Introduction
Causative agents of infectious gastroenteritis
include viruses, bacteria and cryptosporidium. Subsequent to
a short incubation period following oral infection, symptoms such
as vomiting, diarrhea, abdominal pain and fever appear (1). As causative viruses, rotavirus (RV),
adenovirus (AV) and norovirus (NV) are common, and NV is the most
dominant. Differences in the viral epidemic pattern can be observed
among countries, regions and climates (2).
Histo-blood group antigens (HBGAs) have been
recognized as receptor tissue for NV that is associated with
infection of the host. Lewis-positive individuals can have HBGAs on
the surface of epithelial cells and NV have an affinity through
blood-type substances, which are expressed in intestinal epithelial
cells (3). As HBGAs are considered to
act as NV receptors and lead to NV susceptibility, we hypothesized
that in addition to genetics, co-infection in the gastrointestinal
tract could be associated with this mechanism. The present study
aimed to investigate the association between bacterial infection
and co-infection in acute gastroenteritis and HBGAs, and further
studies are also important to evaluate the results.
Patients and methods
Baseline characteristics of
subjects
The study is a single-center retrospective cohort
study. All the clinical and laboratory data and preoperative
backgrounds were reviewed from patient medical records from Toho
University School of Medicine, Omori Hospital (Tokyo, Japan). A
total of 370 patients with acute gastroenteritis who presented with
diarrhea (age, 14–89 years; mean age, 37.0 years) (Table I) were recruited. The male/female ratio
was 20/17. Single infection (bacteria or virus), co-infection with
two viruses, and co-infection with one virus and one bacterium were
statistically analyzed.
 | Table I.Baseline characteristics of the
subjects. |
Table I.
Baseline characteristics of the
subjects.
| Characteristics | Total, n=370 |
|---|
| Male, n | 200 |
| Female, n | 170 |
| Median age (range),
year | 37 (14–89) |
| Norovirus, n | 93 |
| Rotavirus, n | 92 |
| Adenovirus, n | 95 |
| Bacteria, n | 96 |
| Virus + virus, n | 62 |
| Virus + bacteria,
n | 46 |
Diagnosis of acute
gastroenteritis
All the patients were diagnosed with acute
gastroenteritis according to acute onset and acute diarrhea without
any diarrhea-inducing baseline illness. Other symptoms such as
nausea, fever or abdominal pain were not considered.
Examination tools
Patient stool samples were collected and kits were
used to examine for antigens of NV, RV and AV. Stool cultures for
pathogenic bacteria were also performed using the same stool
samples. The examination tool for NV in Japan is QUICKNAVI-NORO™
(Otsuka Pharmaceutical Co., Tokyo, Japan). The measurement
principle is immunochromatography and the targets are NV genogroups
I and II. The sensitivity and specificity were 81.6 and 96.9%,
respectively, compared to the previously used reverse transcription
polymerase chain reaction (RT-PCR) method. The examination tool for
RV and AV is BD Rota/Adeno Examan Stick™ (BD Biosciences, San
Diego, CA, USA). The measurement principle is immunochromatography
and the targets are AV B3, B7, F40, F41, RV AG1WA and AG3SA11.
Compared to the previously used kit, the sensitivity and
specificity were ~95%. Stool cultures for pathogenic bacteria were
ordered without any species targeted under routine measurement.
Statistical analysis
All the clinical and laboratory data were collected
from patient medical records. Continuous variables are expressed as
the mean ± standard deviation unless otherwise stated and were
compared using the Mann-Whitney U test. Categorical variables were
compared using the χ2 test. Cox proportional hazards
regression analysis was used to identify variables that were
significant predictors of survival. P<0.05 was considered to
indicate a statistically significant difference. All the
statistical analyses were performed using SPSS version 11.0 (SPSS,
Inc., Chicago, IL, USA).
Ethics
The present study was approved by the Institutional
Review Board of Toho University Omori Medical Center (project
approval number: 20–106).
Results
Viral and bacterial content of each
infection
In total, 88 of the 376 subjects (23.4%) were
positive for a virus only, and 50 (13.3%) were positive for
bacteria only. The presence of bacteria and a virus was detected in
46 (47.9%) of the 96 bacterial gastroenteritis cases (Table II). Fourteen species of bacteria were
detected, including Campylobacter [33/96 (36.4%)], enteropathogenic
Escherichia coli [E.coli; 31/96 (34.1%)] and enterotoxigenic
E. coli [30/96 (33.0%)] (Fig. 1). The
mean ages of the infected patients were 50.0±24.6, 36.3±15.8 and
38.6±18.3 years, respectively. RV gastroenteritis was detected in
92 patients (24.7%; co-infection 83.7%), AV gastroenteritis in 95
(25.5%; co-infection 86.3%), and NV co-infection gastroenteritis in
93 (24.9%; co-infection 35.4%). Of the co-infection with a virus
and bacteria cases, 27.2% had RV, 27.4% had AV and 19.4% had NV.
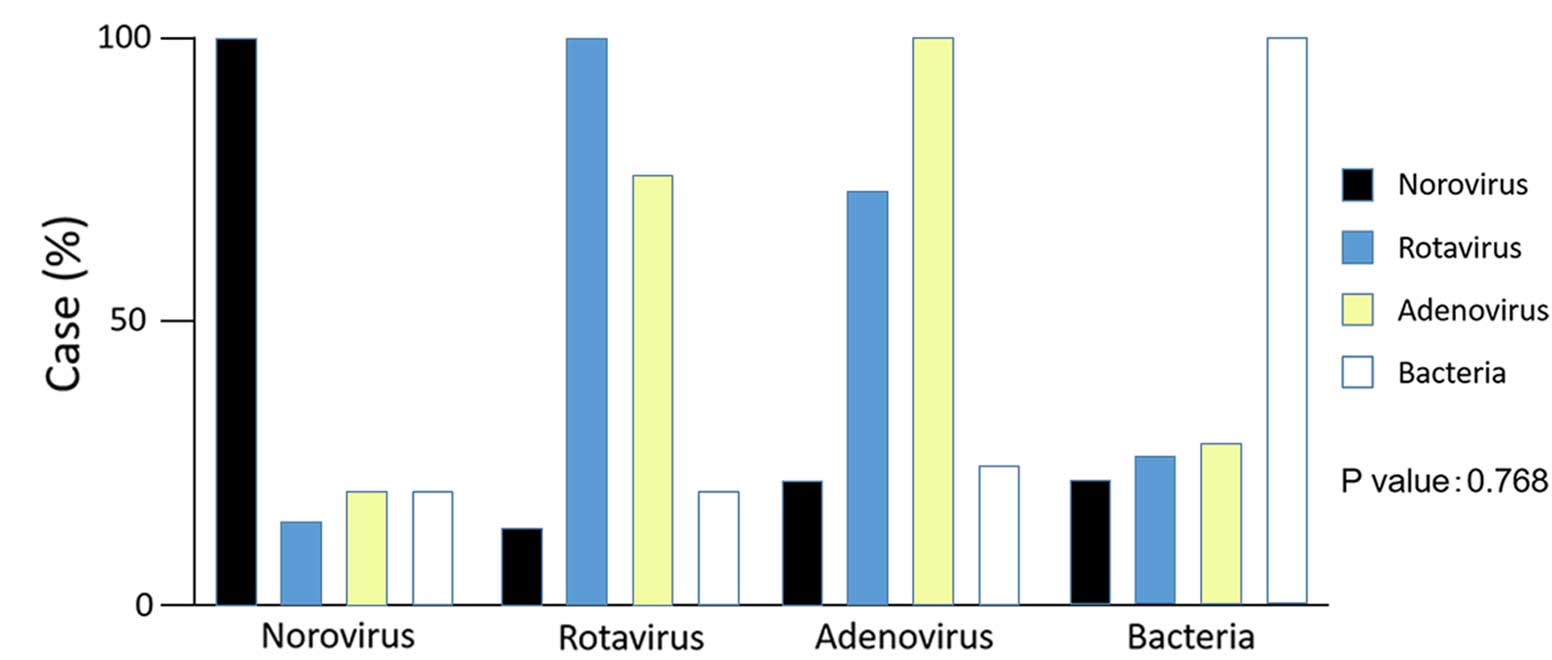
Co-infection with bacteria and NV was not significant in all viral
infections (P=0.768) (Fig. 2). The
correlation between the serum values of C-reactive protein (CRP)
and white blood cells (WBCs) and co-infection was analyzed.
Co-infection was evaluated by WBC count not by CRP (P=0.03). In
terms of the ABO histo-blood group type and NV infection, the
frequency in O-type patients was not significantly increased
(P=0.052), although the cumulative number of patients was the
largest among the ABO blood groups (Table III).
 | Table II.Viral and bacterial content of each
infection. |
Table II.
Viral and bacterial content of each
infection.
| Content of each
infection | Cases, n |
|---|
| NV total | 93 |
| Only
NV | 60 |
| NV +
AV | 4 |
| NV + RV +
AV | 11 |
| NV +
bacteria | 15 |
| NV + AV +
bacteria | 1 |
| NV + RV +
AV + bacteria | 2 |
| RV total | 92 |
| Only
RV | 15 |
| RV +
AV | 41 |
| RV + NV +
AV | 11 |
| RV +
bacteria | 5 |
| RV + AV +
bacteria | 18 |
| RV + NV +
AV + bacteria | 2 |
| AV total | 95 |
| Only
AV | 13 |
| AV +
NV | 4 |
| AV +
RV | 41 |
| AV + NV +
RV | 11 |
| AV +
bacteria | 5 |
| AV + NV +
bacteria | 1 |
| AV + RV +
bacteria | 18 |
| AV + NV +
RV + bacteria | 2 |
| Bacteria total | 96 |
| Only
bacteria | 50 |
| Bacteria
+ NV | 15 |
| Bacteria
+ RV | 5 |
| Bacteria
+ AV | 5 |
| Bacteria
+ NV + AV | 1 |
| Bacteria
+ RV + AV | 18 |
| Bacteria
+ NV + RV + AV | 2 |
 | Table III.Norovirus infection analysis by the
ABO blood type. |
Table III.
Norovirus infection analysis by the
ABO blood type.
| Infection | O, n | A, n | B, n | AB, n |
|---|
|
Norovirus+ | 28 | 26 | 14 | 1 |
|
Norovirus− | 34a | 37 | 22 | 17 |
| Total | 62 | 63 | 36 | 18 |
Discussion
Causative agents of infectious gastroenteritis
include viruses, bacteria and cryptosporidium. Subsequent to
a short incubation period following oral infection, symptoms such
as vomiting, diarrhea, abdominal pain and fever appear (1). As causative viruses, RV, AV and NV are
common, and NV is the most dominant. Differences in the viral
epidemic pattern can be observed among countries, regions and
climates (2).
RV belongs to the Reoviradae family and
infects the intestinal tract. RVs can be divided into groups A
through C, with group B being the most common cause of severe
diarrhea in adults. Although rare, the association of RV with
diseases outside the intestinal tract, such as the central nervous
system, has been suggested. The distribution of viruses in
extraintestinal organs has been confirmed in animal experiments
(4–6). AV
belongs to the Adenoviridae family. AV infections often
present as conjunctivitis, tonsillitis (which may appear to be
identical to strep throat and cannot be distinguished from strep
except by throat culture), ear infection or croup. AV types 40 and
41 can also cause gastroenteritis (7).
NV is classified as part of the Caliciviridae family and
infects the intestinal tract. It is broadly classified into
genogroups I and II (8,9).
The RT-PCR/enzyme-linked immonosorbent assay method
has been approved as a diagnostic kit and has prevailed over the
conventional immune electron microscopy/radioimmunoassay method.
HBGAs have been recognized as receptor tissue for NV that is
associated with infection of the host (3). ABO blood group substances and Lewis
blood-type substances are expressed in intestinal epithelial cells
in ~84% of Japanese individuals. Lewis-positive individuals can
have HBGAs on the surface of epithelial cells. NV is reported to
have an affinity to these HBGAs and can recognize them as
receptors. It has been reported that the incidence of infection in
individuals with blood types A and O, and secretion-type
individuals is higher than that of the individuals with blood type
B and non-secretion-type individuals (10). In the present study, this significant
difference could not be confirmed.
For all infectious diseases, not just viral
gastroenteritis, a rapid serological diagnosis is important. The
prevention of infectious spread is important, and the accurate
differential diagnosis of bacteria or other causes is required. It
is essential for prompt measures to be taken to protect against
outbreaks in facilities. The basic rapid diagnostic method is the
antigen-antibody reaction, in which soluble viral protein antigen
and antibody react in the ion chromatography method. The
sensitivity and specificity maintain an adequate level (>90%)
with the aforementioned kits (i.e., QUICKNAVI-NORO™ and BD
Rota/Adeno Examan Stick™), and are adequate rapid diagnostic
methods. Rapid diagnostic methods can contribute to the suppression
of infectious spread.
In the developed world, Campylobacter jejuni
is the primary cause of bacterial gastroenteritis, with half of
these cases associated with exposure to poultry (11). E. coli and Salmonella
shigella are other types of source bacteria (12). When food becomes contaminated with
bacteria and remains at room temperature for a period of several
hours, the bacteria multiply and increase the risk of infection in
those who consume the food. Certain foods commonly associated with
illness include raw or undercooked meat, poultry, seafood and eggs;
raw sprouts; unpasteurized milk and soft cheeses; and fruit and
vegetable juice (13). In the present
study, Campylobacter and E. coli were mainly
detected, consistent with the previous study.
The frequency of sporadic NV infection is greater
than that of RV and AV. Co-infection of RV and AV occurred
frequently. As such, RV and AV may not be able to function well
alone and may require a co-worker to trigger an infection. The
involvement of HBGAs can be considered to heighten the single
infection rate of NV; however, the exact association remains to be
elucidated and further research is required. Although it has been
thought that NV is specific for co-infection with a bacterial
infection compared to RV and AV, this was not demonstrated in the
present study. We assume that indigenous bacteria in the intestinal
tract and bacterial infection is associated with viral infection.
The increasing number of NV infections may be associated with
indigenous bacteria in the intestinal tract and bacterial
infection. HBGAs are believed to affect the transmission and
infection processes of NV and bacteria. Further studies of bacteria
and their binding capacity to viruses will provide new insights
(14). The presence of blood
group-active enteric bacteria, such as E.coli, Salmonella
and Klebsiella, has been reported and ~50% of the strains
inhibited anti-blood group ABO agglutinins (15). The blood group activities of enteric
bacteria were attributed to the presence of HBGA-like O antigen in
lipopolysaccharide (LPS) (16).
Bacterial LPS has been shown to have a key role in the infection of
mouse mammary tumor virus. The gut microbiota in the human
intestine also affects the likelihood of infection with human
enteric viruses (17). Elevation in
the levels of antigen in the intestine is believed to provide an
opportunity for NV infection and could also affect other
gastroenteritis virus infections, such as RV (18,19). NV is
believed to be specific for co-infection with bacteria; however in
the present study, it is suggested that co-infection with bacteria
is not confined to NV. The serum values of WBCs and the CRP of
co-infection cases (virus and bacteria) are higher than those of
single infection (virus). In case of elevated serum values of
inflammatory markers, the involvement of bacterial infection should
be considered.
The Norwalk virus strain 68, which is the prototype
of NV, is absorbed in blood type A and O antigen and secretion-type
antigen (20). The blood group
antigens are also expressed in intestinal epithelial cells, not
just on the surface of human red blood cells. A previous study
reported that the villi of the jejunum affected by NV are flattened
and atrophied (21). In vitro
binding assay using virus-like particles (VLPs) has revealed that
the incidence of infection in individuals with blood type A and O
and secretion-type individuals are higher than that of individuals
with blood type B and non-secretion-type individuals (22). Evaluation is difficult as the diagnosis
of Lewis blood type was not performed in Omori Hospital. This only
pertains to the Norwalk virus strain 68, and it is clear that not
all NV strains recognize the blood group antigen in the same manner
as the Norwalk virus strain 68.
Infection was shown to occur equally in individuals
with A, B and O blood types in the GII/4 genotype, which can be
severe and can spread easily (23,24). In the
present study, there was no significant difference in the infection
rate among ABO blood types in NV infection; the results were
similar for RV and AV. It is not clear whether HBGAs are only
involved in absorption between cells and NV, or if they invade the
intestinal tract. Clarification of the mechanism of NV infection
beginning with the adsorption to HBGAs requires elucidation in
future studies.
In conclusion, co-infection of bacteria and virus
occurred frequently in the gastrointestinal tract. The ABO blood
phenotype expression was not significant in the present series of
NV infections and the results did not suggest the affinity of NV
for specific bacteria. However, it was suggested that there could
be another pathway leading to infectious diarrheal disease in
addition to the correlation between the genetically determined HBGA
expression of an individual and their susceptibility to an enteric
virus, particularly NV.
References
|
1
|
Bryce J, Boschi-Pinto C, Shibuya K and
Black RE: WHO Child Health Epidemiology Reference Group. WHO
estimates of the causes of death in children. Lancet.
365:1147–1152. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parashar UD, Gibson CJ, Bresee JS and
Glass RI: Rotavirus and severe childhood diarrhea. Emerg Infect
Dis. 12:304–306. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kageyama T, Kojima S, Fukushi S, Hoshino
FB, Shinohara M, Uchida K, Natori K, Takeda N and Katayama K:
Genogroup-specific PCR primers for detection of Norwalk-like
viruses. J Virol Methods. 100:107–114. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Azevedo MS, Yuan L, Jeong KI, Gonzalez A,
Nguyen TV, Pouly S, Gochnauer M, Zhang W, Azevedo A and Saif LJ:
Viremia and nasal and rectal shedding of rotavirus in gnotobiotic
pigs inoculated with Wa human rotavirus. J Virol. 79:5428–5436.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Crawford SE, Patel DG, Cheng E, Berkova Z,
Hyser JM, Ciarlet M, Finegold MJ, Conner ME and Estes MK: Rotavirus
viremia and extraintestinal viral infection in the neonatal rat
model. J Virol. 80:4820–4832. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fenaux M, Cuadras MA, Feng N, Jaimes M and
Greenberg HB: Extraintestinal spread and replication of a
homologous EC rotavirus strain and a heterologous rhesus rotavirus
in BALB/c mice. J Virol. 80:5219–5232. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Waddell G, Whelan G and Bock G: Novel
diarrhoea viruses. New York: Wiley. 631987.
|
|
8
|
Okada M, Ogawa T, Kaiho I and Shinozaki K:
Genetic analysis of noroviruses in Chiba Prefecture, Japan, between
1999 and 2004. J Clin Microbiol. 43:4391–4401. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hansman GS, Natori K, Shirato-Horikoshi H,
Ogawa S, Oka T, Katayama K, Tanaka T, Miyoshi T, Sakae K, Kobayashi
S, et al: Genetic and antigenic diversity among noroviruses. J Gen
Virol. 87:909–919. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hutson AM, Atmar RL, Graham DY and Estes
MK: Norwalk virus infection and disease is associated with ABO
histo-blood group type. J Infect Dis. 185:1335–1337. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Galanis E: Campylobacter and
bacterial gastroenteritis. CMAJ. 177:570–571. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Webb A and Starr M: Acute gastroenteritis
in children. Aust Fam Physician. 34:227–231. 2005.PubMed/NCBI
|
|
13
|
Nyachuba DG: Foodborne illness: Is it on
the rise? Nut Rev. 68:257–269. 2010. View Article : Google Scholar
|
|
14
|
Miura T, Sano D, Suenaga A, Yoshimura T,
Fuzawa M, Nakagomi T, Nakagomi O and Okabe S: Histo-blood group
antigen-like substances of human enteric bacteria as specific
adsorbents for human noroviruses. J Virol. 87:9441–9451. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Springer GF, Wiiliamson P and Brandes WC:
Blood group activity of gram-negative bacteria. J Exp Med.
113:1077–1093. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Andersson M, Carlin N, Leontein K,
Lindquist U and Slettengren K: Structural studies of the
O-antigenetic polysaccharide of Escherichia coli O86, which
possesses blood-group B activity. Carbonhydr Res. 185:211–223.
1989. View Article : Google Scholar
|
|
17
|
Kane M, Case LK, Kopaskie K, Kozlova A,
MacDearmid C, Chervonsky AV and Golovkina TV: Successful
transmission of a retrovirus depends on the commensal microbiota.
Science. 334:245–249. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu L, Crawford SE, Czako R,
Cortes-Penfield NW, Smith DF, Le Pendu J, Estes MK and Prasad BV:
Cell attachment protein VP8* of a human rotavirus specifically
interacts with A-type histo-blood group antigen. Nature.
485:256–259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang P, Xia M, Tan M, Zhong W, Wei C,
Wang L, Morrow A and Jiang X: Spike protein VP8* of human rotavirus
recognize histo-blood group antigens in a type-specific manner. J
Virol. 86:4833–4843. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shirato-Horikoshi H and Takeda N:
Interaction between norovirus and human histo-blood group antigens.
Uirusu. 57:181–189. 2007.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Graham DY, Jiang X, Tanaka T, Opekun AR,
Madore HP and Estes MK: Norwalk virus infection of volunteers: New
insights based on improved assays. J Infect Dis. 170:34–43. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lindesmith L, Moe C, Marionneau S, Ruvoen
N, Jiang X, Lindblad L, Stewart P, LePendu J and Baric R: Human
susceptibility and resistance to Norwalk virus infection. Nat Med.
9:548–553. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Okada M, Tanaka T, Oseto M, Takeda N and
Shinozaki K: Genetic analysis of noroviruses associated with
fatalities in healthcare facilities. Arch Virol. 151:1635–1641.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rockx BH, Vennema H, Hoebe CJ, Duizer E
and Koopmans MP: Association of histo-blood group antigens and
susceptibility to Norovirus infections. J Infect Dis. 191:749–754.
2005. View
Article : Google Scholar : PubMed/NCBI
|