Introduction
Renal cell carcinoma (RCC) is the most common
malignant tumor of the kidney and incidence has been increasing
worldwide (1). The pathogenesis and
diagnosis of the prognosis for patients with RCC has remained poor,
regardless of the extensive clinical trials that have been
performed (2–4). There are numerous components that
comprise the tumor environment, each of which allows for an
advantageous environment for survival (5). Oxidative stress has been linked to the
progression of mutations and cancer. In the case of a cancer cell,
a significant imbalance between reactive oxygen species/reactive
nitrogen species production and antioxidant defense can explain all
the findings associated with tumor growth and a state of high
oxidative stress, such as loss of redox homeostasis (6). Hypoxia is a general characteristic of
solid tumors (7). Increased
glutathione (GSH) contents have been observed in a number of
different human cancer tissues, including breast, brain, colon,
pancreas, lungs, head and neck cancer and leukemia (8–14).
High intracellular GSH levels are important contributors to
pathologies, such as cellular transformation, resistance to
radiation and antineoplastic treatments in cancer cells (15).
GSH is synthesized de novo in a two-step
process catalyzed by glutamate cysteine ligase (GCL, EC 6.3.2.2),
previously known as γ-glutamylcysteine synthetase and GSH
synthetase (GS; EC 6.3.2.3). GCL catalyzes the first and
rate-limiting step, in which glutamate is ligated with cysteine to
form γ-glutamylcysteine (γ-GC), which is rapidly linked to glycine
to form GSH via the action of GS (16). GCL is a heterodimeric enzyme
consisting of a catalytic subunit (GCLc) and a modulatory subunit
(GCLm), which are encoded by two genes (17). GCLc performs all the enzymatic
activity and its catalytic efficiency is increased by the covalent
interaction with GCLm (18,19). GSH can be depleted through the
specific downregulation of the GCL levels by hammerhead ribozyme.
Ribozymes have been shown to deplete GSH levels by inhibiting GCLc
and GCLm, and enhancing anticancer drug sensitivity (20). The aim of the present study was to
evaluate the change of GCL expression and activity in RCC
patients.
Materials and methods
Patients and controls
A total of 46 patients with clear cell RCC
fulfilling the RCC criteria of World Health Organization, revised
in 2004 (21), were enrolled for
the study. The tumor tissue and adjacent normal tissue were sampled
from all the patients through surgery. Staging of 46 RCC patients,
including 12 of stage I, 10 of stage II, 18 of stage III and six of
stage IV, was assessed by the tumor-node metastasis (TNM) staging
system for kidney cancer revised by American Joint Committee on
Cancer (22). In total, 46 RCC
patients were confirmed through pathology. All the patients signed
an informed consent form prior to the initiation of the study. All
the samples from the patients were analyzed and approved by the
Ethics Committee of Qingdao Municipal Hospital (Qingdao, Shandong,
China).
GCL activity assay
GCL activity was determined by the fluorescence
assay described by Chen et al (23). Tumor tissue and adjacent tissues for
comparison from RCC patients were homogenized in 20 mmol/l
Tris-HCl, 1 mmol/l EDTA, 250 mmol/l sucrose, 20 mmol/l sodium
borate and 2 mmol/l serine [TES/SB buffer (w/v=1/4)] and sonicated
at 100 W for 60 sec. The mixtures were centrifuged at 10,000 × g at
4°C for 10 min and the supernatants were collected. The samples
were centrifuged again at 15,000 × g at 4°C for 20 min and the
supernatants were collected. The protein concentrations were
determined using the bicinchoninic acid (BCA) Protein Assay kit
(Beyotime Institute of Biotechnology, Shanghai, China), with bovine
serum albumin as the standard.
For the GCL activity assay, 30 μl supernatant
aliquots were mixed with 30 μl GCL-reaction cocktail [400 mmol/l
Tris-HCl, 40 mmol/l adenosine triphosphate (ATP), 40 mmol/l
L-glutamic acid, 2 mmol/l EDTA, 20 mmol/l sodium borate, 2 mmol/l
serine and 40 mmol/l MgCl2]. After incubation at 37°C
for 5 min, 30 μl 30 mmol/l cysteine solution (dissolved in TES/SB
buffer) was added and the mixtures were incubated at 37°C for 13
min. The enzymatic reaction in the mixtures was stopped by
precipitation of proteins with 200 mmol/l 5-sulfosalicylic acid
(SSA). After placing the samples on ice for 20 min, the mixtures
were centrifuged at 2,000 × g at 4°C for 10 min. Following
centrifugation, 20 μl of each supernatant that contained the γ-GC
product was added to a 96-well plate designed for fluorescence
detection. For each assay, 20 μl γ-GC standards containing 5 μl GCL
reaction cocktail, 5 μl 200 mmol/l SSA, 5 μl H2O and 5
μl γ-GC standard solution (0, 20, 40, 60, 80, 100, 120 or 140 μM
γ-GC in TES/SB buffer) was added to each well of the same 96-well
plate to generate the standard curve. Subsequently, 180 μl
2,3-naphthalenedicarboxyaldehyde (NDA) was added into each well.
After incubation in the dark at room temperature for 30 min, the
formation of NDA-γ-GC was measured (472 nm excitation/528 nm
emission) using a fluorescent plate reader (GENios Plus; Tecan
Group Ltd., Männedorf, Switzerland). The production of γ-GC in each
sample was calculated with a standard curve. The values are
expressed in millimoles per min per milligram protein.
Western blotting
The protein contents of the GCLc and GCLm subunits
of GCL were determined by western blotting, as described by Chen
et al (23). Briefly, the
tumor and adjacent tissues for comparison from RCC patients were
homogenized, respectively, in DNase buffer containing 20 mmol/l
Tris-HCl (pH 6.8), 1 mmol/l CaCl2, 5 mmol/l
MgCl2 and 150 U/ml DNase I (Takara Bio, Inc., Dalian,
China). The homogenates were placed on ice for 40 min and mixed
with the same volume of urea buffer containing 6 M urea, 2% sodium
dodecyl sulfate (SDS) and 20 mmol/l Tris-HCl (pH 6.8). The mixtures
were centrifuged at 600 × g at 4°C for 15 min and the supernatants
were collected. The protein concentrations in the supernatants were
determined using the BCA Protein Assay kit as aforementioned. The
supernatants containing equal amounts of protein (20 μg for GCLc
and 50 μg for GCLm as determined by the linear responses of
respective antibody) were loaded onto 12% SDS-polyacrylamide gels
and separated by electrophoresis using Mini-Vertical Gel
Electrophoresis Units (Bio-Rad, Hercules, CA, USA). The proteins
that were resolved on the gels were transferred to polyvinylidene
difluoride (PVDF) membranes using a Trans-Blot Semi-Dry Transfer
Cell (Trans-Blot; Bio-Rad) at 10 V for 30 min.
The protein-bound PVDF membranes were incubated
overnight at 4°C with the polyclonal GCLc antibody (1:3,000; Abcam,
Cambridge, UK) or monoclonal GCLm antibody (1:3,000; Abcam). The
blots that were probed with the GCLc or GCLm antibody were
incubated with the second antibody, goat anti-rabbit immunoglobulin
G (1:3,000; Beijing Kangwei Technology Group Co., Ltd., Beijing,
China) conjugated with horseradish peroxidase, at room temperature
for 1 h. The 5-bromo-4-chloro-3′-indolyl-phosphate
p-toluidine-nitroblue tetrazolium chloride (Beijing Kangwei
Technology Group Co., Ltd., Beijing, China) substrate was used for
colorimetric visualization of the immunoreactions on the membranes.
The immunoblots were imaged using a JS-680D automatic gel imaging
analyzer (Shanghai Peiqing Science and Technology Co., Ltd.,
Shanghai, China). The band intensity of all the samples was
normalized using glyceraldehyde 3-phosphate dehydrogenase as the
standard. The intensity of the immunoreactions on the blots was
quantified using Quantity One software (SensiAnsys; Shanghai
Peiqing Science and Technology Co., Ltd., Shanghai, China).
Statistical analysis
Statistical analysis was performed using the SPSS
13.0 software (SPSS, Inc., Chicago, IL, USA). Data are expressed as
the mean ± standard deviation. The difference between the subject
groups was analyzed using student’s t-test independently.
Correlations analysis was performed using Spearman’s rank test.
P<0.05 was considered to indicate a statistically significant
difference. All the figures were generated with the GraphPad Prism
software, version 5.0 (GraphPad Software, Inc., La Jolla, CA,
USA).
Results
Changes in enzymatic activity of GCL in
RCC
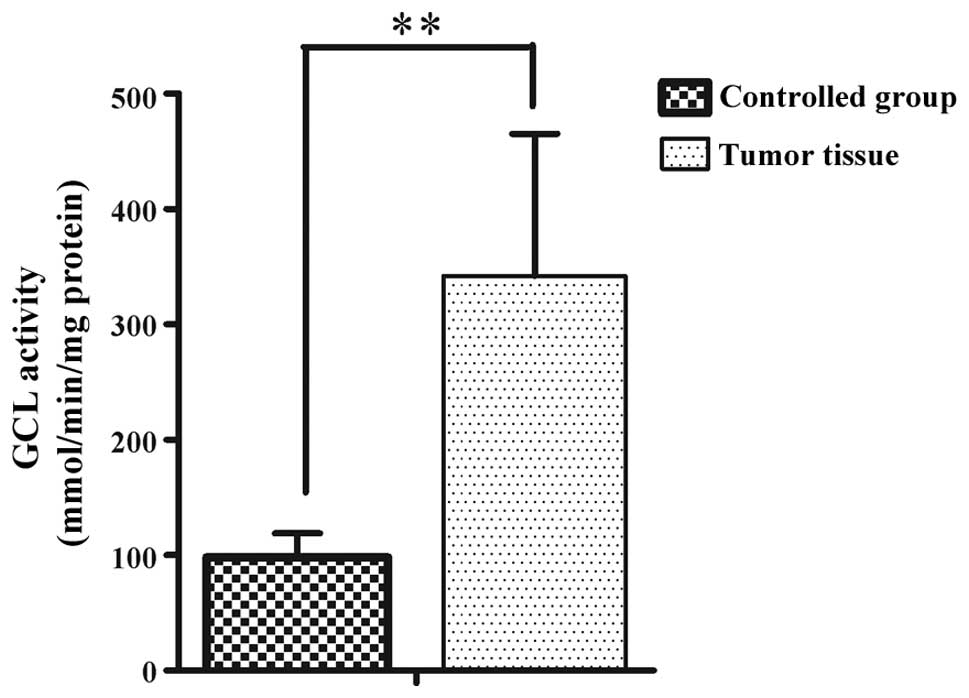
GCL activity from 46 RCC patients was analyzed. The
average GCL activity in the tumor tissue from the RCC patients was
342±123 mmol/min/mg protein, which significantly increased compared
to the adjacent normal tissue (98±21 mmol/min/mg protein)
(P<0.01) (Fig. 1). The
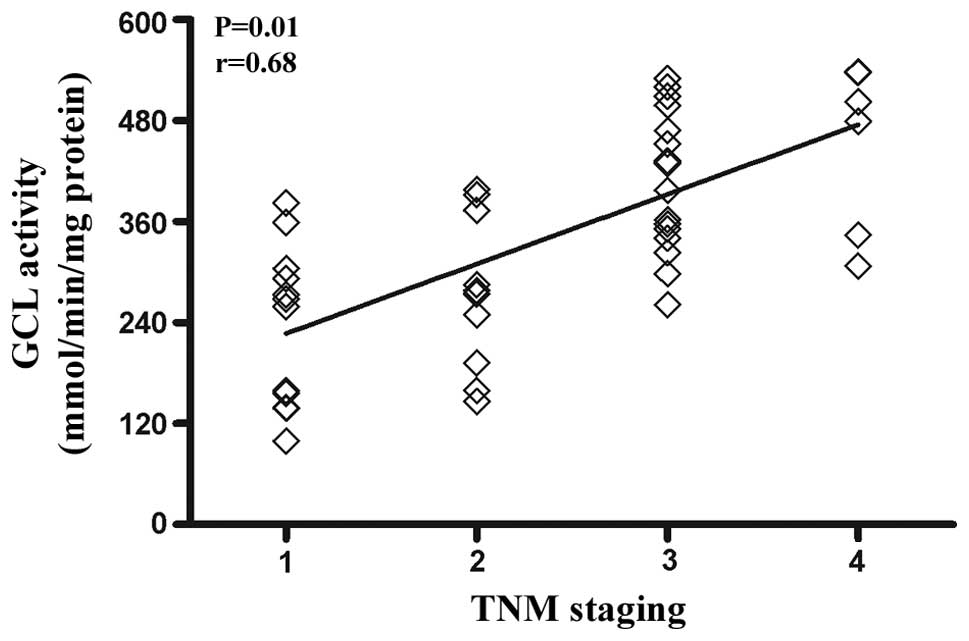
association between the enzymatic activity of GCL and TNM staging
was analyzed. The results demonstrated that the GCL activity levels
positively correlated with TNM staging (r=0.68, P<0.01)
(Fig. 2).
Changes to the protein contents of GCL
subunits in RCC
To understand why the GCL activity in the tumor
tissue of RCC patients changed, the protein content of the two GCL
subunits was determined.
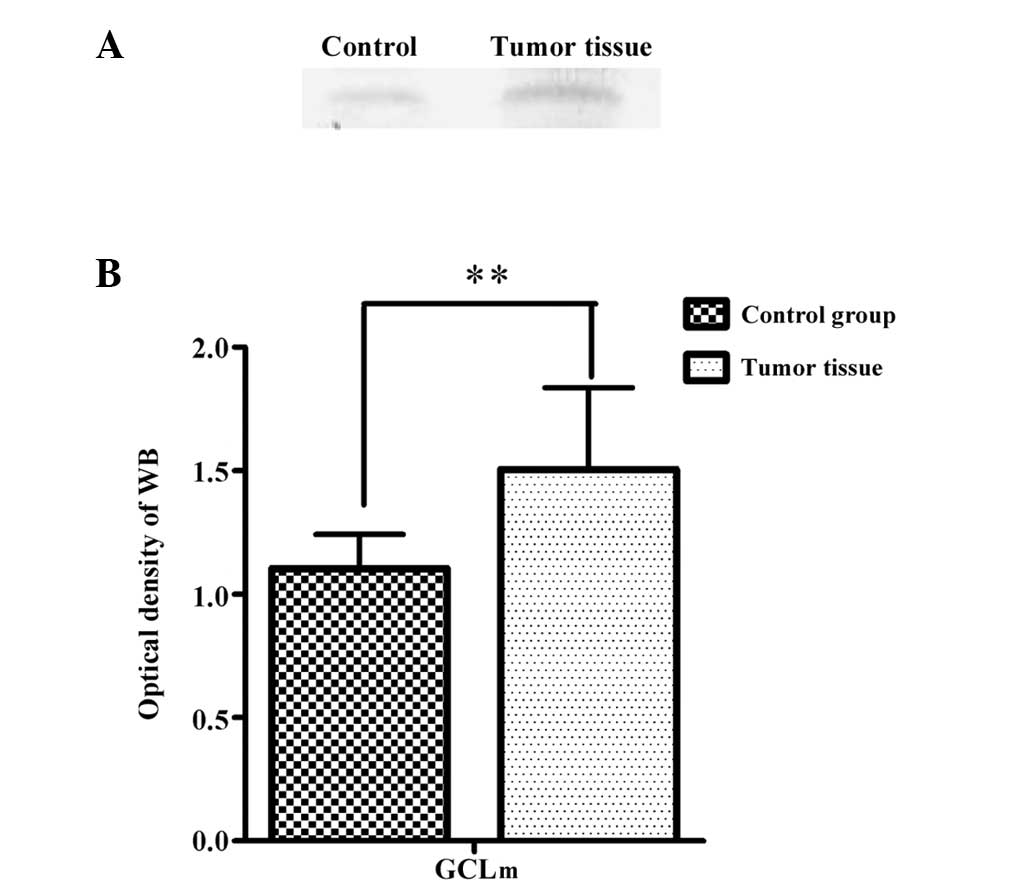
The modifier subunit of GCL lowers the Km
(increases the affinity) for glutamate and ATP, and increases the
concentration of GSH required for GCL inhibition (Ki)
(24). The representative
immunoblots (A) and a summary of the densitometric analysis (B) of
GCLm from the RCC tumor tissue and adjacent normal tissue are shown
in Fig. 3. In the tumor tissue, the
relative GCLm contents were greater (1.506±0.33, P<0.01)
compared to the values observed in the adjacent control tissue
(1.103±0.14).
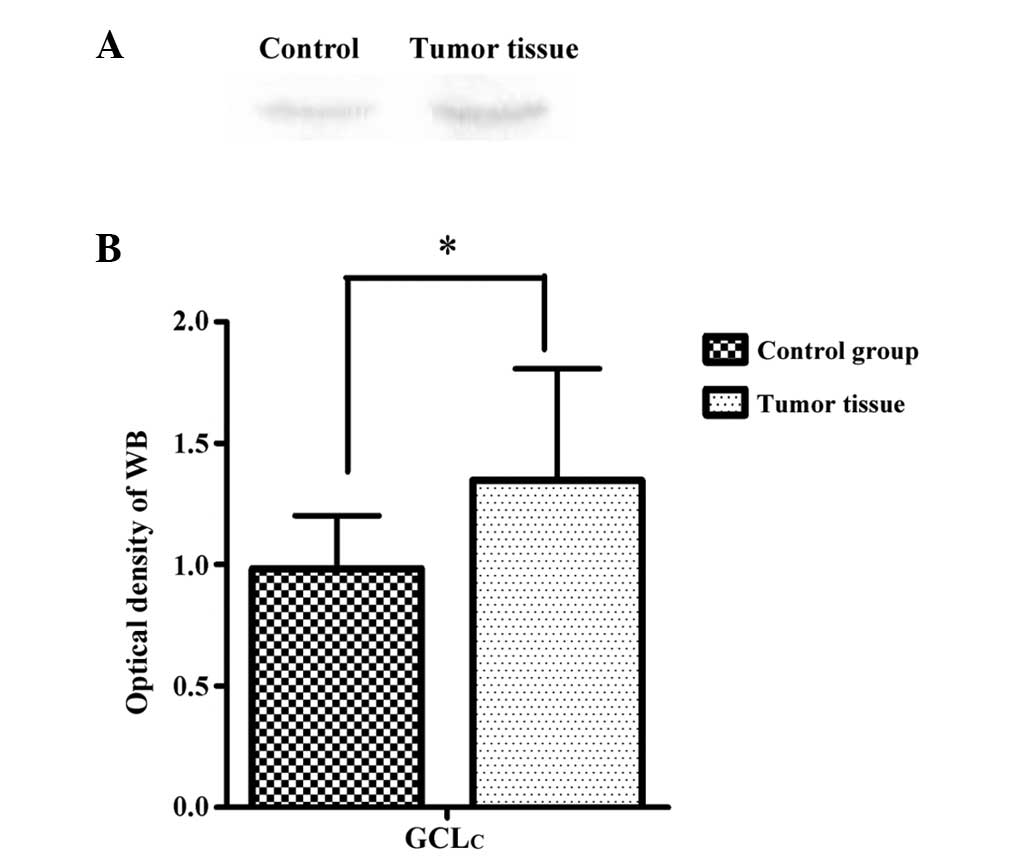
The representative immunoblots (A) and densitometric
analysis (B) of GCLc from the RCC tumor tissue and adjacent normal
tissue are shown in Fig. 4. The
relative content of GCLc in the RCC tumor tissue was significantly
increased (1.348±0.46, P<0.05) compared to the values observed
in the adjacent control tissue (0.983±0.22).
Taken together, all these results showed that the
changes in the protein content of the GCL subunits were found in
the tumor tissue from the RCC patients.
Discussion
GSH is a significant intracellular antioxidant that
generates a number of important roles within a cell, including drug
detoxification, maintenance of the redox state and cellular
protection from damage by free radicals, peroxides and toxins
(15). Consequently, GSH has a
critical role in several human diseases, including cancer and
cardiovascular diseases (25). In
the present study, the change in GCL protein expression and
enzymatic activity was reported through western blotting and an
activity assay in RCC patients, and through the positive
correlation between GCL activity and TNM staging of RCC.
High intracellular GSH levels are significant
contributors to pathologies, such as cellular transformation,
resistance to radiation and antineoplastic treatments in cancer
cells (15). The loss of
intracellular GSH is an early indicator of cell death progression
in response to different apoptotic stimuli and it is believed to be
part of the changes involved in the generation of a permissive
apoptotic environment that is necessary for the activation of
apoptotic enzymes (26). A previous
study has focused on utilizing the oxidative vulnerabilities of
cancer cells and has led to the development of drugs that are
specifically designed to disrupt the cellular redox balance and
enhance the clinical effects of existing chemotherapy agents
(27). The roles of the
intracellular and extracellular redox state in the induction and
maintenance of oxidative stress that is associated with cancer and
metastasis via the activation of survival pathways, disruption of
cell-death signaling and increase in cell proliferation has been
shown previously (28).
In the present study, the protein expression levels
of GCLc and GCLm and the enzymatic activity of GCL were shown to be
significantly increased in the tumor tissue of the RCC patients,
which may be an indicator of oxidative stress in RCC. Taken
together, these results show the cause and consequence of the
severity of the disease. These results suggest that the levels of
GCL activity may be involved in RCC pathogenesis.
GCL catalyzes the first and rate-limiting step, in
which glutamate is ligated with cysteine to form γ-GC, which is
rapidly linked to glycine to form GSH via the action of GS
(16). The present study showed
that there is a good correlation between the GSH levels and GCL,
which is the primary determinant of the rate of GSH synthesis
(29). GSH-dependent detoxifying
reactions protect cells from oxidative damage (30). In the last two decades, there has
been much progress in the understanding of the roles of GSH in
cancer cell biochemistry (31). GSH
constitutes a major antioxidant defense system and together with
the GSH-related enzymes, GSH is critical in protecting cells
against free radical damage and controlling the tumor cell response
to additional cancer therapies, such as irradiation and
chemotherapy (32). Although there
are potential benefits for cancer therapy using a selective
GSH-depletion strategy, such a methodology has remained elusive
thus far (28).
In conclusion, the present study showed that GCL
enzymatic activity was upregulated and positively correlated with
TNM staging in RCC patients. The results indicate that increasing
GCL activity may correlate with the disease severity of RCC. The
data provide support that oxidative stress may be a target for
therapy or pharmacological agents of the RCC disease. The study
indicates that the protein expression levels of GCLc and GCLm are
increased in the renal tumor tissue from RCC patients compared to
the adjacent controls. However, more in vitro and in
vivo mechanistic studies are required to investigate the
relationship between GSH and pathogenesis of RCC.
References
|
1
|
Shah D, Aggarwal A, Bhatnagar A, Kiran R
and Wanchu A: Association between T lymphocyte sub-sets apoptosis
and peripheral blood mononuclear cells oxidative stress in systemic
lupus erythematosus. Free Radic Res. 45:559–567. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lusini L, Tripodi SA, Rossi R, et al:
Altered glutathione anti-oxidant metabolism during tumor
progression in human renal-cell carcinoma. Int J Cancer. 91:55–59.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ames BN, Shigenaga MK and Gold LS: DNA
lesions, inducible DNA repair, and cell division: three key factors
in mutagenesis and carcinogenesis. Environ Health Perspect.
101(Suppl 5): 35–44. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moller P and Wallin H: Adduct formation,
mutagenesis and nucleotide excision repair of DNA damage produced
by reactive oxygen species and lipid peroxidation product. Mutat
Res. 410:271–290. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kongara S and Karantza V: The interplay
between autophagy and ROS in tumorigenesis. Front Oncol. 2:1712012.
View Article : Google Scholar
|
|
6
|
Kryston TB, Georgiev AB, Pissis P and
Georgakilas AG: Role of oxidative stress and DNA damage in human
carcinogenesis. Mutat Res. 711:193–201. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eltzschig HK and Carmeliet P: Hypoxia and
inflammation. N Engl J Med. 364:656–665. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yeh CC, Hou MF, Wu SH, et al: A study of
glutathione status in the blood and tissues of patients with breast
cancer. Cell Biochem Funct. 24:555–559. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Suess E, Malessa S, Ungersböck K, et al:
Technetium-99m-d, l-hexaniethylpropyleneamine oxime (HMPAO) uptake
and glutathione content in brain tumors. J Nucl Med. 32:1675–1681.
1991.PubMed/NCBI
|
|
10
|
Grubben MJ, van den Braak CC, Nagengast FM
and Peters WH: Low colonic glutathione detoxification capacity in
patients at risk for colon cancer. Eur J Clin Invest. 36:188–192.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peters WH, van Schaik A, Peters JH and van
Goor H: Oxidised- and total non-protein bound glutathione and
related thiols in gallbladder bile of patients with various
gastrointestinal disorders. BMC Gastroenterol. 7:72007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gupta A, Srivastava S, Prasad R, et al:
Oxidative stress in non-small cell lung cancer patients after
chemotherapy: association with treatment response. Respirology.
15:349–356. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Almadori G, Bussu F, Galli J, et al:
Salivary glutathione and uric acid levels in patients with head and
neck squamous cell carcinoma. Head Neck. 29:648–654. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kearns PR, Pieters R, Rottier MM, Pearson
AD and Hall AG: Raised blast glutathione levels are associated with
an increased risk of relapse in childhood acute lymphocytic
leukemia. Blood. 97:393–398. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Singh S, Khan AR and Gupta AK: Role of
glutathione in cancer pathophysiology and therapeutic
interventions. J Exp Ther Oncol. 9:303–316. 2012.PubMed/NCBI
|
|
16
|
Ziegler DM: Role of reversible
oxidation-reduction of enzyme thiols-disulfides in metabolic
regulation. Annu Rev Biochem. 54:305–329. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang CS, Chang LS, Anderson ME and
Meister A: Catalytic and regulatory properties of the heavy subunit
of rat kidney gamma-glutamylcysteine synthetase. J Biol Chem.
268:19675–19680. 1993.PubMed/NCBI
|
|
18
|
McConnachie LA, Mohar I, Hudson FN, et al:
Glutamate cysteine ligase modifier subunit deficiency and gender as
determinants of acetaminophen-induced hepatotoxicity in mice.
Toxicol Sci. 99:628–636. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dalton TP, Dieter MZ, Yang Y, Shertzer HG
and Nebert DW: Knockout of the mouse glutamate cysteine ligase
catalytic subunit (Gclc) gene: embryonic lethal when homozygous,
and proposed model for moderate glutathione deficiency when
heterozygous. Biochem Biophys Res Commun. 279:324–329. 2000.
View Article : Google Scholar
|
|
20
|
Iida T, Kijima H, Urata Y, et al:
Hammerhead ribozyme against gamma-glutamylcysteine synthetase
sensitizes human colonic cancer cells to cisplatin by
down-regulating both the glutathione synthesis and the expression
of multidrug resistance proteins. Cancer Gene Ther. 8:803–814.
2001. View Article : Google Scholar
|
|
21
|
Eble JN, Sauter G, Epstein JI and
Sesterhenn IA: Pathology and Genetics of Tumours of the Urinary
System and Male Genital Organs. 1st edition. IARC Press; Lyon: pp.
12–43. 2004
|
|
22
|
Edge S, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th edition.
Springer; New York, NY: pp. 103–115. 2010
|
|
23
|
Chen CN, Brown-Borg HM, Rakoczy SG,
Ferrington DA and Thompson LV: Aging impairs the expression of the
catalytic subunit of glutamate cysteine ligase in soleus muscle
under stress. J Gerontol A Biol Sci Med Sci. 65:129–137. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Franklin CC, Backos DS, Mohar I, White CC,
Forman HJ and Kavanagh TJ: Structure, function, and
post-translational regulation of the catalytic and modifier
subunits of glutamate cysteine ligase. Mol Aspects Med. 30:86–98.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Locigno R and Castronovo V: Reduced
glutathione system: role in cancer development, prevention and
treatment (review). Int J Oncol. 19:221–236. 2001.PubMed/NCBI
|
|
26
|
Kern JC and Kehrer JP: Free radicals and
apoptosis: relationships with glutathione, thioredoxin, and the BCL
family of proteins. Front Biosci. 10:1727–1738. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Montero AJ and Jassem J: Cellular redox
pathways as a therapeutic target in the treatment of cancer. Drugs.
71:1385–1396. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Solinas G, Marchesi F, Garlanda C,
Mantovani A and Allavena P: Inflammation-mediated promotion of
invasion and metastasis. Cancer Metastasis Rev. 29:243–248. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maher P: The effects of stress and aging
on glutathione metabolism. Ageing Res Rev. 4:288–314. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yuan J, Zhang Z, Li L and Song W:
Resveratrol affects the expression of glutamate cysteine ligase in
the kidneys of aged rats. Exp Ther Med. 7:1762–1766.
2014.PubMed/NCBI
|
|
31
|
Estrela JM, Ortega A and Obrador E:
Glutathione in cancer biology and therapy. Crit Rev Clin Lab Sci.
43:143–181. 2006. View Article : Google Scholar
|
|
32
|
Backos DS, Franklin CC and Reigan P: The
role of glutathione in brain tumor drug resistance. Biochem
Pharmacol. 83:1005–1012. 2012. View Article : Google Scholar : PubMed/NCBI
|