Introduction
Aging is an inevitable, complex and multifactorial
process that is characterized by the progressive degeneration of
tissues and organ systems in humans (1). Although the aging process is largely
determined by genetics, it is influenced by various environmental
factors, including diet, exercise, smoking, alcohol intake and
exposure to microorganisms, pollutants and ionizing radiation
(1). People of the same age may thus
differ markedly in terms of their physical appearance and
physiological status (1). In addition,
in the majority of developed countries, women typically outlive men
by 7–10 years (1,2). Recent research has also shown that
childhood personality and education, as well as behavioral factors,
contribute to longevity (3).
Physiological changes associated with aging lead to
a decrease in the function of various organ systems (4,5). Given that
aging also increases mortality risk as a function of time, it is
important to understand precisely the anatomic and physiological
changes attributed to the normal aging process. A key medical and
social aim is to extend the healthy life span of humans and to
prevent prolonged immobility or hospitalization of the elderly due
to serious conditions, such as cardiovascular disease, stroke or
fractures.
The present study examined age-related physiological
changes in 13 clinical parameters and their associations with
obesity, hypertension, type 2 diabetes mellitus, dyslipidemia, and
chronic kidney disease (CKD) in community-dwelling Japanese
individuals. The aim of the study was to contribute to the
prevention of these diseases and to promote the achievement of a
healthy long life and successful aging in the elderly.
Subjects and methods
Study population
Study subjects comprised 6,027 community-dwelling
individuals who were recruited to a population-based cohort study
(Inabe Health and Longevity Study) in Inabe City (Mie, Japan). The
Inabe Health and Longevity Study is a longitudinal genetic
epidemiological study of atherosclerotic, cardiovascular and
metabolic diseases (6–9). Detailed methods for the recruitment of
the study subjects and for the collection and storage of medical
examination data and genomic DNA samples, as well as
characteristics of the study subjects (3,352 men, 2,675 women) with
regard to all measurements in a 5-year follow-up, were as described
previously (6).
Examination of age-related changes in
clinical parameters and the prevalence of common complex
diseases
Age-related changes in a total of 13 clinical
parameters, including body mass index (BMI); waist circumference;
systolic, diastolic and mean blood pressure (BP); pulse pressure;
fasting plasma glucose concentration; blood glycosylated hemoglobin
(hemoglobin A1c) content; serum concentrations of
triglycerides, high-density lipoprotein (HDL)-cholesterol and
low-density lipoprotein (LDL)-cholesterol; serum creatinine level;
and estimated glomerular filtration rate (eGFR), were examined.
Age-related changes in the prevalence of obesity, hypertension,
type 2 diabetes mellitus, hypertriglyceridemia,
hypo-HDL-cholesterolemia, hyper-LDL-cholesterolemia and CKD were
also evaluated. Diagnostic criteria for these diseases were as
follows: Subjects with obesity had a BMI of ≥25 kg/m2,
based on the BMI criteria of obesity for Japanese and Asian
populations (10), and control
individuals had a BMI of <25 kg/m2. Subjects with
hypertension either had a systolic BP of ≥140 mmHg or diastolic BP
of ≥90 mmHg (or both) or had been prescribed antihypertensive
medication, and control individuals had a systolic BP of <140
mmHg and diastolic BP of <90 mmHg, as well as no history of
hypertension or of taking antihypertensive medication. Subjects
with type 2 diabetes mellitus either had a fasting plasma glucose
level of ≥126 mg/dl or blood hemoglobin A1c content of
≥6.5% (or both) or had been prescribed antidiabetes medication, and
control individuals had a fasting plasma glucose level of <126
mg/dl and blood hemoglobin A1c of <6.5%, as well as
no history of type 2 diabetes mellitus or of taking antidiabetes
medication. Subjects with hypertriglyceridemia either had a serum
triglyceride concentration of ≥150 mg/dl or had been prescribed
antidyslipidemic medication for hypertriglyceridemia, and control
individuals had a serum triglyceride level of <150 mg/dl, as
well as no history of hypertriglyceridemia or of taking
antidyslipidemic medication. Subjects with hypo-HDL-cholesterolemia
had a serum HDL-cholesterol concentration of <40 mg/dl or had
been prescribed antidyslipidemic medication for
hypo-HDL-cholesterolemia, and control individuals had serum
HDL-cholesterol of ≥40 mg/dl, as well as no history of
hypo-HDL-cholesterolemia or of taking antidyslipidemic medication.
Subjects with hyper-LDL-cholesterolemia had a serum LDL-cholesterol
level of ≥140 mg/dl or had been prescribed antidyslipidemic
medication for hyper-LDL-cholesterolemia, and control individuals
had serum LDL-cholesterol of <140 mg/dl, as well as no history
of hyper-LDL-cholesterolemia or of taking antidyslipidemic
medication. The eGFR was calculated with the use of the simplified
prediction equation derived from that in the Modification of Diet
in Renal Disease Study and proposed by the Japanese Society of
Nephrology: eGFR (ml min−1 1.73 m−2) = 194 ×
[age (years)]−0.287 × [serum creatinine
(mg/dl)]−1.094 (x 0.739 if female) (11). The National Kidney Foundation-Kidney
Disease Outcomes Quality Initiative guidelines recommend a
diagnosis of CKD if eGFR is <60 ml min−1 1.73
m−2 (12). Therefore, the
present study adopted the criterion of an eGFR of <60 ml
min−1 1.73 m−2 for diagnosis of CKD, and
control individuals had an eGFR of ≥60 ml min−1 1.73
m−2 and did not have a history of renal disease.
The study protocol complied with the Declaration of
Helsinki and was approved by the Committees on the Ethics of Human
Research of Mie University Graduate School of Medicine and Inabe
General Hospital. Written informed consent was obtained from all
the subjects.
Statistical analysis
Categorical data according to age were compared
between cases and controls by the χ2 test. Correlations
of quantitative data (clinical parameters) with age were examined
by simple regression analysis with fitting to a straight line or
quadratic curve. Bonferroni's correction was applied to compensate
for multiple comparisons and P<0.0011 (0.05/44) was considered
to indicate a statistically significant difference. Statistical
tests were performed with JMP 5.1 software (SAS Institute Inc.,
Cary, NC, USA).
Results
Age-related changes in BMI and waist
circumference
BMI (Fig. 1A–C) and
waist circumference (Fig. 1D–F) were
significantly (P<0.0011) correlated with age in the longitudinal
data analysis. BMI and waist circumference increased with age up to
~50 years and subsequently declined in men (Fig. 1B and E), whereas the two parameters
increased linearly with age in women (Fig.
1C and F). The R2 values for BMI and waist
circumference were greater in women (0.0216 and 0.0478,
respectively) compared with men (0.0174 and 0.0166, respectively).
The prevalence of obesity was significantly associated with age in
the cross-sectional analysis performed in March 2014 (Fig. 2). The highest prevalence (41.1%) of
obesity was observed in men aged 40–49 years, with the prevalence
in men subsequently decreasing with age (Fig. 2C). In women, the prevalence of obesity
increased gradually with age, reaching a value of 32.2% in those
aged ≥70 years (Fig. 2D).
 | Figure 1.Correlation of body mass index (BMI)
or waist circumference with age. Correlations were examined in
longitudinal data for BMI in (A) all the subjects (27,921
measurements), (B) men (15,548) or (C) women (12,373), as well as
for waist circumference in (D) all subjects (21,358), (E) men
(11,817) or (F) women (9,541). The line in each panel represents a
least-squares plot of the data. (A) P=6.51×10−42,
R2=0.0074, BMI
(kg/m2)=22.8255+0.0074x−0.0016
(x−52.4897)2; (B) P=8.96×10−56,
R2=0.0174, BMI
(kg/m2)=24.7967–0.0165x−0.0022
(x−52.4919)2; (C) P=8.21×10−61,
R2=0.0216, BMI
(kg/m2)=20.0823+0.0406x; (D)
P=4.34×10−60, R2=0.0124, waist
circumference (cm)=76.0696+0.0893x; (E)
P=3.08×10−41, R2=0.0166, waist
circumference (cm)=83.4948+0.0132x−0.0072
(x−52.4919)2; (F) P=1.15×10−103,
R2=0.0478, waist circumference
(cm)=68.4565+0.1776x. x, age (years). |
 | Figure 2.Association of the prevalence of
obesity to age in cross-sectional analysis. The association of (A)
the number or (B–D) percentage of subjects with obesity to age was
examined for (A and B) all subjects (1,805 with obesity, 4,222
controls), as well as for (C) men (1,185 with obesity, 2,167
controls) and for (D) women (620 with obesity, 2,055 controls)
separately. Subjects with obesity and the controls are represented
by closed and open columns, respectively. (A and B)
P=2.87×10−4; (C) P=8.53×10−9; (D)
P=3.63×10−7. |
Age-related changes in BP and pulse
pressure
Systolic, diastolic and mean BP, as well as pulse
pressure, were significantly correlated with age in the
longitudinal data analysis (Fig. 3).
Systolic BP (Fig. 3A), mean BP
(Fig. 3C) and pulse pressure (Fig. 3D) increased linearly with age, whereas
diastolic BP (Fig. 3B) increased with
age up to ~60 years and decreased thereafter. The
R2 values increased according to the rank order
of diastolic BP (0.0445) <mean BP (0.0534) <systolic BP
(0.1081) <pulse pressure (0.1101). The prevalence of
hypertension was significantly associated with age in the
cross-sectional analysis (Fig. 4),
increasing with age to ≤69.9 or 68.5% in men and women,
respectively, aged ≥70 years (Fig. 4C and
D).
 | Figure 3.Correlation of systolic blood
pressure (BP), diastolic BP, mean BP or pulse pressure with age.
Correlations were examined for (A) systolic BP, (B) diastolic BP,
(C) mean BP or (D) pulse pressure in longitudinal data for all the
subjects (27,911 measurements). The line in each panel represents a
least-squares plot of the data. (A) P<1.00×10−64,
R2=0.1081, systolic BP
(mmHg)=97.4221+0.4443x; (B) P=4.75×10−156,
R2=0.0445, diastolic BP
(mmHg)=69.0468+0.1400x−0.0113
(x−52.4897)2; (C) P<1.00×10−64,
R2=0.0534, mean BP (mmHg)=77.2966+0.2433x;
(D) P<1.00×10−64, R2=0.1101, pulse
pressure (mmHg)=30.1858+0.3015x. x, age (years). |
 | Figure 4.Association of the prevalence of
hypertension to age in cross-sectional analysis. The association of
(A) the number or (B-D) percentage of subjects with hypertension to
age was examined for (A and B) all the subjects (2,250 with
hypertension, 3,777 controls), as well as for (C) men (1,408 with
hypertension, 1,944 controls) and for (D) women (842 with
hypertension, 1,833 controls) separately. Subjects with
hypertension and the controls are represented by closed and open
columns, respectively. (A and B) P=1.09×10−218; (C)
P=1.08×10−124; (D) P=9.53×10−99. |
Age-related changes in fasting plasma
glucose concentration and blood hemoglobin A1c
content
The fasting plasma glucose level (Fig. 5A) and blood hemoglobin A1c
content (Fig. 5B) were significantly
correlated with age in the longitudinal data analysis, with the two
parameters gradually increasing with age
(R2=0.0290 and 0.0687, respectively). The
prevalence of type 2 diabetes mellitus was significantly associated
with age in the cross-sectional analysis (Fig. 6), increasing with age in men (Fig. 6C) and women (Fig. 6D). The prevalence of type 2 diabetes in
men was approximately twice that in women for each age group.
Age-related changes in serum
triglyceride concentration
The serum triglyceride concentration was
significantly correlated with age in the longitudinal data analysis
(Fig. 7). It increased with age up to
~50 years and decreased thereafter in men (Fig. 7B), whereas it increased linearly with
age in women (Fig. 7C). The
R2 value for serum triglyceride was greater in
women (0.0576) compared with men (0.0150). The prevalence of
hypertriglyceridemia was significantly associated with age in
cross-sectional analysis (Fig. 8). In
men, it increased with age to a peak of 56.8% at 50–59 years and
decreased thereafter (Fig. 8C),
whereas in women it increased with age to reach a value of 34.9%
for those aged ≥70 years (Fig.
8D).
 | Figure 7.Correlation of serum triglyceride
concentration with age. Correlations were examined for serum
triglyceride concentration in longitudinal data for (A) all the
subjects (28,040 measurements), for (B) men (15,639) and for (C)
women (12,401). The line in each panel represents a least-squares
plot of the data. (A) P=2.12×10−41,
R2=0.0082, serum triglyceride
(mg/dl)=101.5246+0.2975x−0.0379
(x−52.4897)2; (B) P=6.86×10−49,
R2=0.0150, serum triglyceride
(mg/dl)=152.3008–0.2561x−0.0635
(x−52.4919)2; (C) P=6.24×10−162,
R2=0.0576, serum triglyceride
(mg/dl)=34.4820+1.0486x. x, age (years). |
 | Figure 8.Association of the prevalence of
hypertriglyceridemia to age in cross-sectional analysis. The
association of (A) the number or (B-D) percentage of subjects with
hypertriglyceridemia to age was examined in (A and B) all the
subjects (2,058 with hypertriglyceridemia, 3,969 controls), as well
as in (C) men (1,497 with hypertriglyceridemia, 1,855 controls) and
(D) women (561 with hypertriglyceridemia, 2,114 controls)
separately. Subjects with hypertriglyceridemia and the controls are
represented by closed and open columns, respectively. (A and B)
P=1.20×10−23; (C) P=1.52×10−19; (D)
P=5.63×10−31. |
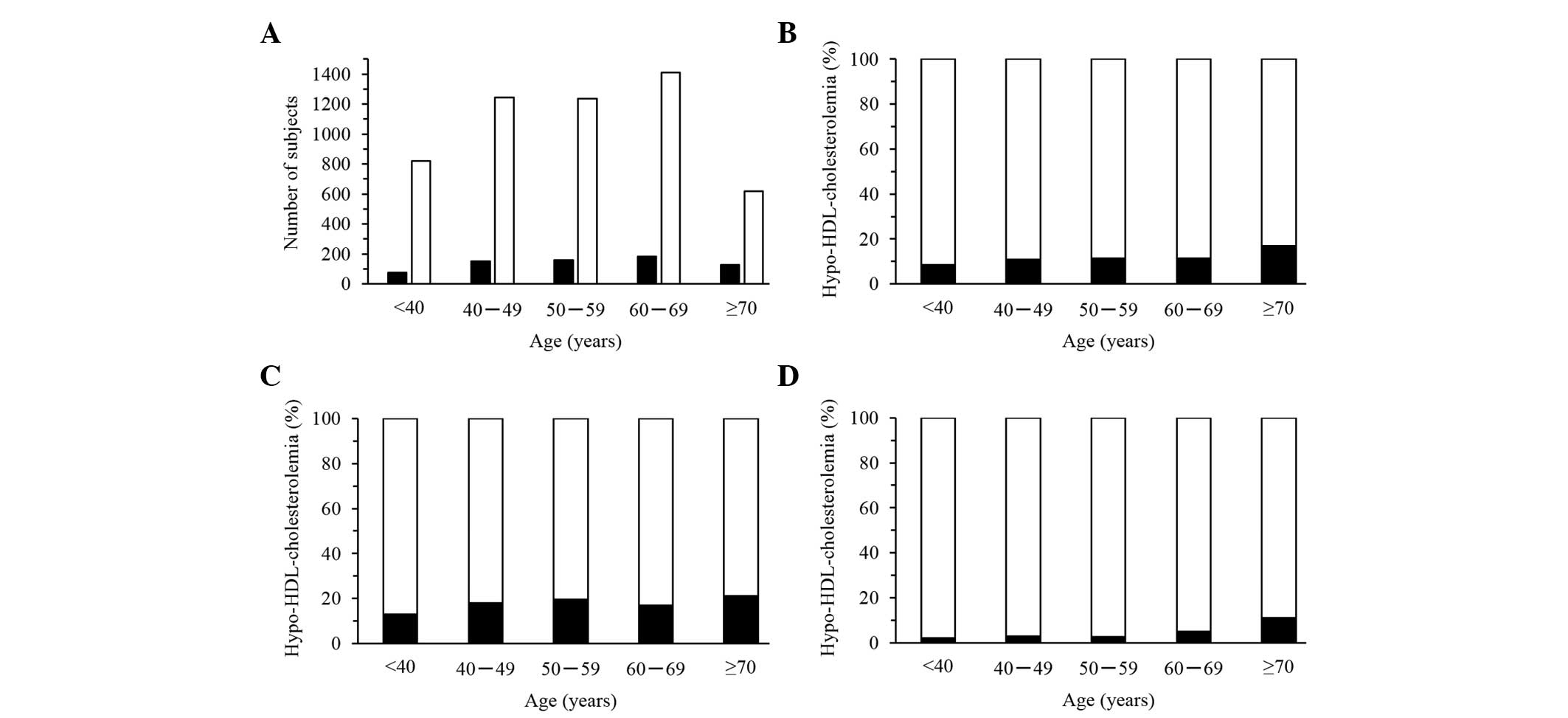
Age-related changes in serum
HDL-cholesterol concentration
The serum concentration of HDL-cholesterol was
significantly correlated with age for women, but not for men, in
longitudinal data analysis (Fig. 9).
Serum HDL-cholesterol increased with age up to ~50 years and
decreased thereafter in women (Fig.
9C). The R2 value for serum HDL-cholesterol
was greater for women (0.0111) compared with men (0.0004). The
prevalence of hypo-HDL-cholesterolemia was significantly associated
with age in women, but not in men, in the cross-sectional analysis
(Fig. 10). In women, the prevalence
of this condition increased gradually with age (Fig. 10D).
 | Figure 9.Correlation of serum high-density
lipoprotein (HDL)-cholesterol concentration with age. Correlations
were examined in longitudinal data for (A) all the subjects (28,005
measurements), for (B) men (15,627) and for (C) women (12,378). The
line in each panel represents a least-squares plot of the data. (A)
P=5.67×10−23, R2=0.0038, serum
HDL-cholesterol (mg/dl)=64.1478–0.0213x−0.0058
(x−52.4897)2; (B) P=0.0136,
R2=0.0004, serum HDL-cholesterol
(mg/dl)=55.5027+0.0241x; (C) P=6.69×10−20,
R2=0.0111, serum HDL-cholesterol
(mg/dl)=74.5306–0.0840x−0.0080
(x−52.4868)2. x, age (years). |
Age-related changes in serum
LDL-cholesterol concentration
The serum concentration of LDL-cholesterol was
significantly correlated with age in the longitudinal data analysis
(Fig. 11). It increased with age up
to ~50 years and decreased thereafter in men (Fig. 11B), whereas it increased linearly with
age in women (Fig. 11C). The
R2 value for serum LDL-cholesterol was greater in
women (0.0591) compared with men (0.0234). The prevalence of
hyper-LDL-cholesterolemia was significantly associated with age in
cross-sectional analysis (Fig. 12).
In men, it increased with age up to a peak of 53.4% at 50–59 years
and decreased thereafter (Fig. 12C),
whereas in women it increased up to a peak of 63.9% at 60–69 years
and subsequently declined (Fig.
12D).
 | Figure 11.Correlation of serum low-density
lipoprotein (LDL)-cholesterol concentration with age. Correlations
were examined in longitudinal data for (A) all the subjects (26,833
measurements), for (B) men (14,997) and for (C) women (11,836). The
line in each panel represents a least-squares plot of the data. (A)
P=1.17×10−147, R2=0.0289, serum
LDL-cholesterol (mg/dl)=117.1722+0.1907x−0.0288
(x−52.4897)2; (B) P=1.64×10−66,
R2=0.0234, serum LDL-cholesterol
(mg/dl)=134.9502–0.1499x−0.0254723
(x−52.4919)2; (C) P=1.12×10−158,
R2=0.0591, serum LDL-cholesterol
(mg/dl)=89.3721+0.6358x. x, age (years). |
 | Figure 12.Association of the prevalence of
hyper-low-density lipoprotein (LDL)-cholesterolemia to age in
cross-sectional analysis. The association of (A) the number or
(B-D) percentage of subjects with hyper-LDL-cholesterolemia to age
was examined in (A and B) all the subjects (2,770 with
hyper-LDL-cholesterolemia, 3,256 controls), as well as in (C) men
(1,539 with hyper-LDL-cholesterolemia, 1,812 controls) and in (D)
women (1,231 with hyper-LDL-cholesterolemia, 1,444 controls)
separately. Subjects with hyper-LDL-cholesterolemia and the
controls are represented by closed and open columns, respectively.
(A and B) P=5.77×10−54; (C) P=1.71×10−11; (D)
P=1.55×10−32. |
Age-related changes in serum
creatinine concentration and eGFR
The serum concentration of creatinine and eGFR were
significantly correlated with age in longitudinal data analysis
(Fig. 13). Serum creatinine (Fig. 13A) and eGFR (Fig. 13B) increased or decreased linearly
with age, respectively (R2=0.0166 and 0.1769,
respectively). The prevalence of CKD was significantly associated
with age in the cross-sectional analysis (Fig. 14), increasing with age to ≤45.1 or
39.6% in men and women, respectively, at ≥70 years (Fig. 14C and D).
Discussion
The present study examined the age-related changes
in 13 clinical parameters and their associations with obesity,
hypertension, type 2 diabetes mellitus, dyslipidemia and CKD in
community-dwelling Japanese individuals. The results indicate that
these clinical parameters and disease prevalence are significantly
associated with age.
Cross-sectional studies with large populations have
shown that body weight and BMI increase gradually during adult
life, ≤50–59 years of age, and subsequently decrease in men and
women (13–15). Given the cross-sectional nature of
these studies, there was a possibility of survival bias due to the
higher mortality rates for obese individuals in middle adulthood
(16). Longitudinal cohort studies
have shown that body weight and BMI increase with age up to ~50
years in men and women, remain unchanged between 50 and 70 years in
men and between 50 and 60 years in women, and subsequently decline
at later ages (17–19). Waist circumference was also found to
increase with age, with the extent of this increase being greater
in women compared with men (20).
Aging is associated with substantial changes in body
composition (21). Fat mass increases
after 20–30 years of age, whereas fat-free mass (reflecting mostly
skeletal muscle) progressively decreases by ≤40% from 20 to 70
years of age (22,23). Whereas fat-free mass is maximal at ~20
years of age and fat mass is maximal at 60–70 years, with the two
parameters declining following these respective ages (22,23). Aging
is also associated with a redistribution of body fat and fat-free
mass. With aging, there is an increase in intra-abdominal fat
relative to subcutaneous or total body fat, as well as a decrease
in peripheral fat-free mass relative to central fat-free mass as a
result of the loss of skeletal muscle (24).
The balance between energy intake and expenditure is
an important determinant of body fat mass (21). Previous studies have suggested that
energy intake either does not change or decreases during aging
(25,26). A decrease in total energy expenditure,
including that attributable to the resting metabolic rate and the
thermic effect of food and physical activity, may therefore be an
important factor in the gradual increase in body fat with age
(27). Physical activity decreases
with age (28) and this decrease has
been estimated to account for about one-half of the decline in
total energy expenditure that occurs with aging (27).
The present results show that BMI, waist
circumference and the prevalence of obesity increased gradually
with age up to ~50 years and decreased thereafter in men, whereas
these parameters increased gradually with age in women. The
age-related change in BMI for men was similar to that observed in
previous cross-sectional and longitudinal studies (13–15,17–19). The
age-related change in waist circumference for women was similar to
previous observations (20), however,
that of the BMI change for women was not consistent with the
results of previous studies (13–15,17–19).
Although the reason for this latter discrepancy is unclear, it may
reflect differences in ethnicity (genetics) or environmental
factors, including dietary habits, physical activity and other
lifestyle aspects.
In the Framingham Heart Study, systolic BP was found
to increase between the ages of 30 and ≥84 years, whereas diastolic
BP increased until the fifth decade and subsequently decreased
slowly from the ages of 60–84. These changes in systolic and
diastolic BP resulted in an increase in pulse pressure with age
(29). An increased pulse pressure due
to elevated systolic BP and decreased diastolic BP in elderly
individuals was shown to be an independent risk factor for
cardiovascular disease (29). An
increased risk of cardiovascular complications associated with an
increased pulse pressure was also demonstrated in a meta-analysis
including the results of several major trials (30). The Prospective Studies Collaboration
examined 61 studies of BP and mortality in 1 million adults with no
previous cardiovascular disease at baseline and identified that BP
was strongly associated with the age-specific mortality of stroke,
coronary artery disease and other vascular diseases (31).
The increase in BP with age is associated with
changes in arterial and arteriolar stiffness. The stiffness of
large arteries is due mostly to arteriosclerotic structural
alterations and calcification, and it leads to earlier reflected
pressure waves from arterioles toward the heart during BP wave
propagation. These pressure waves arrive back during systole,
increasing central systolic BP and widening pulse pressure
(32). Large-artery stiffness and
peripheral vascular resistance contribute to the increase in
systolic BP with age, whereas the increase in diastolic BP at ≤50
years is mostly due to increased peripheral vascular resistance in
small vessels and the subsequent decrease in diastolic BP is
attributable to the increase in large-artery stiffness. Although
peripheral vascular resistance may initiate hypertension, it is the
acceleration of large-artery stiffness that leads to the rise in
systolic BP >50 years of age (32).
The present results show that systolic and mean BP,
pulse pressure and the prevalence of hypertension increased
linearly with age, whereas diastolic BP increased with age up to
~60 years and decreased thereafter. The age-related changes in
systolic and diastolic BP and pulse pressure in the study are thus
consistent with those observed previously (29).
Aging is accompanied by an increase in glucose
intolerance and the prevalence of type 2 diabetes mellitus
(33,34), both of which result from an imbalance
between the body's requirement for insulin (insulin sensitivity)
and its ability to secrete insulin (β-cell function). Glucose
intolerance in the elderly may result from impaired insulin
secretion, increased peripheral insulin resistance or changes in
other hormone systems. Additional factors that affect glucose
tolerance in the elderly include obesity, physical inactivity,
reduced dietary carbohydrate, impaired renal function and
administration of certain drugs (35).
The Baltimore Longitudinal Study of Aging revealed that glucose
tolerance declines prominently >60 years, primarily as a result
of an age-related increase in adiposity and decrease in physical
activity, with the effect of age itself being moderate (36). The finding that impairment of glucose
tolerance develops largely >60 years of age (36) is consistent with the results of several
studies showing that insulin-mediated glucose disposal is also
decreased in such elderly individuals (37–39). Loss of
glucose tolerance with age is thus associated with weight gain and
a sedentary lifestyle, as well as to a loss of insulin secretory
function and insulin sensitivity (40).
The present results show that the fasting plasma
glucose level, blood hemoglobin A1c content and the
prevalence of type 2 diabetes mellitus gradually increased with age
in men and women. Age-related changes in these parameters in the
study are thus consistent with previous observations (36–39).
Longitudinal and cross-sectional analyses of lipid
profiles in a previous study with Japanese individuals showed that
serum triglyceride levels increased until 50 years of age and
subsequently decreased in men, whereas they increased until 70
years of age in women (41,42). An age-related change in serum
HDL-cholesterol level was not observed in men, whereas this
parameter decreased with age in women. In men, the serum
LDL-cholesterol concentration increased until age 60 and remained
unchanged from age 60–80, whereas in women it increased until age
80.
The mechanisms responsible for the development of
dyslipidemia with age remain unclear, however, they may be
associated with changes in the liver sinusoidal endothelium
(pseudocapillarization), an increase in postprandial lipemia,
insulin resistance induced by free fatty acids, growth hormone
deficiency, a decline in androgen levels in men and in estrogen
levels in women and a decrease in peroxisome proliferator-activated
receptor α expression in the liver (43).
The present results show that the serum triglyceride
level increased with age up to ~50 years and decreased thereafter
in men whereas it increased linearly with age in women. The serum
concentration of HDL-cholesterol did not change with age in men,
whereas it increased slightly with age up to ~50 years and
decreased thereafter in women. The serum LDL-cholesterol level
increased with age up to ~50 years and subsequently declined in
men, whereas it increased linearly with age in women. With the
exception of HDL-cholesterol results for women, the age-related
changes in lipid profiles in the study are thus largely consistent
with those observed previously (41,42).
Renal function was previously shown to decrease
gradually >30 years (44). Numerous
individuals thus manifest a progressive decrease in glomerular
filtration rate and renal blood flow with age, although there is
wide interindividual variability. The decline in glomerular
filtration rate is attributable to a reduction in the glomerular
capillary plasma flow rate and the glomerular capillary
ultrafiltration coefficient (44,45). Aging
is also associated with altered responsiveness to vasoactive
stimuli, such that responses to vasoconstrictor stimuli are
enhanced whereas vasodilatory responses are impaired (45). The activities of the renin-angiotensin
and nitric oxide systems also change with age (45). These physiological changes result in
age-related impairment of kidney function and the development of
CKD (45).
The present results show that the serum creatinine
level and the prevalence of CKD increased linearly with age,
whereas eGFR declined. The age-related changes in these parameters
are consistent with previous observations (46,47).
There were limitations to the present study, which
included: i) Substantial percentages of the subjects had undergone
medical treatment for hypertension, type 2 diabetes, dyslipidemia,
or CKD; ii) although the mean follow-up period for the cohort was 5
years, this time varied among individuals (from 1 to 11 years); and
iii) the number of subjects differed among the different age
groups.
In conclusion, the present results indicate that 13
clinical parameters, as well as the prevalence of obesity,
hypertension, type 2 diabetes mellitus, dyslipidemia and CKD, are
significantly associated with age. These age-related changes may
have important practical implications for the clinical management
of elderly individuals. Given that metabolic status is dependent on
age, changes in the responses to commonly administered drugs may
necessitate adjustment of dosage in the elderly. There is also a
requirement for the implementation of a rational diet and exercise
programs in an effort to delay or reverse some of these age-related
physiological changes. These results may prove informative for the
prevention of the common complex diseases examined in the present
study, as well as for more serious conditions such as coronary
artery disease and stroke, and they may therefore contribute to the
achievement of a healthy long life and a successful aging
strategy.
Acknowledgements
The present study was supported by Core Research for
Evolutionary Science and Technology of the Japan Science and
Technology Agency (to Y.Y.), as well as by a Grant-in-Aid for
Scientific Research from the Ministry of Education, Culture,
Sports, Science and Technology of Japan (no. 15H04772 to Y.Y.).
References
|
1
|
Nigam Y, Knight J, Bhattacharya S and
Bayer A: Physiological changes associated with aging and
immobility. J Aging Res. 2012:4684692012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barford A, Dorling D, Davey Smith G and
Shaw M: Life expectancy: Women now on top everywhere. BMJ.
332:8082006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Martin P, Poon LW and Hagberg B:
Behavioral factors of longevity. J Aging Res. 2011:1975902011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rosenthal RA and Kavic SM: Assessment and
management of the geriatric patient. Crit Care Med. 32 (Suppl
4):S92–S105. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dodds C: Physiology of ageing. Anaesth
Intensive Care Med. 7:456–458. 2006. View Article : Google Scholar
|
|
6
|
Yamada Y, Matsui K, Takeuchi I, Oguri M
and Fujimaki T: Association of genetic variants with hypertension
in a longitudinal population-based genetic epidemiological study.
Int J Mol Med. 35:1189–1198. 2015.PubMed/NCBI
|
|
7
|
Yamada Y, Matsui K, Takeuchi I, Oguri M
and Fujimaki T: Association of genetic variants of the alpha-kinase
1 gene with type 2 diabetes mellitus in a longitudinal
population-based genetic epidemiological study. Biomed Rep.
3:347–354. 2015.PubMed/NCBI
|
|
8
|
Yamada Y, Matsui K, Takeuchi I and
Fujimaki T: Association of genetic variants with dyslipidemia and
chronic kidney disease in a longitudinal population-based genetic
epidemiological study. Int J Mol Med. 35:1290–1300. 2015.PubMed/NCBI
|
|
9
|
Yamada Y, Matsui K, Takeuchi I and
Fujimaki T: Association of genetic variants with coronary artery
disease and ischemic stroke in a longitudinal population-based
genetic epidemiological study. Biomed Rep. 3:413–419.
2015.PubMed/NCBI
|
|
10
|
Kanazawa M, Yoshiike N, Osaka T, Numba Y,
Zimmet P and Inoue S: Criteria and classification of obesity in
Japan and Asia-Oceania. Asia Pac J Clin Nutr. 11 (Suppl
8):S732–S737. 2002. View Article : Google Scholar
|
|
11
|
Matsuo S, Imai E, Horio M, Yasuda Y,
Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H and Hishida A:
Collaborators developing the Japanese equation for estimated GFR:
Revised equations for estimated GFR from serum creatinine in Japan.
Am J Kidney Dis. 53:982–992. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
National Kidney Foundation, . K/DOQI
clinical practice guidelines for chronic kidney disease:
Evaluation, classification, and stratification. Am J Kidney Dis. 39
(Suppl 1):S1–S266. 2002.PubMed/NCBI
|
|
13
|
Mokdad AH, Bowman BA, Ford ES, Vinicor F,
Marks JS and Koplan JP: The continuing epidemics of obesity and
diabetes in the United States. JAMA. 286:1195–1200. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Flegal KM, Carroll MD, Ogden CL and
Johnson CL: Prevalence and trends in obesity among US adults,
1999–2000. JAMA. 288:1723–1727. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hedley AA, Ogden CL, Johnson CL, Carroll
MD, Curtin LR and Flegal KM: Prevalence of overweight and obesity
among US children, adolescents, and adults, 1999–2002. JAMA.
291:2847–2850. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Manson JE, Willett WC, Stampfer MJ,
Colditz GA, Hunter DJ, Hankinson SE, Hennekens CH and Speizer FE:
Body weight and mortality among women. N Engl J Med. 333:677–685.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kannel WB, Gordon T and Castelli WP:
Obesity, lipids, and glucose intolerance. The Framingham Study. Am
J Clin Nutr. 32:1238–1245. 1979.PubMed/NCBI
|
|
18
|
Rissanen A, Heliövaara M and Aromaa A:
Overweight and anthropometric changes in adulthood: A prospective
study of 17,000 Finns. Int J Obes. 12:391–401. 1988.PubMed/NCBI
|
|
19
|
Grinker JA, Tucker K, Vokonas PS and Rush
D: Body habitus changes among adult males from the normative aging
study: Relations to aging, smoking history and alcohol intake. Obes
Res. 3:435–446. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Poehlman ET, Toth MJ, Bunyard LB, Gardner
AW, Donaldson KE, Colman E, Fonong T and Ades PA: Physiological
predictors of increasing total and central adiposity in aging men
and women. Arch Intern Med. 155:2443–2448. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Villareal DT, Apovian CM, Kushner RF and
Klein S: American Society for Nutrition; NAASO, The Obesity
Society: Obesity in older adults: Technical review and position
statement of the American Society for Nutrition and NAASO, The
Obesity Society. Am J Clin Nutr. 82:923–934. 2005.PubMed/NCBI
|
|
22
|
Baumgartner RN, Stauber PM, McHugh D,
Koehler KM and Garry PJ: Cross-sectional age differences in body
composition in persons 60+ years of age. J Gerontol A Biol Sci Med
Sci. 50:M307–M316. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gallagher D, Visser M, De Meersman RE,
Sepúlveda D, Baumgartner RN, Pierson RN, Harris T and Heymsfield
SB: Appendicular skeletal muscle mass: Effects of age, gender, and
ethnicity. J Appl Physiol (1985). 83:229–239. 1997.PubMed/NCBI
|
|
24
|
Beaufrère B and Morio B: Fat and protein
redistribution with aging: Metabolic considerations. Eur J Clin
Nutr. 54 (Suppl 3):S48–S53. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hallfrisch J, Muller D, Drinkwater D,
Tobin J and Andres R: Continuing diet trends in men: The Baltimore
Longitudinal Study of Aging (1961–1987). J Gerontol. 45:M186–M191.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Garry PJ, Hunt WC, Koehler KM, Vander Jagt
DJ and Vellas BJ: Longitudinal study of dietary intakes and plasma
lipids in healthy elderly men and women. Am J Clin Nutr.
55:682–688. 1992.PubMed/NCBI
|
|
27
|
Elia M, Ritz P and Stubbs RJ: Total energy
expenditure in the elderly. Eur J Clin Nutr. 54 (Suppl 3):S92–S103.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Williamson DF, Madans J, Anda RF, Kleinman
JC, Kahn HS and Byers T: Recreational physical activity and
ten-year weight change in a US national cohort. Int J Obes Relat
Metab Disord. 17:279–286. 1993.PubMed/NCBI
|
|
29
|
Franklin SS: Ageing and hypertension: The
assessment of blood pressure indices in predicting coronary heart
disease. J Hypertens Suppl. 17:S29–S36. 1999.PubMed/NCBI
|
|
30
|
Staessen JA, Gasowski J, Wang JG, Thijs L,
Den Hond E, Boissel JP, Coope J, Ekbom T, Gueyffier F, Liu L, et
al: Risks of untreated and treated isolated systolic hypertension
in the elderly: Meta-analysis of outcome trials. Lancet.
355:865–872. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lewington S, Clarke R, Qizilbash N, Peto R
and Collins R: Prospective Studies Collaboration: Age-specific
relevance of usual blood pressure to vascular mortality: A
meta-analysis of individual data for one million adults in 61
prospective studies. Lancet. 360:1903–1913. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pinto E: Blood pressure and ageing.
Postgrad Med J. 83:109–114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chang AM and Halter JB: Aging and insulin
secretion. Am J Physiol Endocrinol Metab. 284:E7–E12. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stumvoll M, Goldstein BJ and van Haeften
TW: Type 2 diabetes: Principles of pathogenesis and therapy.
Lancet. 365:1333–1346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stout RW: Glucose tolerance and ageing. J
R Soc Med. 87:608–609. 1994.PubMed/NCBI
|
|
36
|
Shimokata H, Muller DC, Fleg JL, Sorkin J,
Ziemba AW and Andres R: Age as independent determinant of glucose
tolerance. Diabetes. 40:44–51. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fink RI, Kolterman OG, Griffin J and
Olefsky JM: Mechanisms of insulin resistance in aging. J Clin
Invest. 71:1523–1535. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rowe JW, Minaker KL, Pallotta JA and Flier
JS: Characterization of the insulin resistance of aging. J Clin
Invest. 71:1581–1587. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rosenthal M, Doberne L, Greenfield M,
Widstrom A and Reaven GM: Effect of age on glucose tolerance,
insulin secretion, and in vivo insulin action. J Am Geriatr Soc.
30:562–567. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Reaven G: Age and glucose intolerance:
Effect of fitness and fatness. Diabetes Care. 26:539–540. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kuzuya M, Ando F, Iguchi A and Shimokata
H: Changes in serum lipid levels during a 10 year period in a large
Japanese population. A cross-sectional and longitudinal study.
Atherosclerosis. 163:313–320. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kuzuya M, Ando F, Iguchi A and Shimokata
H: Effect of smoking habit on age-related changes in serum lipids:
A cross-sectional and longitudinal analysis in a large Japanese
cohort. Atherosclerosis. 185:183–190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu HH and Li JJ: Aging and dyslipidemia:
A review of potential mechanisms. Ageing Res Rev. 19:43–52. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhou XJ, Rakheja D, Yu X, Saxena R, Vaziri
ND and Silva FG: The aging kidney. Kidney Int. 74:710–720. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Weinstein JR and Anderson S: The aging
kidney: Physiological changes. Adv Chronic Kidney Dis. 17:302–307.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yamagata K, Ishida K, Sairenchi T,
Takahashi H, Ohba S, Shiigai T, Narita M and Koyama A: Risk factors
for chronic kidney disease in a community-based population: A
10-year follow-up study. Kidney Int. 71:159–166. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Coresh J, Selvin E, Stevens LA, Manzi J,
Kusek JW, Eggers P, Van Lente F and Levey AS: Prevalence of chronic
kidney disease in the United States. JAMA. 298:2038–2047. 2007.
View Article : Google Scholar : PubMed/NCBI
|