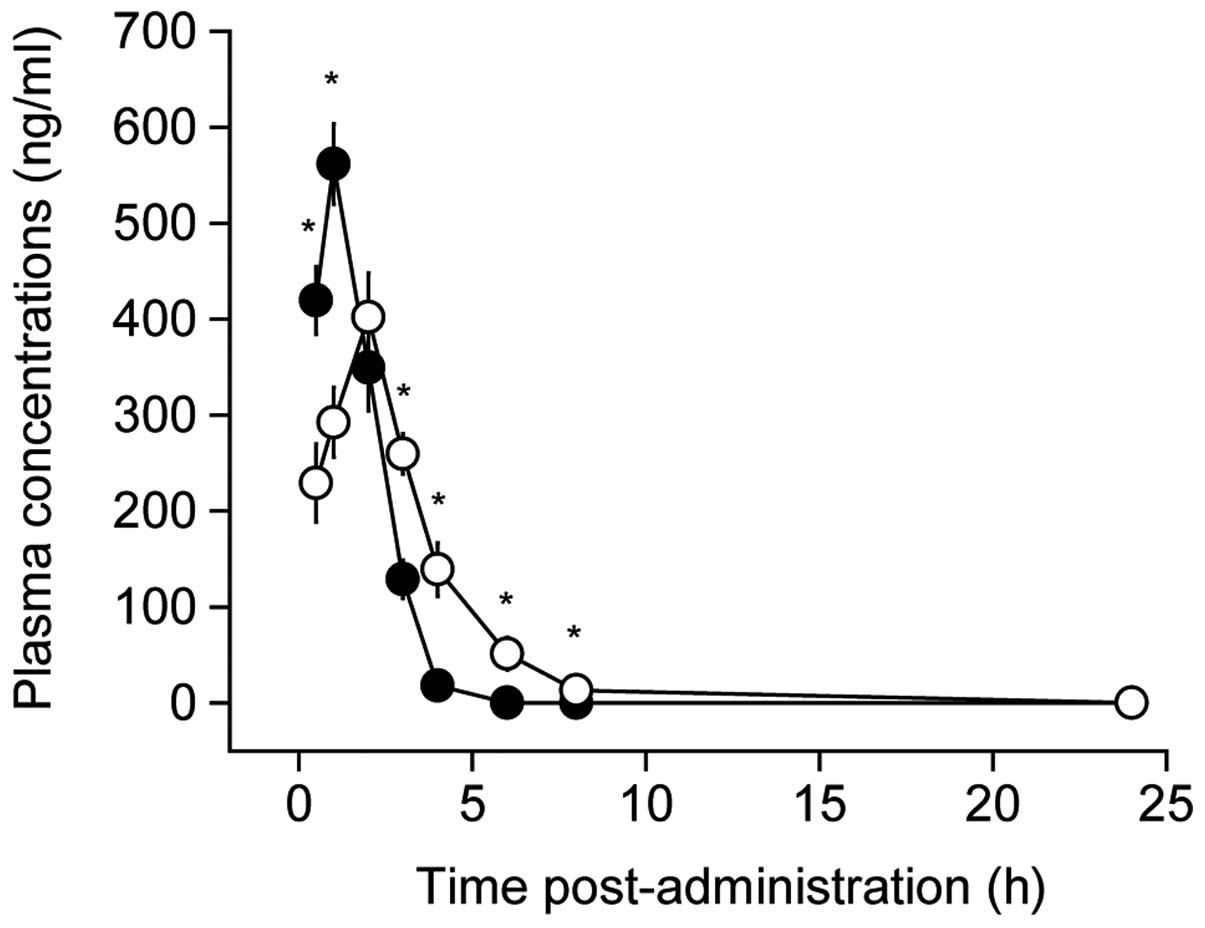
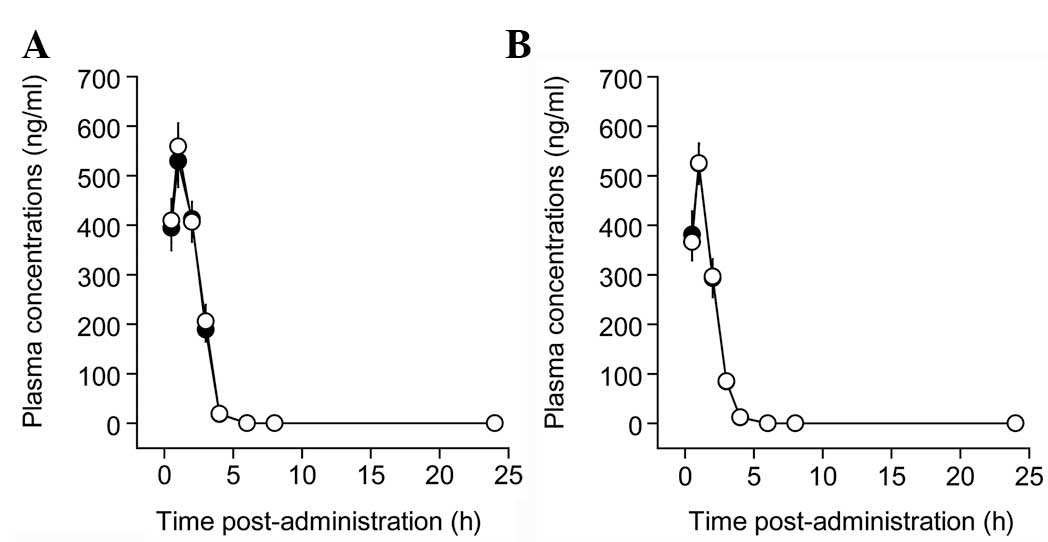
|
1
|
Kearney PM, Whelton M, Reynolds K, Whelton
PK and He J: Worldwide prevalence of hypertension: a systematic
review. J Hypertens. 22:11–19. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nesbitt SD: Antihypertensive combination
therapy: optimizing blood pressure control and cardiovascular risk
reduction. J Clin Hypertens (Greenwich). 9(Suppl 4): 26–32. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Salahuddin A, Mushtaq M and Materson BJ:
Combination therapy for hypertension 2013: an update. J Am Soc
Hypertens. 7:401–407. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakao N, Yoshimura A, Morita H, Takada M,
Kayano T and Ideura T: Combination treatment of angiotensin-II
receptor blocker and angiotensin-converting-enzyme inhibitor in
non-diabetic renal disease (COOPERATE): a randomised controlled
trial. Lancet. 361:117–124. 2003. View Article : Google Scholar
|
|
5
|
Todd PA and Fitton A: Perindopril. A
review of its pharmacological properties and therapeutic use in
cardiovascular disorders. Drugs. 42:90–114. 1991.PubMed/NCBI
|
|
6
|
Cleland JG, Tendera M, Adamus J, et al:
The perindopril in elderly people with chronic heart failure
(PEP-CHF) study. Eur Heart J. 27:2338–2345. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Waeber B, de la Sierra A and Ruilope LM:
The ADVANCE trial: clarifying the role of perindopril/indapamide
fixed-dose combination in the reduction of cardiovascular and renal
events in patients with diabetes mellitus. Am J Cardiovasc Drugs.
9:283–291. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Santa Cruz Biotechnology. Material safety
data sheet of perindopril (sc-205799). http://pdf.analysis1.org/perindopril-santa-cruz-biotechnology-w1778uri.
Accessed on December 6, 2010
|
|
9
|
Clark LT: Safety profile of perindopril.
Am J Cardiol. 88:36i–40i. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ji HF, Li XJ and Zhang HY: Natural
products and drug discovery. Can thousands of years of ancient
medical knowledge lead us to new and powerful drug combinations in
the fight against cancer and dementia? EMBO Rep. 10:194–200.
2009.PubMed/NCBI
|
|
11
|
Scheid V, Bensky B, Ellis A and Barolet R:
Chinese Herbal Medicine: Formulas and Strategies. 2nd edition.
Eastland Press; Seattle, WA: pp. 724–728. 2009
|
|
12
|
Liu IM, Tzeng TF, Liou SS and Chang CJ:
The amelioration of streptozotocin diabetes-induced renal damage by
Wu-Ling-San (Hoelen Five Herb Formula), a traditional Chinese
prescription. J Ethnopharmacol. 124:211–218. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He L, Rong X, Jiang JM, Liu PQ and Li Y:
Amelioration of anti-cancer agent adriamycin-induced nephrotic
syndrome in rats by Wulingsan (Gorei-San), a blended traditional
Chinese herbal medicine. Food Chem Toxicol. 46:1452–1460. 2008.
View Article : Google Scholar
|
|
14
|
Ding XQ, Pan Y, Wang X, Ma YX and Kong LD:
Wuling san ameliorates urate under-excretion and renal dysfunction
in hyperuricemic mice. Chin J Nat Med. 11:214–221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu YP: Chinese Materia Medica: Chemistry,
Pharmacology, and Applications. Harwood Academic; Amsterdam: pp.
311–344. 1998
|
|
16
|
DeVane CL: Pharmacokinetics (2nd edition,
revised and expanded), M. Gibaldi and D. Perrier (vol. 15 of Drugs
and the Pharmaceutical sciences), Marcel Dekker, New York, 1982.
Biopharmaceutics and Drug Disposition. 4:201. 1983. View Article : Google Scholar
|
|
17
|
Chiou WL: Critical evaluation of the
potential error in pharmacokinetic studies of using the linear
trapezoidal rule method for the calculation of the area under the
plasma level - time curve. J Pharmacokinet Biopharm. 6:539–546.
1978. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brown CL, Backhouse CI, Grippat JC and
Santoni JP: The effect of perindopril and hydrochlorothiazide alone
and in combination on blood pressure and on the renin-angiotensin
system in hypertensive subjects. Eur J Clin Pharmacol. 39:327–332.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Scalbert E, Abdon D, Devissaguet M and
Juggi JS: Interaction between an angiotensin converting enzyme
inhibitor, perindopril, and a thiazide diuretic in the
spontaneously hypertensive rat. Can J Cardiol. 8:381–386.
1992.PubMed/NCBI
|
|
20
|
Morin JP, Thomas N, Toutain H, Borghi H
and Fillastre JP: Treatment with an angiotensin converting enzyme
inhibitor may increase the nephrotoxicity of gentamicin in rats.
Pathol Biol (Paris). 37:652–656. 1989.(In French).
|
|
21
|
Vipond AJ, Bakewell S, Telford R and
Nicholls AJ: Lithium toxicity. Anaesthesia. 51:1156–1158. 1996.
View Article : Google Scholar
|
|
22
|
Christensen S, Shalmi M, Hansen AK and
Marcussen N: Effects of perindopril and hydrochlorothiazide on the
long-term progression of lithium-induced chronic renal failure in
rats. Pharmacol Toxicol. 80:132–141. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vandenburg MJ, Stephens JD, Resplandy G,
Dews IM, Robinson J and Desche P: Digoxin pharmacokinetics and
perindopril in heart failure patients. J Clin Pharmacol.
33:146–149. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Johnston D and Duffin D: Drug-patient
interactions and their relevance in the treatment of heart failure.
Am J Cardiol. 70:109C–112C. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Setiawati E, Deniati SH, Yunaidi DA, et
al: Bioequivalence study of two perindopril erbumine tablet
formulations in healthy volunteers. Arzneimittelforschung.
61:234–238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Devissaguet JP, Ammoury N, Devissaguet M
and Perret L: Pharmacokinetics of perindopril and its metabolites
in healthy volunteers. Fundam Clin Pharmacol. 4:175–189. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Georgakakou S, Kazanis M and Panderi I:
Hydrophilic interaction liquid chromatography/positive ion
electrospray ionization mass spectrometry method for the
quantification of perindopril and its main metabolite in human
plasma. Anal Bioanal Chem. 397:2161–2170. 2010. View Article : Google Scholar
|
|
28
|
Lecocq B, Funck-Brentano C, Lecocq V, et
al: Influence of food on the pharmacokinetics of perindopril and
the time course of angiotensin-converting enzyme inhibition in
serum. Clin Pharmacol Ther. 47:397–402. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grislain L, Mocquard MT, Dabe JF, et al:
Interspecies comparison of the metabolic pathways of perindopril, a
new angiotensin-converting enzyme (ACE) inhibitor. Xenobiotica.
20:787–800. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Begg EJ, Robson RA, Bailey RR, Lynn KL,
Frank GJ and Olson SC: The pharmacokinetics and pharmacodynamics of
quinapril and quinaprilat in renal impairment. Br J Clin Pharmacol.
30:213–220. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Resplandy G and Genissel P:
Pharmacokinetics of perindopril in high-risk populations. J
Cardiovasc Pharmacol. 18(Suppl 7): S9–S18. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Parker E, Aarons L, Rowland M and
Resplandy G: The pharmacokinetics of perindoprilat in normal
volunteers and patients: influence of age and disease state. Eur J
Pharm Sci. 26:104–113. 2005. View Article : Google Scholar : PubMed/NCBI
|