Introduction
Necrotizing enterocolitis (NEC) is one of the most
damaging intra-abdominal emergencies in premature infants and is
the cause of significant mortality and morbidity, including
neurodevelopmental impairment, particularly in extreme preterm
neonates requiring surgery for the illness (1–3).
Although the pathogenesis of NEC remains elusive, the principal
initiating events are believed to involve gut ischemia, formula
feeding and intestinal colonization with opportunistic pathogens.
These perinatal insults weaken the integrity of the immature gut
barrier, resulting in bacterial translocation and activation of
innate immune responses (4).
Cyclooxygenase-2 (COX-2) is a rate-limiting enzyme
in the synthesis of prostanoids from their precursor, arachidonic
acid. Inhibitors of COX, such as glucocorticoids and nonsteroidal
anti-inflammatory drugs, have been implicated as an NEC risk factor
(5,6). By contrast, COX-2 is proinflammatory
and therefore, irregularly high COX-2 levels may be pathogenic
during intestinal inflammation (7).
These data indicate that COX-2 may play protective and deleterious
roles in NEC. However, the exact role of COX-2 in the pathogenesis
of NEC has not been fully elucidated. In a previous study of COX-2
in NEC, no correlation was found between COX-2 expression and
intestinal injury severity (8).
Therefore, the aim of the present study was to
investigate the dynamic change and the potential role of
COX-2 mRNA in neonatal rat with lipopolysaccharide
(LPS)-induced intestinal injury, and to define whether NEC is
associated with the expression of COX-2 mRNA in the mucosa
of the affected intestine tissue.
Materials and methods
Animal model
Wistar rats, <24 h in age (mean weight, 6.24±0.81
g), were administered an intraperitoneal (IP) injection of 5 mg/kg
Escherichia coli O55:B5 endotoxin
(LPS; Sigma-Aldrich, St. Louis, MO, USA) or a similar volume of
saline (9–11). All the pups were sacrificed at 1, 3,
6, 12 or 24 h after receiving LPS IP (n=8). The control pups (n=8)
were sacrificed at 1 h after saline IP. The pups that succumbed
prior to the collection of the specimens were excluded from the
study.
Specimens collection
All the surviving animals were sacrificed via
decapitation. The gastrointestinal (GI) tract was carefully
removed. The small intestine was subsequently divided into two
halves: jejunum and ileum. A 3-cm segment of distal ileum, which
was 4 cm proximal to the ileocecal valve, from each animal was cut
and fixed for histological evaluation of NEC. The remainder of the
ileum was snap-frozen at −80°C for mRNA measurement.
Experimental methods and analysis
marker
NEC evaluation
The segment of distal ileum was harvested, fixed in
4% paraformadehyde, embedded in paraffin, microtome-sectioned at 5
μm and counterstained with hematoxylin and eosin for histological
evaluation of intestinal injury. Histological changes in the ileum
were scored by a blinded investigator and were assigned a NEC score
on a scale 0–4 as follows: 0, normal, intact villous epithelium
with normal histology; 1, mild villous edema with epithelial
sloughing confined to the tips of the villi; 2, mild midvillous
necrosis; 3, moderate midvillous necrosis with crypts still readily
detectable; and 4, severe necrosis of entire villi with complete
absence of epithelial structures (11,12).
Reverse transcription-polymerase chain
reaction (RT-PCR) for COX-2 and β-actin
Total RNA was extracted using the Biotragents™
reagent (Sino-American Biotechnology, Co., Luoyang, China) and 2 μl
RNA was used to synthesize cDNA in the presence of an oligo dT
15-primer, RNase inhibitor and the avian myeloblastosis virus
reverse transcriptase in a final volume of 20 μl. Sequence-specific
oligonucleotide primers (Bioasia Biotechnology, Co., Ltd.,
Shanghai, China) were designed according to rat podocin as follows:
COX-2 sense, 5′-TTC AAA TGA GAT TGT GGG AAA ATT GCT-3′; and
antisense, 5′-AGA TCA TCT CTG CCT GAG TAT CTT T-3′ and
β-actin sense, 5′-CAC CCT GTG CTG CTC ACC GAG GCC-3′; and
antisense, 5′-CCA CAC AGA TGA CTT GCG CTC AGG-3′. The expected size
of amplification was 305 base pairs (bp) for COX-2 and 314
bp for β-actin. PCR was performed in a 25-μl reaction
system, which contained 3 μl cDNA, 17.1 μl ddH2O, 2.5 μl
10X PCR buffer, 2 μl 2.5 mmol/l dNTPs, 0.2 μl Taq DNA polymerase
[Takara Biotechnology (Dalian), Co., Ltd., Dalian, China] and 0.1
μl of each primer. Amplification cycles of COX-2 were 95°C
for 1.5 min, followed by 45 cycles at 94°C for 45 sec, 55°C for 45
sec, 72°C for 1.5 min and terminated by a final extension of 72°C
for 10 min.
The PCR products were subjected to electrophoresis
with 2% agarose gel and stained with ethidium bromide. The band
intensity was determined by gel image analysis system (Kodak 1D;
Eastman Kodak, Rochester, NY, USA). The relative mRNA
concentrations were normalized for β-actin. The expression
levels of COX-2 mRNA were calculated by dividing the
intensity of the internal control, β-actin.
Statistical analysis
Software SPSS 19.0 for Windows (SPSS, Inc., Chicago,
IL, USA) was used in all the statistical tests. Comparisons between
the groups were calculated using one-way analysis of variance and
all the data are expressed as mean ± standard deviation. P<0.05,
was considered to indicate a statistically significant difference.
The degree of correlation was described using the Spearman’s
rank-correlation test.
Results
Incidence and severity of NEC
Using the histological scoring system, tissues with
histological scores ≥2+ were designated positive for NEC. In the
LPS-injected group, 52.5% (21/40) showed significant (P<0.01)
pathological changes in ileal structure characterized as moderate
(2+), severe (3+) or full necrosis (4+) compared to only a 0% (0/8)
incidence of NEC in the control group. The most deteriorating
change was at 12 h and the incidence of NEC was 87.5% (7/8). There
was severe necrosis of the entire villi with complete absence of
epithelial structures. The degree of ileal damage was also
significantly (P<0.05) increased in the LPS-injected compared to
the control group (Table I).
 | Table IScores of the lesion on distal ileum
morphology and COX-2 mRNA of neonatal rats. |
Table I
Scores of the lesion on distal ileum
morphology and COX-2 mRNA of neonatal rats.
| Groups | Scores | COX-2
mRNA |
|---|
| Control (n=8) | 0.12±0.17 | 0.14±0.03 |
| LPS (n=8) |
| 1 h | 1.28±0.62a | 0.25±0.04a |
| 3 h | 1.75±0.74a | 0.93±0.01a |
| 6 h | 1.98±0.75a | 1.01±0.07a |
| 12 h | 2.85±0.41a | 1.24±0.01a |
| 24 h | 2.35±0.63a | 1.35±0.08a |
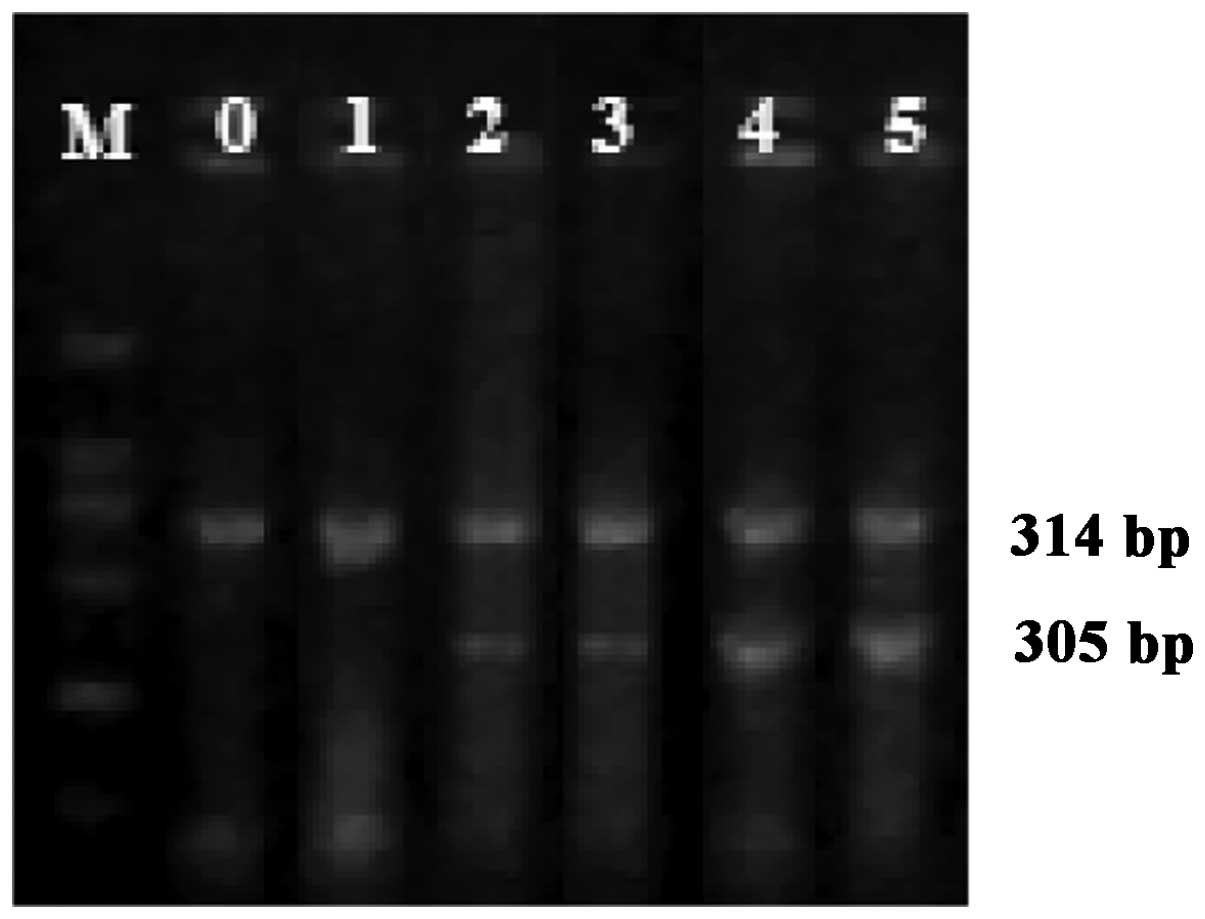
Expression of COX-2 mRNA
The expression of COX-2 mRNA was
significantly upregulated following LPS treatment (P<0.05)
(Table I and Fig. 1). There was a significantly positive
correlation between COX-2 mRNA expression and the grade of
intestinal injury at 1, 3, 6 and 12 h (γ=0.892, 0.855, 0.770 and
0.877; P<0.05). There was a significantly negative correlation
between COX-2 mRNA and severity of NEC only at 24 h
(γ=−0.769, P<0.05).
Discussion
In the present study, significantly lower levels of
COX-2 mRNA were detected in the ileal tissue in a cohort of
control rats. The COX-2 mRNA expression was significantly
increased following LPS injection. There was a significantly
positive correlation between the expression of COX-2 mRNA
and the degree of intestinal injury within 12 h after LPS
injection. These results showed that an increased expression of
ileal COX-2 mRNA results in a significant increase of the
incidence and severity of NEC. The COX-2 mRNA expression was
not significantly decreased at 24 h, and there was a significantly
negative correlation with the severity of intestinal injury.
NEC is predominately a disease of premature infants.
In recent years, its incidence has become more prevalent with the
increasing survival of low-birth weight premature infants (13). The pathogenesis of NEC continues to
be investigated, however, the unifying hypothesis includes mucosal
injury of the small intestine, followed by bacterial translocation
and an amplified inflammatory response to endotoxin (14).
The COX enzymes are critical in the biosynthesis of
prostanoids, play significant roles in the gut and are key for
intestinal epithelium maintenance (15). COX, the enzyme that catalyzes the
first two steps in the biosynthesis of the prostaglandins from
arachidonic acid, exists in two isoforms. COX-1 is constitutively
expressed throughout the GI tract and, at least in the absence of
damage or inflammation, is the major source of prostaglandin
synthesis in these tissues (16).
The inducible form, COX-2, is either undetectable or expressed at
extremely low levels in the healthy GI tract of humans and various
animals (15). However, in response
to various proinflammatory stimuli, COX-2 is rapidly induced. An
increase in COX-2 protein expression was noted in the perforated
intestinal sections of all 36 neonates examined in the study by
Chung et al (13). High
intestinal COX levels have been identified in an animal model of
NEC (7,13).
COX-2 was initially regarded as a target for
anti-inflammatory drugs. Suppression of the activity of this enzyme
reduces edema formation and hyperalgesia. Therefore, COX-2 is also
a major contributor to the processes that result in resolution of
inflammation. A study of paw edema in COX-2-deficient and wild-type
mice identified the significance of COX-2 in the resolution of
inflammation (17).
COX-knockout mice are susceptible to intestinal disorders
(18). The deficiency is correlated
with enhanced intestinal epithelial permeability, which results in
exaggerated bacterial translocation and increased mortality during
peritonitis-induced sepsis (19).
Grosfeld et al (6) reported a cytoprotective role for
prostaglandin E1, showing an increased NEC risk and bowel
perforation in premature infants with patent ductus arteriosus
(PDA) receiving indomethacin (INDO). Mortality was higher in the
PDA/INDO group with NEC compared to the PDA/INDO infants without
NEC. There was a significant association between toll-like
receptor-4 (TLR4) signaling and COX-2 expression in the gut.
TLR4 and MyD88 signaling are required for optimal proliferation and
protection against apoptosis in the injured intestine (17). MyD88 deficiency has been shown to
aggravate intestinal ischemia/reperfusion injury and inhibit
increases in COX-2 expression and prostaglandin E2 synthesis
during the development of injury (20). LPS-induced COX-2 expression
stimulates the proliferation of colonocytes and repair of colonic
epithelium, therefore LPS stimulation of COX-2 was protective in
experimental NEC (7). The
expression of COX isoforms in the duodenum is upregulated by
feeding and inhibition of COX-1 or COX-2 induces ulcers in the
duodenum, indicating that the two isoforms play a critical role in
the protection of the intestinal mucosa (21).
The outcome following the development of selective
COX-2 inhibitors, with a purpose to reduce inflammation whilst
sparing the GI tract from injury, was a series of discoveries that
indicated a crucial role of COX-2 in GI mucosal defense and repair.
There are low levels of COX-2 expression in the
healthy GI tract, however, it also significantly contributes to
mucosal immunity and to the ability of the mucosa to resist injury
induced by luminal irritants. The COX-2 gene quickly
responds to stress and the downstream products of this enzyme are
potent lipid mediators that increase the resistance to injury and
regulate the dynamics of inflammation and resolution (22).
Combined, these data indicate that a quick induction
of COX-2 is a general response to luminal irritation that is
aimed at increasing mucosal resistance to injury and at priming for
the preparation of mucosal repair in the event that injury does
occur (22). Therefore, resolution
of inflammation is a critical process in restoring homeostasis and
COX-2 plays a crucial role in this process.
The present data showed that the expression of
COX-2 mRNA was significantly upregulated following LPS
injection. The aforementioned studies indicate that COX-2 plays key
roles in the ability of the GI mucosa to respond to injury.
COX-2 mRNA expression was significantly upregulated with the
repair of intestinal injury at 24 h, suggesting the induction of
COX-2 activity participates in the exacerbation of the
injury and resolution of inflammation (23).
In conclusion, COX-2 plays a significant role
in neonatal rats with LPS-induced intestinal injury and repair
processes. Caution should be exerted concerning the potential
therapeutic uses of specific inhibitors or promoters of COX-2 at
the optimal phase of inflammation and further information is
required to define the role of the COX/prostaglandin pathway in the
pathogenesis of NEC.
References
|
1
|
Stoll BJ, Hansen NI, Bell EF, et al;
Eunice Kennedy Shriver National Institute of Child Health and Human
Development Neonatal Research Network. Neonatal outcomes of
extremely preterm infants from the NICHD Neonatal Research Network.
Pediatrics. 126:443–456. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin PW and Stoll BJ: Necrotising
enterocolitis. Lancet. 368:1271–1283. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fanaroff AA, Stoll BJ, Wright LL, et al;
NICHD Neonatal Research Network. Trends in neonatal morbidity and
mortality for very low birthweight infants. Am J Obstet Gynecol.
196:147.e1–e8. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Emami CN, Chokshi N, Wang J, et al: Role
of interleukin-10 in the pathogenesis of necrotizing enterocolitis.
Am J Surg. 203:428–435. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guthrie SO, Gordon PV, Thomas V, et al:
Necrotizing enterocolitis among neonates in the United States. J
Perinatol. 23:278–285. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grosfeld JL, Chaet M, Molinari F, et al:
Increased risk of necrotizing enterocolitis in premature infants
with patent ductus arteriosus treated with indomethacin. Ann Surg.
224:350–357. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bergholz R, Zschiegner M, Eschenburg G, et
al: Mucosal loss with increased expression of IL-6, IL-8, and COX-2
in a formula-feeding only neonatal rat model of necrotizing
enterocolitis. J Pediart Surg. 48:2301–2307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Grishin AV, Wang J, Potoka DA, et al:
Lipopolysaccharide induces cyclooxygenase-2 in intestinal
epithelium via a noncanonical p38 MAPK pathway. J Immunol.
176:580–588. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Premer DM, Goertz R, Georgieff MK, et al:
Muscle proteolysis and weight loss in a neonatal rat model of
sepsis syndrome. Inflammation. 26:97–101. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qureshi FG, Leaphart C, Cetin S, et al:
Increased expression and function of integrins in enterocytes by
endotoxin impairs epithelial restitution. Gastroenterology.
128:1012–1022. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu H, Zhu B and Xue XD: Role of neuronal
nitric oxide synthase and inducible nitric oxide synthase in
intestinal injury in neonatal rats. World J Gastroenterol.
12:4364–4368. 2006.PubMed/NCBI
|
|
12
|
Hammerman C, Goldschmidt D, Caplan MS, et
al: Protective effect of bilirubin in ischemia-reperfusion injury
in the rat intestine. J Pediatr Gastroenterol Nutr. 35:344–349.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chung DH, Ethridge RT, Kim S, et al:
Molecular mechanisms contributing to necrotizing enterocolitis. Ann
Surg. 233:835–842. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Petrosyan M, Guner YS, Williams M, et al:
Current concepts regarding the pathogenesis of necrotizing
enterocolitis. Pediatr Surg Int. 25:309–318. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kargman S, Charleson S, Cartwright M, et
al: Characterization of prostaglandin G/H synthase 1 and 2 in rat,
dog, monkey, and human gastrointestinal tracts. Gastroenterology.
111:445–454. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wallace JL, Bak A, McKnight W, et al:
Cyclooxygenase 1 contributes to inflammatory responses in rats and
mice: implications for gastrointestinal toxicity. Gastroenterology.
115:101–109. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fukata M, Chen A, Klepper A, et al: Cox-2
is regulated by Toll-like receptor-4 (TLR4) signaling: role in
proliferation and apoptosis in the intestine. Gastroenterology.
131:862–877. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ishikawa TO, Oshima M and Herschman HR:
Cox-2 deletion in myeloid and endothelial cells, but not in
epithelial cells, exacerbates murine colitis. Carcinogenesis.
32:417–426. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fredenburgh LE, Velandia MM, Ma J, et al:
Cyclooxygenase-2 deficiency leads to intestinal barrier dysfunction
and increased mortality during polymicrobial sepsis. J Immunol.
187:5255–5267. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Watanabe T, Kobata A, Tanigawa T, et al:
Activation of the MyD88 signaling pathway inhibits
ischemia-reperfusion injury in the small intestine. Am J Physiol
Gastrointest Liver Physiol. 303:G324–G334. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Satoh H, Amagase K, Ebara S, et al:
Cyclooxygenase (COX)-1 and COX-2 both play an important role in the
protection of the duodenal mucosa in cats. J Pharmacol Exp Ther.
344:189–195. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wallace JL and Devchand PR: Emerging roles
for cyclooxygenase-2 in gastrointestinal mucosal defense. Br J
Pharmacol. 145:275–282. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wallace JL: COX-2: a pivotal enzyme in
mucosal protection and resolution of inflammation. Scientific World
Journal. 6:577–588. 2006. View Article : Google Scholar : PubMed/NCBI
|