Introduction
Telomeres are distinctive structures consisting of a
specific DNA sequence (TTAGGG)n and associated binding
proteins that cap the ends of linear chromosomes (1). Telomeres enable cells to distinguish
chromosomal ends from natural double-strand breaks in the genome
(2). Due to the lack of telomerase or
other mechanisms to maintain telomere length, telomeres undergo
erosion following each cell cycle due to the replicating problem of
the linear DNA telomere shortening to a critical length resulting
in loss of telomere protection, which leads to cell cycle arrest
and loss of cell viability (3).
However, the human cancer cells have a relatively stable telomere
length, which indicate its modulating role of telomeres in
biology.
Our previous results indicated that there was a
significant negative correlation of telomere length and
radiosensitivity in the same tissue-derived cell lines, so
telomeres may be used as a predictor of radiosensitivity (4). Additionally, the expression of protection
of telomeres 1 (POT1) was significantly upregulated by
3.348-fold in the radioresisitant cancer cells compared to the
radiosensitive cells through a cDNA microarray containing 14,000
human genes (5). All the above
indicate that there may be a close association between POT1,
telomere length and radiosensitivity in human cancer cells.
POT1, as a 3′ single-stranded overhang telomeric
DNA-binding protein, has been identified in fission yeast and
humans (6). A recent study indicated
that each POT1 binds to one telomeric repeat and coats the entire
single-stranded overhang of the telomere. In subsequent genetic and
biochemical studies, the role of POT1 for telomere length
maintenance and telomere capping has been identified. In addition
to the full-length POT1 protein (also termed variant v1), at least
four other isoforms (termed v2, v3, v4 and v5) are generated from
the human POT1 gene due to RNA alternative splicing, in
which the POT1 v1 and v5 variants have been widely studied
(7,8).
Regardless of the extensive studies conducted in the
biological realm for POT1, the role of the POT1 level in
radiosensitivity and telomere regulation in human cancer cells
remains unclear. In the present study, the variant expression of
POT1 v1 and v5 was investigated and its association with
telomere length and radiosensitivity was explored in colon and
gastric cancer cells.
Materials and methods
Cell culture
Five colon cancer cell lines (LOVO, colo205, HCT15,
HCT116 and HT29) and five gastric cancer cell lines (AGS, SGC7901,
MKN-45, MKN-28 and SNU-1) were obtained from the China Center for
Type Culture Collection (Wuhan, China). All the cells were cultured
in RPMI-1640 medium (Invitrogen Life Technologies, Carlsbad, CA,
USA) supplemented with 10% fetal calf serum (GE Healthcare Life
Sciences HyClone Laboratories, Logan, UT, USA) at 37°C in a
humidified atmosphere containing 5% CO2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cultured cells using the
TRIzol® reagent (Invitrogen Life Technologies) and first-strand cDNA
was synthesized using RevertAid first strand cDNA synthesis kit
(MBI Fermentas, Vilnius, Lithuania) according to the manufacturer's
instructions. To quantify full length POT1 mRNA levels,
RT-qPCR was performed using 2 µl cDNA with SYBR-Green I (Takara
Bio, Inc., Shiga, Japan) in a total volume of 50 µl with the primer
5′-CAGGAGCTG ACGTGGAAGAT-3′ (forward) and 5′-ATGTATTGTTCC
TTGTATAAGAAATGGTGC-3′ (reverse). After enzyme activation for 10 min
at 95°C, 40 three-step cycles were performed (30 sec at 94°C, 30
sec at 60°C and 20 sec at 72°C). RT-qPCR for POT1 v5 variant
was performed as described above with primers
5′-CATCGGCTACAAAATCTG-3′ and 5′-ACCAT TTTCTCTTGGTCTCAG-3.′ β-actin
expression was measured in all the samples as an endogenous control
with primers. Threshold cycles (Ct) of β-actin were used to
calculate the Ct values, which were corrected for input cDNA. The
average ΔCt value was used to calculate the ΔΔCt values. Relative
mRNA expression was calculated with the formula: 2 EXP (-ΔΔCt) ×
100% and all the mRNA levels were indicated using the formula
(target gene mRNA of sample/β-actin of sample) ×100. All the
samples were measured in triplicate in two separate
experiments.
Measurement of telomere length
PCR reactions were performed using a method by
Cawthon (9) by aliquoting 15 µl of
master mix into each reaction well of a 96-well plate compatible
with the M×3000P qPCR system (Agilent Technologies, Santa Clara,
CA, USA) containing ∼20 ng DNA diluted in pure water, for a final
volume of 25 µl/reaction. Five concentrations of a reference DNA
sample (the ‘Standard DNA’) were prepared by serial dilution and
analyzed in duplicate in every 96-well plate, and these reactions
provided the data for the generation of the standard curves used
for relative quantitation. All the experimental DNA samples were
assayed in triplicate. The final concentrations of reagents in the
PCR reaction with SYBR-Green I (Takara Bio, Inc.) were 10 mmol/l
Tris-HCl (pH 8.3), 50 mmol/l KCl 3 mmol/l MgCl2, 0.2
mmol/l each deoxynucleotide, 1 mmol/l dithiothreitol and 1 M
betaine. Each 25 µl reaction received 0.625 U AmpliTaq Gold DNA
polymerase (Applied Biosystems, Inc., Foster City, CA, USA). For
multiplex RT-qPCR, the telomere primer pair telg and telc (final
concentration of 900 nM each), were combined either with the
albumin primer pair albu and albd (final concentration of 900 nM
each), or with the β-globin primer pair hbgu and hbgd (final
concentration of 500 nM each) in the master mix. All the primer
sequences and the rationale for their design are presented in the
results section. The thermal cycling profile was stage 1: 15 min at
95°C; stage 2: 2 cycles of 15 sec at 94°C and 15 sec at 49°C; and
stage 3: 32 cycles of 15 sec at 94°C, 10 sec at 62°C, 15 sec at
74°C with signal acquisition, 10 sec at 84°C and 15 sec at 88°C
with signal acquisition. The 74°C reads provided the Ct values for
the amplification of the telomere template, and the 88°C reads
provided the Ct values for the amplification of the scg template.
Following the completion of thermal cycling and raw data
collection, two standard curves were generated for each plate; one
for the telomere signal and one for the scg signal. The T/S ratio
for an experimental DNA sample is T, which is the ‘Standard DNA’
that matches the experimental sample for copy number of the
telomere template in nanograms, divided by S, which is the
‘Standard DNA’ that matches the experimental sample for copy number
of the scg in nanograms. As each experimental sample was assayed in
triplicate, three T/S results were obtained for each sample;
therefore, the final reported result for a sample in a given run is
the average of the three T/S values. Average T/S is expected to be
proportional to the average telomere length/cell. Samples with a
T/S >1.0 have an average telomere length greater than that of
the ‘Standard DNA’ samples, and those with a T/S <1.0 have an
average telomere length shorter than that of the ‘Standard
DNA’.
Colony-forming assay
Cells were trypsinized at 37°C for 5–10 min and
pipetted eight times to keep the clumped cells as a single cell
suspension. The single cell suspension was adjusted and seeded into
25 cm2 flasks at various densities based on the results
of the pre-experiments. Subsequently, the cells were left to settle
overnight and were exposed to irradiation at room temperature,
followed by immediate incubation at 37°C, 5% CO2 for
14–20 days. Following fixation and staining with 1% w/v crystal
violet (Sigma-Aldrich, St. Louis, MO, USA) in dehydrated alcohol,
colonies of >50 cells were scored. Surviving fractions (SF2)
were evaluated relative to 0 Gy radiation-treated controls.
Statistical analysis
The statistical analyses were performed using SPSS
17.0 software (SPSS, Inc., Chicago, IL, USA) and assessed by the
Mann-Whitney U test and Spearman's rank correlation test for
equality of variances. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression levels of full length POT1
and POT1 v5 mRNA
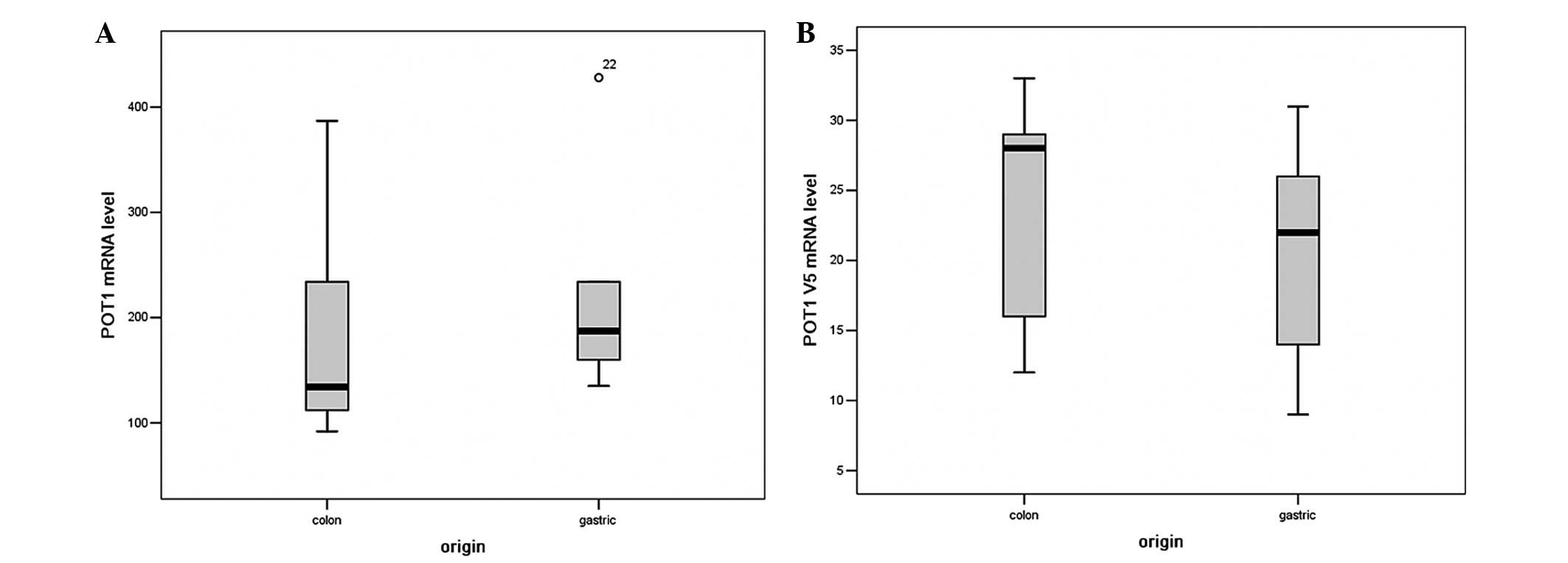
Transcript levels of full length POT1 and
POT1 v5 mRNA were determined by RT-qPCR in all 10 cancerous
cell samples. In all these cell lines, full length POT1 mRNA
levels with a mean value of 198±54 ranged from 118 to 428, and had
a significant difference when compared to each other. The expression
difference of full length POT1 mRNA levels in different
tissue-derived cancer cells is shown in Fig. 1A. In addition, the POT1 v5 mRNA
levels with a mean value of 18±5 was in the range from 9 to 33 in
all cancer cells. The expression of POT1 in different cancer
cell types was relatively stable compared to the high variation of
human telomerase reverse transcriptase. The expression difference
of POT1 v5 mRNA levels in different tissue background cancer
cells is shown in Fig. 1B.
Expression levels of POT1 mRNA and
telomere length
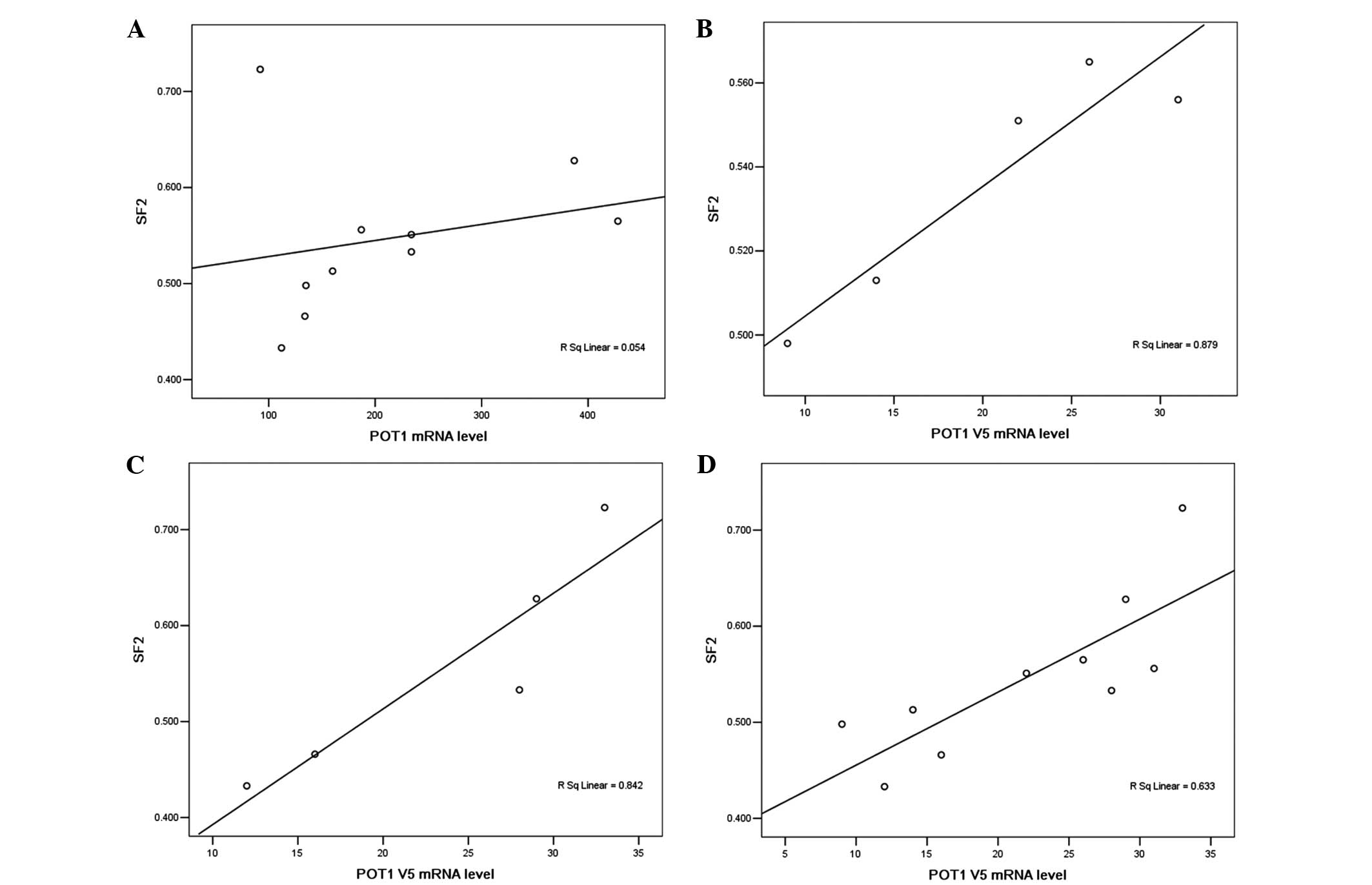
There was an extremely weak correlation between the
full length POT1 level and telomere length
(R2=0.284, P<0.05). In particular, the linear
correlation between them in colon cancer cells and gastric cancer
cells was investigated. The mRNA levels of POT1 are
positively correlated to telomere length in human gastric
adenocarcinoma cell lines (Fig. 2A).
However, a correlation in the colon cancer types was not found. The
mRNA levels of POT1 are positively correlated to telomere
length in human colon cell lines if HT29 is excluded from the group
(P<0.05, Fig. 2B). No significant
associations were observed between telomere length and POT1
v5 mRNA levels.
Expression levels of POT1 mRNA levels
and radiosensitivity
SF2 at 2 Gy was used as an index of clonogenic
cellular radiosensitivity. The present results showed a correlation
between radiosensitivity and POT1 mRNA levels in the 10
carcinoma cell lines in which linear regression analysis was used
to establish a determination coefficient (r2) of 0.054
for the association between POT1 mRNA levels and
radiosensitivity (SF2) (P>0.05). Evidently, different
radiosensitivities of cancer cell lines did not depend on the
levels of full length POT1 mRNA (Fig. 3A). Of note, there was a significant
correlation between the POT1 v5 variant level and
radiosensitivity in gastric cancer (Fig.
3B) and colon cancer cells (Fig.
3C). These results suggest that the POT1 v5 level is a
critical factor in the regulation of radiosensitivity in colon and
gastric adenocarcinoma cell lines. However, the correlation
coefficient of only 0.633 (Fig. 3D)
also suggests the presence of additional factors in the process of
radiosensitivity regulation.
Discussion
Numerous findings suggested that human POT1 protein
may function in telomere length regulation rather than in
POT1 gene regulation, or more specifically the G-overhang
(10–12). Previous studies have described the
effects of perturbing POT1 function on telomere length in murine,
galline and immortal human cells with constitutive telomerase
expression (13–15). Certain data suggest that long telomere
length appears to be a protective factor to the damaging effects of
ionizing radiation (16). This finding
solved a major challenge in cell biology: The limited replicative
life span of non-transformed human cells known as the ‘Hayflick
limit’. These results would verify the well-known association
between telomere and genome stability. Short telomeres are likely
to interfere with the efficient repair of double strand breaks in
the genome, resulting in a higher sensitivity to ionizing radiation
(17). Increasing evidence suggests
that POT1 functions in telomere overhang protection, telomeres' DNA
damage signaling and cell cycle progression (18,19).
Other data indicate that POT1 is essential to
maintain the normal structure at telomeric single-stranded
overhangs, protect against apoptosis and prevent chromosomal
instability and senescence (20–22). In the
two cases, POT1 was found to be essential for the prevention of
activating a catastrophic DNA damage response (23). The radiosensitivity of cancer cells is
known to depend on the DNA damage response and its consequence. In
the present study, POT1 mRNA levels (POT1 v1 and v5
variant) were inversely associated with the radiosensitivity in
gastric cancer cells. Adequate telomere length, telomerase activity
and T-loop formation play important roles in maintenance of
telomere function and when only one mechanistic factor is
compromised, such as lack of functional telomerase or telomere
shortening, the other components of the capping system can
compensate.
Telomere dysfunction appears to increase the
frequency of genetically initiated DNA damage response (24). Telomeres eliciting a DNA damage
response (DDR) were still able to repress end-to-end fusions
through the retention of POT1 at the dysfunctional chromosome end
(25). In cell extracts, POT1 and its
binding partner, tripeptidyl peptidase I, are required to prevent
non-homologous end joining-dependent telemetric DNA fusions,
suggesting that these proteins directly inhibit the ligation
reaction (26). Recent studies have
uncovered an apparent paradox: Several proteins involved in DNA
damage processing and checkpoint responses are recruited to
telomeres in every cell cycle and are required for end protection,
although DNA repair is prevented (27). In this setting, telomere dysfunction
resulting in a DDR with defiant POT1 leads to end-to-end
chromosome fusions and indicate more radiosensitive to ionizing
radiation.
The present study demonstrates that POT1 mRNA
levels modulate telomere length or radiosensitivity in vitro
in colon and gastric adenocarcinoma cancer cells, and these
findings confirm the conclusion that the POT1 v1 or v5
variant mRNA level can act as a biomarker of radiosensitivity of
cancer cells upon ionizing radiation. The reduced POT1
expression levels are assumed to reflect the telomere dysfunction
and may serve as a possible predictor of individual
radiosensitivity and carcinogen. Due to the lack of a rapid and
high-throughput biological measurement to quantify POT1 mRNA
levels, this assumption remains to be demonstrated. More in
vivo studies in human cancer cells are also required to
reinforce this assumption.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 30770643).
References
|
1
|
de Lange T: Shelterin: The protein complex
that shapes and safeguards human telomeres. Genes Dev.
19:2100–2110. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stewart SA, Ben-Porath I, Carey VJ,
O'Connor BF, Hahn WC and Weinberg RA: Erosion of the telomeric
single-strand overhang at replicative senescence. Nat Genet.
33:492–496. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maser RS and DePinho RA: Connecting
chromosomes, crisis, and cancer. Science. 297:565–569. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tang T, Zhou FX, Lei H, Yu HJ, Xie CH,
Zhou YF and Liu SQ: Increased expression of telomere-related
proteins correlates with resistance to radiation in human laryngeal
cancer cell lines. Oncol Rep. 21:1505–1509. 2009.PubMed/NCBI
|
|
5
|
Zhou FX, Xiong J, Luo ZG, Dai J, Yu HJ,
Liao ZK, Lei H, Xie CH and Zhou YF: cDNA expression analysis of a
human radiosensitive-radioresistant cell line model identifies
telomere function as a hallmark of radioresistance. Radiat Res.
174:550–557. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lei M, Podell ER and Cech TR: Structure of
human POT1 bound to telomeric single-stranded DNA provides a model
for chromosome end-protection. Nat Struct Mol Biol. 11:1223–1229.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Colgin LM, Baran K, Baumann P, Cech TR and
Reddel RR: Human POT1 facilitates telomere elongation by
telomerase. Curr Biol. 13:942–946. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu L, Multani AS, He H, Cosme-Blanco W,
Deng Y, Deng JM, Bachilo O, Pathak S, Tahara H, Bailey SM, et al:
Pot1 deficiency initiates DNA damage checkpoint activation and
aberrant homologous recombination at telomeres. Cell. 126:49–62.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cawthon RM: Telomere length measurement by
a novel monochrome multiplex quantitative PCR method. Nucleic Acid
Res. 37:e212009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Opresko PL, Mason PA, Podell ER, Lei M,
Hickson ID, Cech TR and Bohr VA: POT1 stimulates RecQ helicases WRN
and BLM to unwind telomeric DNA substrates. J Biol Chem.
280:32069–32080. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baumann P, Podell E and Cech TR: Human
Pot1 (protection of telomeres) protein: Cytolocalization, gene
structure, and alternative splicing. Mol Cell Biol. 22:8079–8087.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sedelnikova OA, Horikawa I, Zimonjic DB,
Popescu NC, Bonner WM and Barrett JC: Senescing human cells and
ageing mice accumulate DNA lesions with unrepairable double-strand
breaks. Nat Cell Biol. 6:168–170. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Michishita E, Park JY, Burneskis JM,
Barrett JC and Horikawa I: Evolutionarily conserved and
nonconserved cellular localizations and functions of human SIRT
proteins. Mol Biol Cell. 16:4623–4635. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baumann P and Cech TR: Pot1, the putative
telomere end-binding protein in fission yeast and humans. Science.
292:1171–1175. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Churikov D, Wei C and Price CM: Vertebrate
POT1 restricts G-overhang length and prevents activation of a
telomeric DNA damage checkpoint but is dispensable for overhang
protection. Mol Cell Biol. 26:6971–6982. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rangarajan A and Weinberg RA: Opinion:
Comparative biology of mouse versus human cells: modelling human
cancer in mice. Nat Rev Cancer. 3:952–959. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu X, Amos CI, Zhu Y, Zhao H, Grossman BH,
Shay JW, Luo S, Hong WK and Spitz MR: Telomere dysfunction: A
potential cancer predisposition factor. J Natl Cancer Inst.
95:1211–1218. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Widmann TA, Herrmann M, Taha N, Konig J
and Pfreundschuh M: Short telomeres in aggressive non-Hodgkin's
lymphoma as a risk factor in lymphomagenesis. Exp Hematol.
35:939–946. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ohali A, Avigad S, Ash S, Goshen Y, Luria
D, Feinmesser M, Zaizov R and Yaniv I: Telomere length is a
prognostic factor in neuroblastoma. Cancer. 107:1391–1399. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hakin-Smith V, Jellinek DA, Levy D,
Carroll T, Teo M, Timperley WR, McKay MJ, Reddel RR and Royds JA:
Alternative lengthening of telomeres and survival in patients with
glioblastoma multiforme. Lancet. 361:836–838. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Avigad S, Naumov I, Ohali A, Jeison M,
Berco GH, Mardoukh J, Stark B, Ash S, Cohen IJ, Meller I, et al:
Short telomeres: A novel potential predictor of relapse in Ewing
sarcoma. Clin Cancer Res. 13:5777–5783. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shen J, Terry MB, Gurvich I, Liao Y, Senie
RT and Santella RM: Short telomere length and breast cancer risk: A
study in sister sets. Cancer Res. 67:5538–5544. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Halaschek-Wiener J, Vulto I, Fornika D,
Collins J, Connors JM, Le ND, Lansdorp PM and Brooks-Wilson A:
Reduced telomere length variation in healthy oldest old. Mech
Ageing Dev. 129:638–641. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gertler R, Rosenberg R, Stricker D,
Friederichs J, Hoos A, Werner M, Ulm K, Holzmann B, Nekarda H and
Siewert JR: Telomere length and human telomerase reverse
transcriptase expression as markers for progression and prognosis
of colorectal carcinoma. J Clin Oncol. 22:1807–1814. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hackett JA and Greider CW: Balancing
instability: Dual roles for telomerase and telomere dysfunction in
tumorigenesis. Oncogene. 21:619–626. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Greider CW: Telomere length regulation.
Annu Rev Biochem. 65:337–365. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Doksani Y and de Lange T: The role of
double-strand break repair pathways at functional and dysfunctional
telomeres. Cold Spring Harb Perspect Biol. 6:a0165762014.
View Article : Google Scholar : PubMed/NCBI
|