Introduction
In addition to their role in controlling lipid
levels, the pleiotropic effects of statins, including the
anti-arrhythmia effects and prevention of cardiac remodeling, have
attracted significant attention (1,2). A
large number of studies have focused on left ventricular remodeling
following myocardial infarction (MI) and the treatment effect of
statins (3–6). In addition to ventricular arrhythmia,
atrial arrhythmia and in particular, atrial fibrillation (AF),
represent a common occurrence following MI (7) and studies have shown that statins can
exert preventive and therapeutic effects on atrial arrhythmias
(8). With the goal to reveal the
mechanisms underlying the preventive and therapeutic effects of
statins on post-MI atrial arrhythmia, the changes were investigated
in post-MI atrial structural remodeling mediated by rosuvastatin in
the present study.
Materials and methods
Drugs and reagents
The main drugs and reagents used in the study
included rosuvastatin (H20060406; AstraZeneca, London, UK), the
hydroxyproline assay kit and Masson staining kit (Njjcbio, Nanjing,
China), an anti-type I collagen antibody (Abcam, Cambridge, UK) and
the Bradford Protein Assay kit (Blkwbio, Beijing, China).
Animals and grouping
Healthy New Zealand white rabbits of either gender,
weighing 2.2±0.3 kg, were purchased from the Experimental Animal
Center of Shandong University of Traditional Chinese Medicine
(Shandong, China). The animal research protocol complied with ‘The
Guide for the Care of Use of Laboratory Animals’ published by the
National Institutes of Health (publication no. 85-23, revised 1996)
and was approved by the Animal Care Committee of Medical College of
Shandong University. The 30 surviving rabbits were randomly divided
into the sham operation control group (S; n=8), the MI group (MI;
n=7), the low-dose rosuvastatin group [Rs; 2.5
mg/(kg/d), n=7] and the high-dose rosuvastatin group
[Rl; 5 mg/(kg/d), n=8]. The S group received thoracotomy
only. Ligation of the left anterior descending coronary artery was
performed to induce MI. The rabbits in the Rs and
Rl groups received intragastric administration of
rosuvastatin following MI for 8 weeks, while the rabbits in the S
and MI groups did not receive any drug intervention.
Establishment of the rabbit MI
model
The rabbits were anesthetized with 3% sodium
pentobarbital (40 mg/kg) by intraperitoneal injection and were
fixed on a small animal surgery table. Following a routine
preoperative electrocardiogram (ECG), a longitudinal incision was
made along the left sternal border, using the 3rd and 4th
intercostal spaces as a midpoint, to fully expose the heart.
Subsequent to opening the pericardium, the heart was lifted to
expose the anterior descending left coronary artery. At the
midpoint, ligation was conducted using a 6–0 suture. The operators
directly observed that the anterior and apical left ventricular
myocardium gradually turned to a dull and even pale color, which
was accompanied with a weakened pulse. ECG showed a gradual ST
segment elevation in I and aVL, indicating the formation of MI.
Fully intraoperative hemostasis was achieved, followed by a
layer-by-layer sternal closure subsequent to the ligation. For the
S group, a suture was placed under the left anterior descending
coronary artery without ligation. To prevent postoperative wound
infection, all the rabbits were injected with penicillin at 100,000
U/d for 3 consecutive days.
Detection of the left ventricular
ejection fraction (LVEF) and left atrial diameter (LAD)
Cardiac ultrasound was performed 8 weeks
postoperatively using an ultrasound probe (5–12 MHz). The left
ventricular end-systolic diameter, end-diastolic diameter, LVEF and
LAD were measured. Real-time video recorded during the measuring
was documented for future analysis. The final data represented the
average of 3 parallel measurements.
Specimen collection
Subsequent to measuring their weights 8 weeks
postoperatively, the rabbits were anesthetized by intraperitoneal
injection of 3% sodium pentobarbital (40 mg/kg), followed by
thoracotomy. The heart was quickly harvested and rinsed with
saline. The large blood vessels, right atrium, left ventricle and
right ventricle were removed. The left atrium was separated and
divided into two portions; one portion was stored at −80°C for the
measurement of collagen and type I collagen protein levels and
another portion was fixed with 4% paraformaldehyde, embedded in
paraffin and sectioned for hematoxylin and eosin (HE) and Masson
staining.
Measurement of muscle hydroxyproline
and collagen content
The content of myocardial hydroxyproline was
measured to derive the content of myocardial collagen. First, 30–50
mg of left atrial tissue was homogenized and processed according to
the manufacturer's instructions provided with the hydroxyproline
assay kit. Subsequently, the supernatant in each tube was collected
and the optical density (OD) value at 550 nm was measured with a
spectrophotometer. The content of collagen (mg/l) was calculated
according to the following formula: Collagen content = [(OD value
of the sample tube - OD value of the blank tube) / (OD value of the
standard tube - OD value of the blank tube)] x 7.46 x dilution
factor.
Detection of type I collagen
expression in left atrial myocardial tissue by western blot
analysis
The tissue stored at −80°C, as mentioned in
‘Specimen collection,’ was weighed and dissolved in lysate buffer.
The proteins were extracted, quantified, separated by SDS-PAGE
electrophoresis and electrotransferred following denaturation. The
nitrocellulose (NC) membrane bearing the transferred proteins was
incubated with the blocking solution at room temperature for 1 h.
Subsequently, the NC membrane was placed in a hybridization bag,
agitated in the primary antibody solution at room temperature for
30 min and incubated in a 4°C refrigerator overnight. Subsequent to
washing the polyvinylidene fluoride (PVDF) membrane with
Tris-Buffered saline and Tween-20 (TBST) 3 times, with 10 min for
each wash, the secondary antibody was added, followed by an
incubation at room temperature for 60 min. Subsequent to washing
with TBST 3 times for 10 min each, the PVDF membrane was developed
using chromogenic substrate solution in a darkroom and the image
was captured to measure the gray value for data analysis. The GAPDH
level served as the internal control.
Statistical analysis
The SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA)
statistical package was applied for statistical analysis. All the
data are expressed as mean ± standard deviation. Comparisons among
multiple groups were performed using an analysis of variance and
pairwise comparisons were carried out using the least significant
difference method. P<0.05 was considered to indicate a
statistically significant difference.
Results
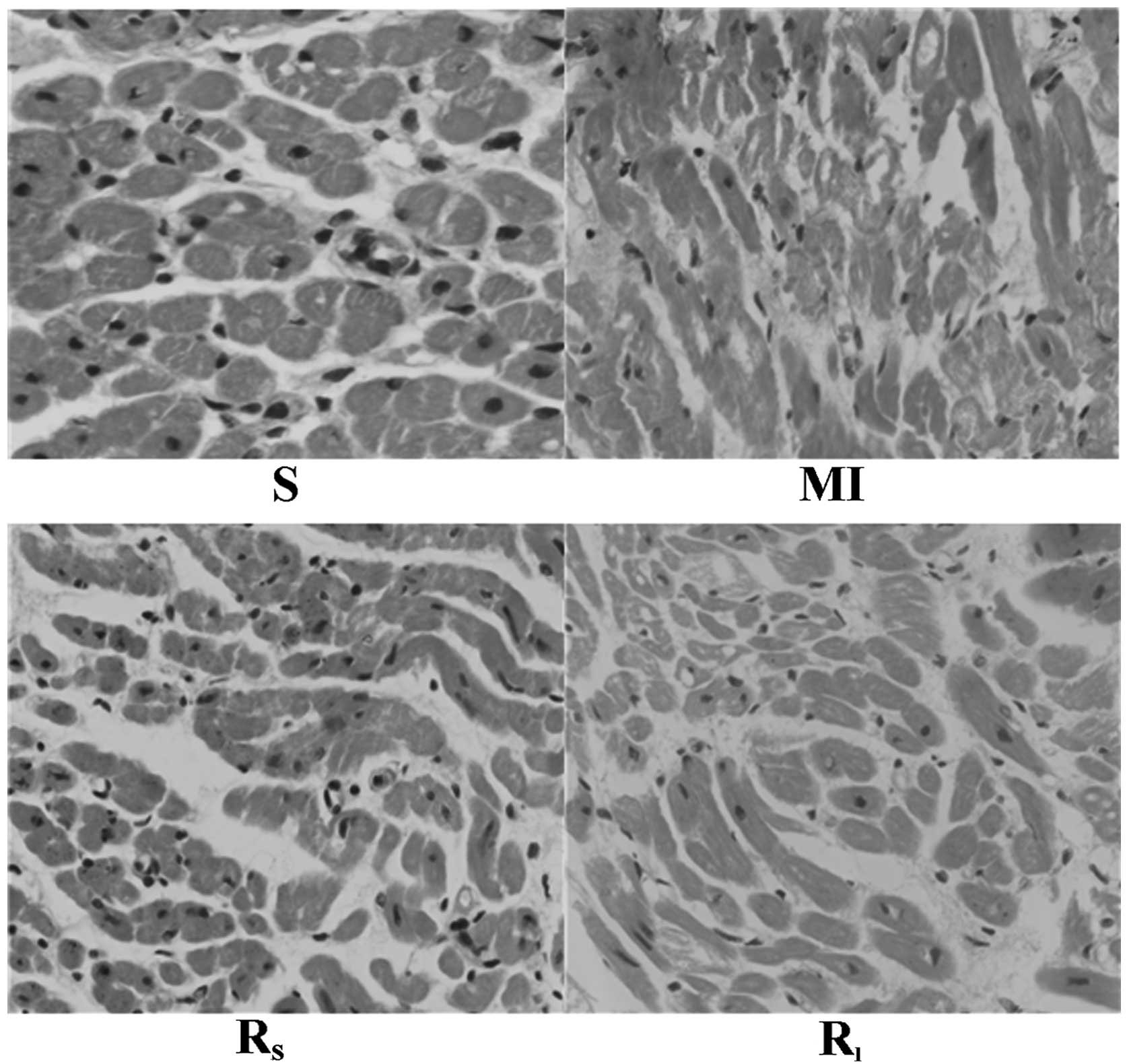
Atrial myocytes histomorphological
changes of the rabbits in each group
HE staining showed that the atrial myocytes in the S
group were arranged regularly and that infiltration of a small
amount of inflammatory cells had occurred, although neither
myocardial necrosis and hypertrophy nor stromal hyperplasia was
observed. In the MI group, the number of atrial myocytes was
decreased, their arrangement was irregular, the nuclei were of
different sizes, with a few dissolved or ruptured, and there was an
accumulation of interstitial collagen. Compared to the MI group,
the Rs and Rl groups exhibited reduced
myocardial necrosis and reduced infiltration of inflammatory cells,
with a significant improvement in compensatory hypertrophy, stromal
hyperplasia and collagen accumulation in myocardial cells, as shown
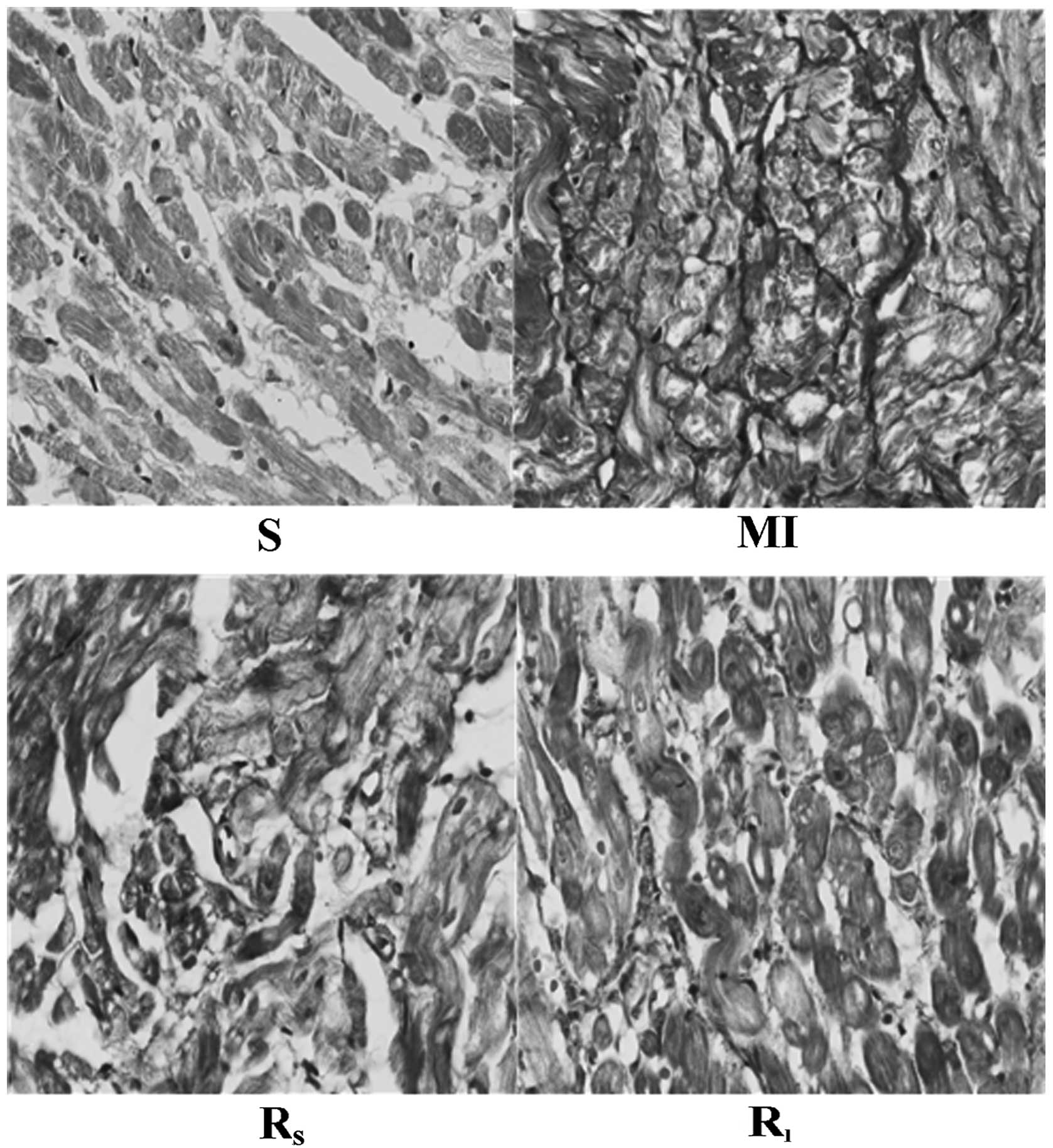
in Fig. 1. Masson staining showed
normal cardiac tissue stained in red and collagen fiber stained in
blue. The expression of collagen fiber in the left atrial tissue of
MI rabbits significantly increased, with fibrosis and interstitial
collagen accumulation in certain cardiomyocytes, as shown in
Fig. 2.
Comparison of LAD, LVEF and
cardiomyocyte collagen content in the rabbits
The LAD and the left atrial collagen content in the
MI group were significantly greater than those observed in the S
group (P<0.01), while the LVEF value in the MI group was
significantly lower than that in the S group (P<0.01). The LAD
and the left atrial collagen content in the Rs and
Rl groups were significantly lower than those in the MI
group (P<0.01), while the LVEF values in these groups were
significantly higher than that in the MI group (P<0.01).
Compared to the Rs group, all the indicators in the
Rl group were significantly lower, although the
differences were not statistically significant, as shown in
Table I.
 | Table IEffect of rosuvastatin on left atrial
diameter (LAD), left ventricular ejection fraction (LVEF) and
collagen content in the rabbits with myocardial infarction
(MI). |
Table I
Effect of rosuvastatin on left atrial
diameter (LAD), left ventricular ejection fraction (LVEF) and
collagen content in the rabbits with myocardial infarction
(MI).
| Indexes | S group | MI group | Rs
group | Rl
group |
|---|
| Quality of the heart,
g | 4.01±0.56 | 8.12±1.11 | 0.71±0.10 | 21.32±3.46 |
| LAD, mm | 6.98±0.62a |
11.22±1.51a | 0.36±0.11a | 29.79±5.50a |
| LVEF, % | 5.01±0.37b |
9.85±1.60b | 0.64±0.10b | 25.15±3.59b |
| Collagen, mg/l | 4.86±0.41b |
9.28±0.99b | 0.62±0.09b | 24.59±3.58b |
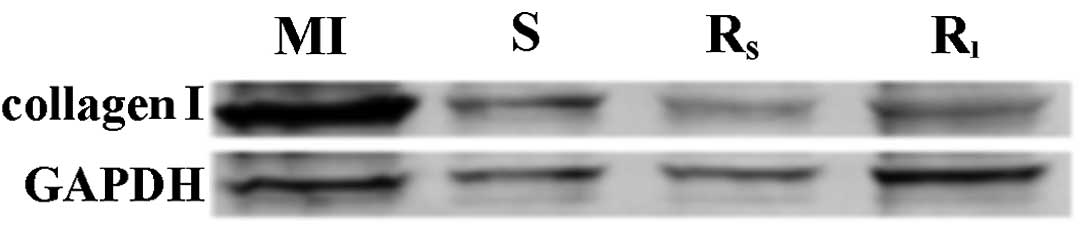
Expression of left atrial type I
collagen
The expression of left atrial type I collagen in the
MI group was significantly higher than that observed in the S group
(P<0.01) and the expression of left atrial type I collagen in
the Rs and Rl groups was significantly lower
compared to the MI group (P<0.05). However, the difference
between the Rs and Rl groups was not
statistically significant (P>0.05), as shown in Fig. 3.
Discussion
Atrial structural remodeling involves changes in
cells and the extracellular matrix and is most prominently
manifested by atrial interstitial fibrosis (6). Interstitial fibrosis mainly occurs in
the extracellular matrix and collagen is the most important
component of the myocardial extracellular matrix, which is
dominated by type I collagen in the heart. The present study
revealed the following results: i) The atrial myocytes of rabbits
in the MI group showed hypertrophy and hyperplasia, irregular
arrangement, karorrhexis and karyolysis, as well as deformation and
necrosis; ii) the MI groups also revealed accumulations of
interstitial collagen and significantly increased biomarkers of
myocardial fibrosis, such as myocardial collagen and the expression
of type I collagen; and iii) significant expansion of the LAD was
observed in the MI group. These results indicated that atrial
structural remodeling occurred following MI and this remodeling may
be associated with the following aspects. First, ventricular
systolic function is severely impaired subsequent to MI, as
supported by the data in the present study showing that the LVEF
value of the MI rabbits was significantly reduced. This impairment
in ventricular systolic function may further lead to ventricular
diastolic dysfunction, thereby causing a high left atrial pressure
and atrial hypertrophy and ultimately inducing atrial remodeling.
Second, the levels of myocardial matrix metalloproteinase (MMP)-3
and −9 and tissue inhibitor of metalloproteinase are upregulated
following MI (9). This increase in
MMP expression is associated with the metabolic dysregulation of
extracellular myocardial interstitial type I collagen and the
degradation of collagen, which can promote atrial fibrosis and lead
to the occurrence of post-MI atrial remodeling. Third, excessive
oxidative stress is an important driving factor of myocardial
remodeling (10) and the oxidative
stress of cardiomyocytes increases following MI, thereby promoting
atrial structural remodeling. Atrial structural remodeling can not
only impair atrial systolic and diastolic function but also provide
a matrix for impulse reentry, leading to AF and other atrial
arrhythmias. In addition, a number of studies have also shown that
the excessively high pressure and sharp dilatation of the left
atrium following MI increases the susceptibility to AF (11,12).
A previous study has also confirmed that statins
have anti-arrhythmia effects and can improve post-MI cardiac
remodeling (13). In this study,
after applying rosuvastatin treatment for 8 weeks, the structure
and morphology of impaired atrial cells were significantly
improved, indicating that rosuvastatin effectively improved atrial
structural remodeling following MI. The possible mechanisms for the
beneficial effects of statins on atrial structural remodeling after
MI include the following: i) Statins can activate the peroxisome
proliferator-activated receptors-α and -γ, extracellular MMP-9 and
tissue protein S, thereby inhibiting cardiac hypertrophy and the
development of fibrosis (14); ii)
statins can reduce the type I collagen level in cardiomyocytes and
fibrotic regions by inhibiting transforming growth factor-β, which
is an important factor promoting myocardial fibrosis, affecting the
production of extracellular matrix by inducing the expression of
type I collagen and fibronectin (15); and iii) the results of the present
study showed that the LVEF value increased after rosuvastatin
treatment in MI rabbits, indicating that rosuvastatin plays a role
in improving impaired left ventricular systolic function. The study
by Tsai et al (16) also
showed that statins inhibited the renin-angiotensin-aldosterone
system to reduce ventricular remodeling and improve impaired left
ventricular systolic function following MI, thereby reducing high
left atrial pressure and left atrial hypertrophy. Taken together,
these results indicate that rosuvastatin can not only directly
improve atrial structural remodeling but also indirectly improve
atrial structural remodeling by reversing ventricular structural
remodeling following MI. In addition, statins can mitigate atrial
remodeling by inhibiting oxidative stress pathways, such as those
mediated by guanylate protein A/guanylate kinase (17). Thus, statin-mediated improvement of
atrial structural remodeling following MI may represent an
important mechanism underlying their preventive and therapeutic
effects on atrial arrhythmia following MI, particularly for AF.
The present study also investigated the effects of
rosuvastatin on atrial remodeling at different doses. Although the
results of corresponding indicators revealed that a high dose of
rosuvastatin could partly improve atrial structural remodeling than
a low dose, the differences were not statistically significant.
This finding may be associated with the short treatment time or the
insufficient dose difference between groups.
Acknowledgements
The authors thank the American Journal Experts (AJE)
for their helpful critical reading of this manuscript and have
received an AJE ‘Editorial Certificate’ for language editing. The
present study was supported by the National Natural Science
Foundation of China (grant no. 81270237) and the Foundation of
Shandong Province (grant no. 2012ZRB14226 and no. ZR2010HL008).
References
|
1
|
Abuissa H, O'Keefe JH and Bybee KA:
Statins as anti-arrhythmics: a systematic review part II: effects
on risk of ventricular arrhythmias. Clin Cardiol. 32:549–552. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ma YX, Li WH and Xie Q: Rosuvastatin
inhibits TGF-beta1 expression and alleviates myocardial fibrosis in
diabetic rats. Pharmazie. 68:355–358. 2013.PubMed/NCBI
|
|
3
|
Paraskevaidis IA, Iliodromitis EK,
Ikonomidis I, et al: The effect of acute administration of statins
on coronary microcirculation during the pre-revascularization
period in patients with myocardial infraction. Atherosclerosis.
223:184–189. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hayashidani S, Tsutsui H, Shiomi T, et al:
Fluvastatin, a 3-hydroxy-3-methylglutaryl coenzyme a reductase
inhibitor, attenuates left ventricular remodeling and failure after
experimental myocardial infarction. Circulation. 105:868–873. 2002.
View Article : Google Scholar
|
|
5
|
Jiang BH, Tardif JC, Sauvageau S, et al:
Beneficial effects of atorvastatin on lung structural remodeling
and function in ischemic heart failure. J Card Fail. 16:679–688.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Allessie M, Ausma J and Schotten U:
Electrical, contractile and structural remodeling during atrial
fibrillation. Cardiovasc Res. 54:230–246. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pinho-Gomes AC, Reilly S, Brandes RP and
Casadei B: Targeting inflammation and oxidative stress in atrial
fibrillation: role of 3-hydroxy-3-methylglutaryl-coenzyme a
reductase inhibition with statins. Antioxid Redox Signal.
20:1268–1285. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kumagai K, Nakashima H and Saku K: The
HMG-CoA reductase inhibitor atorvastatin prevents atrial
fibrillation by inhibiting inflammation in a canine sterile
pericarditis model. Cardiovasc Res. 62:105–111. 2004. View Article : Google Scholar
|
|
9
|
Aronson D, Mutlak D, Bahouth F, et al:
Restrictive left ventricular filling pattern and risk of new-onset
atrial fibrillation after acute myocardial infarction. Am J
Cardiol. 107:1738–1743. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McCarty MF: Practical prevention of
cardiac remodeling and atrial fibrillation with full-spectrum
antioxidant therapy and ancillary strategies. Med Hypotheses.
75:141–147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alasady M, Shipp NJ, Brooks AG, et al:
Myocardial infarction and atrial fibrillation: importance of atrial
ischemia. Circ Arrhythm Electrophysiol. 6:738–745. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bode F, Katchman A, Woosley RL and Franz
MR: Gadolinium decreases stretch-induced vulnerability to atrial
fibrillation. Circulation. 101:2200–2205. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang J, Xu Y, Pan L, Chen T, Chen Z and
Zhao R: Effect of simvastatin on collagen I deposition in
non-infarcted myocardium: role of NF-κB and osteopontin. Can J
Physiol Pharmacol. 88:1026–1034. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qin YM, Ye P, He JQ, Sheng L, Wang LY and
Du J: Simvastatin inhibited cardiac hypertrophy and fibrosis in
apolipoprotein E-deficient mice fed a ‘Western-style diet’ by
increasing PPAR α and γ expression and reducing TC, MMP-9, and Cat
S levels. Acta Pharmacol Sin. 31:1350–1358. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shyu KG, Wang BW, Chen WJ, Kuan P and Hung
CR: Mechanism of the inhibitory effect of atorvastatin on endoglin
expression induced by transforming growth factor-beta1 in cultured
cardiac fibroblasts. Eur J Heart Fail. 12:219–226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsai CT, Lai LP, Kuo KT, et al:
Angiotensin II activates signal transducer and activators of
transcription 3 via Rac1 in atrial myocytes and fibroblasts:
implication for the therapeutic effect of statin in atrial
structural remodeling. Circulation. 117:344–355. 2008. View Article : Google Scholar
|
|
17
|
Briones AM, Rodriguez-Criado N, Hernanz R,
et al: Atorvastatin prevents angiotensin II-induced vascular
remodeling and oxidative stress. Hypertension. 54:142–149. 2009.
View Article : Google Scholar : PubMed/NCBI
|