Introduction
Heavy metal intoxication and/or oxidative stress are
implicated in numerous chronic diseases, including metabolic and
neurological disorders. Environmental contamination by heavy metals
such as cadmium (Cd) and mercury (Hg) is a serious international
concern as these heavy metals can enter the food chain, accumulate
in fishes and animals, and thus, endanger human health (1–5). According
to the World Health Organization, the limit of tolerable intake for
Cd, methylmercury and inorganic Hg in an adult human is 7, 1.6 and
4 µg/kg of body weight per week, respectively (6).
One of the possible mechanisms underlying the
effects of Cd and Hg is oxidative cytotoxicity. Cd and Hg
interaction with the carboxyl and sulfhydryl group of proteins
results in free radical generation (7–10). In
addition, Cd and Hg can deplete antioxidant levels, resulting in an
imbalance between oxidant and antioxidant ability. An increase in
reactive oxygen species levels results in oxidation of
polyunsaturated fatty acids, causing neuronal damage due to high
acid content in the nervous system. In addition, our previous study
demonstrated that Cd administration significantly increased lipid
peroxidation and reduced levels of antioxidant enzymes such as Cu,
Zn-superoxide dismutase (SOD1), catalase and glutathione peroxidase
(11).
Dendropanax morbifera Léveille (DML), which
belongs to the family Araliaceae, is an endemic species found in
the southwestern region of South Korea (12). Recently, studies have investigated the
effects of DML on various conditions, including their antioxidant
and antidotal capacities (11,13). For example, a methanol extract of the
debarked stem of DML contains abundant phenolic and flavonoid
compounds, which show strong antioxidant activities (13). In addition, our previous study
demonstrated that DML extracts facilitate Cd excretion and suppress
Cd-induced lipid peroxidation in hippocampal homogenates (11). However, to the best of our knowledge,
no clinical study has examined the possible health benefits of a
DML extract on heavy metal and antioxidant levels in human
subjects.
The aim of the present randomized, double-blind,
placebo-controlled cross-over study was to examine the effects of
repeated application of DML extract on the antioxidant status and
oxidative damage in healthy adults.
Subjects and methods
Preparation of the test product from
the leaf extract of DML
The DML extract used was manufactured by a good
manufacturing practices-certified company (Natural F&P Corp.,
Cheonwon, Korea). Fresh leaves from DML were obtained from
Hambakjae Biopharm Co., Ltd. (Jeju, Korea). The plant was
authenticated by two practitioners of traditional Asian medicine.
Leaves from the plant samples (15 kg) were chopped, blended and
soaked in 2 liters of 80% ethanol, and refluxed three times at 20°C
for 2 h. The insoluble materials were removed by centrifugation at
10,000 × g for 30 min, and the resulting supernatant was
concentrated and freeze-dried to obtain a powder. For the test
product preparation, DML, stearin magnesium and silicon dioxide
were mixed. For the placebo, microcrystalline cellulose 102 was
mixed with lactose powder, and dextrin was added to obtain a tablet
form. Food colors (red, yellow and blue) were also added to produce
the same color as the test product (Table
I). The raw materials used in the test product and placebo were
placed in a mixer and blended sufficiently to ensure homogeneity;
subsequently, these were made into tablet at 300 mg/tablet.
 | Table I.Composition of Dendropanax
morbifera Léveille (DML) extracts and placebo tablets. |
Table I.
Composition of Dendropanax
morbifera Léveille (DML) extracts and placebo tablets.
| Composition | DML | Placebo (%) |
|---|
| Dendropanax
extracts | 98.000 |
|
| Cellulose 102 |
| 59.852 |
| Lactose powder |
| 39.600 |
| Stearin
magnesium | 1.000 |
|
| Dextrin |
| 0.400 |
| Silicon dioxide | 1.000 |
|
| Food color
(red/yellow/blue) |
| 0.148 |
| Total | 100.000 | 100.000 |
Stability testing for the test product
and placebo
The test product and placebo were assessed for
stability; moisture content (%); coliform group; lead (mg/kg),
arsenic (mg/kg) and sodium (mg/100 g) levels; and disintegration by
outsourcing to ‘Korea Health Supplements Association’ (Seongnam,
South Korea). All test results were acceptable as per the
requirements set by the Food Sanitation Act. No preservative was
added to the test product.
Study approval and sample target group
selection
In accordance with the Functional Foods for Health
Act (http://www.mfds.go.kr/files/upload/eng/FOOD_SANITATION_ACT.pdf),
all issues associated with human trials were approved by the
Institutional Review Board for Human Trials of Hallym University
(Hwaseong, Korea) (IRB approval no. 2014-074); the study was
conducted in agreement with the Declaration of Helsinki and
performed in accordance with International Conference on
Harmonization guidelines. In addition, the study was
retrospectively registered at the WHO Primary Registries in
Republic of Korea (Clinical Research Information Service)
(registration no. KCT0001472, May 8, 2015).
Subjects were male and female volunteers aged 23–65
years (n=61). The subjects were informed of the details of the
trial, and only those who fully understood the purpose of trial
participation and gave consent for participation were selected as
subjects. All the subjects were considered suitable for the trial
as they had normal liver functions, as assessed by measuring
glutamate-oxaloacetate transaminase (AST) and glutamate-pyruvate
transaminase (ALT) levels in the blood. Exclusion criteria
consisted of intestinal absorption abnormalities and liver
functions. Subjects with ALT or AST >60 U/T were also excluded.
One subject was excluded due to high levels of ALT and AST. The
others (n=60) (Fig. 1) were randomly
assigned to treatment groups, minimizing any intentional elements
of group assignment, in a double-blind procedure to ensure that the
subjects did not know which group they were assigned to (test group
or comparative control group) (Fig.
1). The sample size of a minimum 9 per treatment group would
provide ≥95% power to detect a difference of Cd values in the serum
based on the previous animal study (11).
Analytical procedures
For heavy metal, lipid peroxidation and antioxidant
analysis, the assay was performed prior and subsequent to
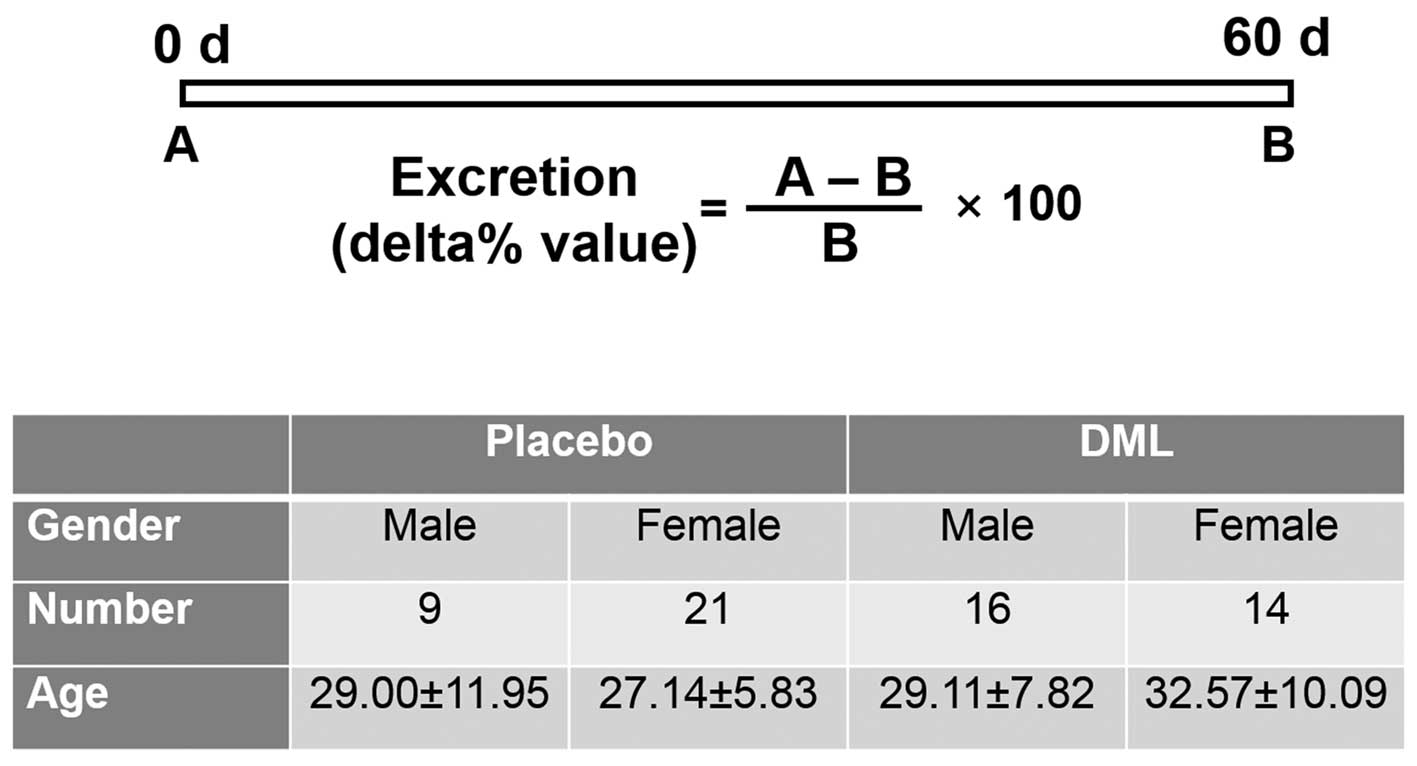
administration of the test product and the placebo (Fig. 2). All the subjects were instructed to
ingest the tablets after breakfast over a 60-day period. The
placebo group was comparable in all characteristics to the DML
group. Food and water intake was not limited.
Measurement of Cd, Hg and
malondialdehyde (MDA) levels and SOD1 activity in the serum
By vein puncture with a syringe, blood samples were
collected in tubes containing ethylenediaminetetraacetic acid. The
tubes were immediately centrifuged at 4°C and 500 × g for 10 min to
obtain serum and plasma for measuring Cd, Hg and MDA levels and
SOD1 activity. These parameters were measured using routine
automated methods. Adverse experiences were rated by the
investigators for intensity and their association to the DML
extract. Efficacy and safety measurements were conducted at a
Hallym University Dongtan Sacred Heart Hospital (Dongtan, South
Korea) laboratory and at a Seoul National University College of
Veterinary Medicine (Seoul, South Korea) by technicians or graduate
students blinded to the placebo and treatment groups.
Statistical analysis
The measured Hg and Cd levels were calculated into
excretion ratio (delta% value), as shown in Fig. 2. The MDA levels and SOD1 activities 60
days after placebo or DML consumption are shown. All the data are
expressed as mean ± standard error of the mean. To determine the
effects of the DML tablet, differences between the means of the two
groups were statistically analyzed using two-way analysis of
variance with repeated measures and Bonferroni's post hoc test
using GraphPad Prism 5.01 (GraphPad Software, La Jolla, CA, USA)
software.
Results
Patient characteristics
The baseline characteristics and biochemical values
of the subjects did not significantly differ between the placebo
and DML groups (data not shown). In addition, no significant
differences between male and female participants were
identified.
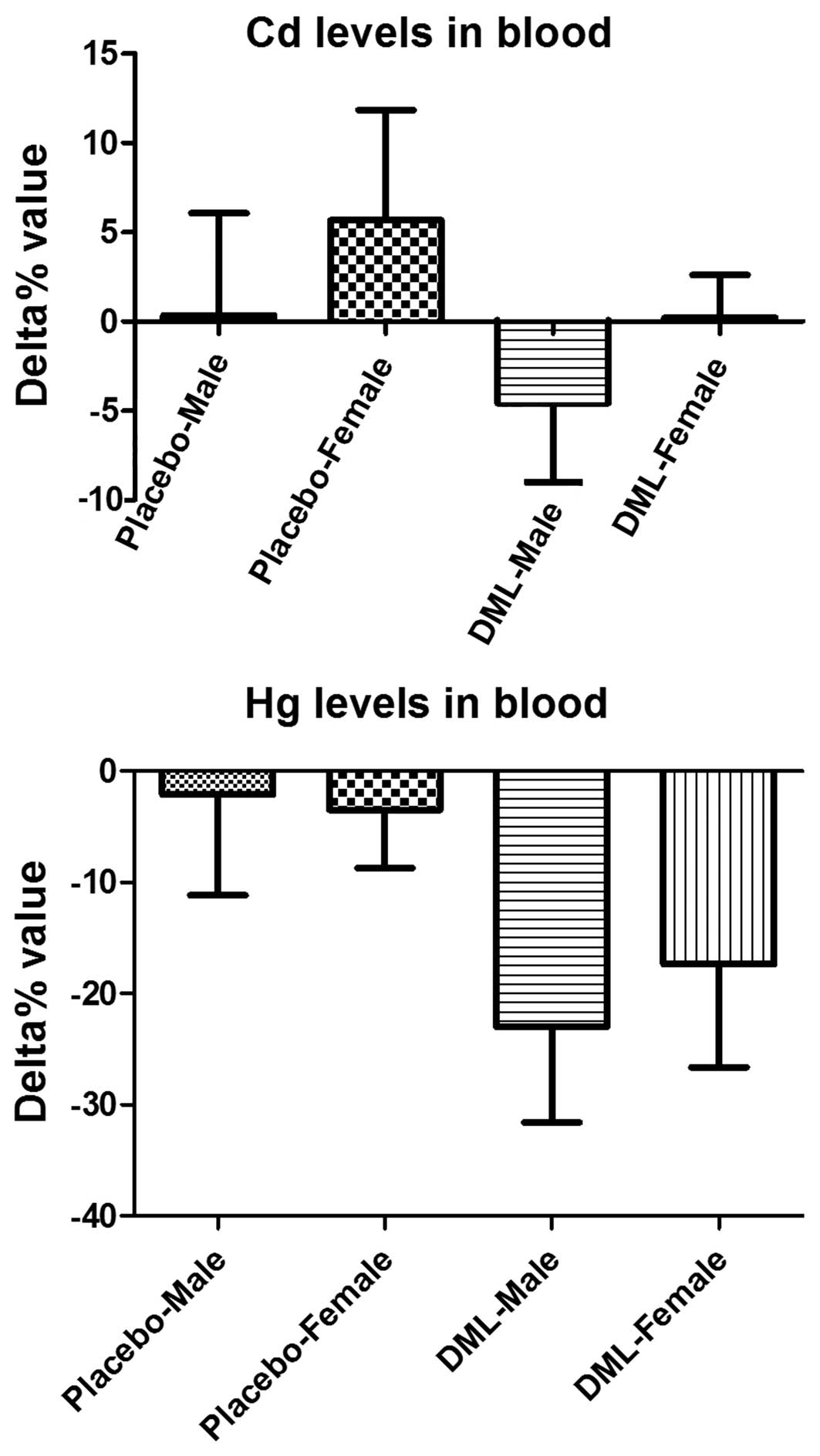
Cd and Hg excretion
Serum Cd levels were increased in the male and
female subjects in the placebo group and decreased in the DML
group; however, no significant difference between the two groups
was identified (P=0.3008) (Fig. 3).
Serum Hg levels decreased in all the groups, but serum levels were
more prominently decreased in the DML-male and DML-female groups
compared to the placebo-male and placebo-female groups,
respectively (P=0.1336 and P=0.1937, respectively) (Fig. 3).
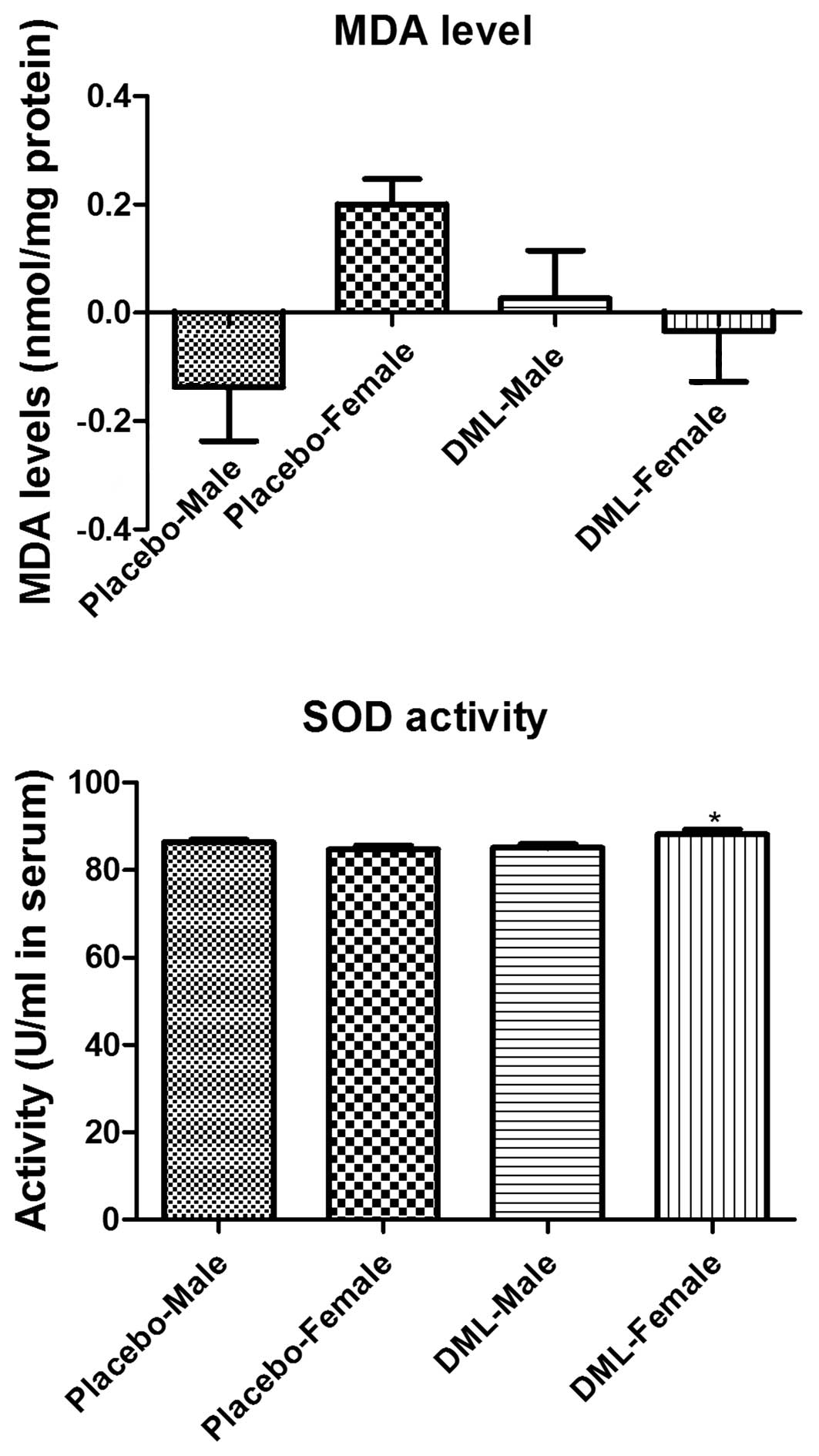
Oxidative stress biomarkers
Serum MDA levels were increased in the DML-male
group compared to the placebo-male group (P=0.3125), while these
were decreased in the DML-female group compared to the
placebo-female group (P=0.0842) (Fig.
4); however, no significant differences were identified.
Serum SOD1 activity was increased in DML-treated
groups, but the SOD1 activity was significantly increased in the
DML-female group compared to the placebo-female group (P=0.02031).
However, no significant difference in SOD1 activity was observed
between the placebo-male and DML-male group (P=0.2031) (Fig. 4).
Discussion
Several lines of evidence demonstrate that DML and
its components reduce reactive oxygen species generation and have
antioxidant effects (13,14). In addition, our previous study
demonstrated that DML facilitates Cd excretion and exerts
antioxidant effects against Cd-induced oxidative damage (11). The present study attempted to use DML
to facilitate Cd and Hg excretion in humans and to increase the
antioxidant capacity in the serum, as Cd and Hg have been shown to
inhibit antioxidant enzymes (15–17).
No significant decrease in serum Cd or Hg levels was
observed. However, the consumption of the DML tablet for 2 months
facilitated excretion of Cd to 4.6% in male subjects and Hg to 23.0
and 17.3% in male and female subjects, respectively. This result
suggests that consumption of DML tablet facilitates Cd and Hg
excretion from the serum. The facilitated excretion rate is
relatively low for Cd; however, the facilitation is extremely
important as cadmium is tightly bound to metallothionein, which is
almost completely reabsorbed in the renal tubules and accumulates
in the body over the lifetime with a biological half-life of ≤38
years (6).
Heavy metals contribute to oxidative processes
(18,19), and the levels of reactive oxygen
species are important factors associated with the development of
chronic disease (20,21). In the present study, the levels of MDA,
a well-known product of lipid peroxidation (22,23), were
measured in human subjects. MDA levels were decreased in the DML
group compared to the placebo group. This result was consistent
with our previous study showing that supplementation with the DML
suppressed Cd-induced increase of MDA levels, as well as protein
carbonylation due to modified proteins in the rat hippocampus
(11). In the present study, the DML
significantly increased SOD levels in the female treatment group
compared to the female placebo group; this suggests that treatment
with the extract for 2 months increases the antioxidant capacity in
the blood. This is consistent with our previous study showing that
administration of DML increased SOD1 activity in normal healthy
rats (11).
In conclusion, administration of DML has positive
effects on excretion of Cd and Hg from the blood and increases
antioxidant capacity by decreasing MDA levels and increasing SOD
activity in the serum.
Acknowledgements
The present study was supported by the High
Value-added Food Technology Development Program, Ministry for
Agriculture, Food and Rural Affairs, Republic of Korea (grant no.
112106-022-HD020).
References
|
1
|
Kim NS and Lee BK: National estimates of
blood lead, cadmium, and mercury levels in the Korean general adult
population. Int Arch Occup Environ Health. 84:53–63. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vieira C, Morais S, Ramos S, Delerue-Matos
C and Oliveira MB: Mercury, cadmium, lead and arsenic levels in
three pelagic fish species from the Atlantic Ocean: Intra- and
inter-specific variability and human health risks for consumption.
Food Chem Toxicol. 49:923–932. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jain RB: Effect of pregnancy on the levels
of blood cadmium, lead, and mercury for females aged 17–39 years
old: Data from National Health and Nutrition Examination Survey
2003–2010. J Toxicol Environ Health A. 76:58–69. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tyrrell J, Melzer D, Henley W, Galloway TS
and Osborne NJ: Associations between socioeconomic status and
environmental toxicant concentrations in adults in the USA: NHANES
2001–2010. Environ Int. 59:328–335. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Storelli MM, Barone G, Cuttone G, Giungato
D and Garofalo R: Occurrence of toxic metals (Hg, Cd and Pb) in
fresh and canned tuna: Public health implications. Food Chem
Toxicol. 48:3167–3170. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Agency for Toxic Substances and Disease
Registry: Toxicological profile for cadmium. US Department of
Health and Human Services. NTIS Report (Atlanta, GA). 1999.No.
PB/89/194476/AS.
|
|
7
|
Ates I, Suzen HS, Aydin A and Karakaya A:
The oxidative DNA base damage in testes of rats after
intraperitoneal cadmium injection. Biometals. 17:371–377. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shanker G, Aschner JL, Syversen T and
Aschner M: Free radical formation in cerebral cortical astrocytes
in culture induced by methylmercury. Brain Res Mol Brain Res.
128:48–57. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gałazyn-Sidorczuk M, Brzóska MM, Jurczuk M
and Moniuszko-Jakoniuk J: Oxidative damage to proteins and DNA in
rats exposed to cadmium and/or ethanol. Chem Biol Interact.
180:31–38. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gonçalves JF, Fiorenza AM, Spanevello RM,
Mazzanti CM, Bochi GV, Antes FG, Stefanello N, Rubin MA, Dressler
VL, Morsch VM, et al: N-acetylcysteine prevents memory deficits,
the decrease in acetylcholinesterase activity and oxidative stress
in rats exposed to cadmium. Chem Biol Interact. 186:53–60. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim W, Kim DW, Yoo DY, Jung HY, Nam SM,
Kim JW, Hong SM, Kim DW, Choi JH, Moon SM, et al: Dendropanax
morbifera Léveille extract facilitates cadmium excretion and
prevents oxidative damage in the hippocampus by increasing
antioxidant levels in cadmium-exposed rats. BMC Complement Altern
Med. 14:4282014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han S, Jung Y, Ko M, Oh Y, Koh S, Kim M
and Oh M: Phylogenetic relationships of the Dendropanax
morbifera and D-trifidus based on PCR-RAPD. Korean J Genet.
20:173–181. 1998.
|
|
13
|
Hyun TK, Kim MO, Lee H, Kim Y, Kim E and
Kim JS: Evaluation of anti-oxidant and anti-cancer properties of
Dendropanax morbifera Léveille. Food Chem. 141:1947–1955.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park SE, Sapkota K, Choi JH, Kim MK, Kim
YH, Kim KM, Kim KJ, Oh HN, Kim SJ and Kim S: Rutin from
Dendropanax morbifera Leveille protects human dopaminergic
cells against rotenone induced cell injury through inhibiting JNK
and p38 MAPK signaling. Neurochem Res. 39:707–718. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Amara S, Douki T, Garrel C, Favier A, Ben
Rhouma K, Sakly M and Abdelmelek H: Effects of static magnetic
field and cadmium on oxidative stress and DNA damage in rat cortex
brain and hippocampus. Toxicol Ind Health. 27:99–106. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Agrawal S, Flora G, Bhatnagar P and Flora
SJ: Comparative oxidative stress, metallothionein induction and
organ toxicity following chronic exposure to arsenic, lead and
mercury in rats. Cell Mol Biol (Noisy-le-grand). 60:13–21.
2014.PubMed/NCBI
|
|
17
|
Martinez CS, Escobar AG, Torres JG, Brum
DS, Santos FW, Alonso MJ, Salaices M, Vassallo DV, Peçanha FM,
Leivas FG, et al: Chronic exposure to low doses of mercury impairs
sperm quality and induces oxidative stress in rats. J Toxicol
Environ Health A. 77:143–154. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stohs SJ and Bagchi D: Oxidative
mechanisms in the toxicity of metal ions. Free Radic Biol Med.
18:321–336. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ercal N, Gurer-Orhan H and Aykin-Burns N:
Toxic metals and oxidative stress part I: Mechanisms involved in
metal-induced oxidative damage. Curr Top Med Chem. 1:529–539. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Basu S: F2-isoprostanes in human health
and diseases: From molecular mechanisms to clinical implications.
Antioxid Redox Signal. 10:1405–1434. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Galanis A, Karapetsas A and
Sandaltzopoulos R: Metal-induced carcinogenesis, oxidative stress
and hypoxia signalling. Mutat Res. 674:31–35. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Romero FJ, Bosch-Morell F, Romero MJ,
Jareño EJ, Romero B, Marín N and Romá J: Lipid peroxidation
products and antioxidants in human disease. Environ Health
Perspect. 106(Suppl 5): 1229–1234. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Del Rio D, Stewart AJ and Pellegrini N: A
review of recent studies on malondialdehyde as toxic molecule and
biological marker of oxidative stress. Nutr Metab Cardiovasc Dis.
15:316–328. 2005. View Article : Google Scholar : PubMed/NCBI
|