Introduction
Severe burns that cover a large total body surface
area result in serious risks to the patient in terms of morbidity
and mortality, and in most cases should be treated in a specialized
burns unit (1). Modern treatments for
severe burns include skin replacement therapy using biomaterials or
grafts, prevention of infection using antibiotics and sterile
conditions, treatment of sepsis, and targeted protein therapies
(2,3). In
addition to the pain and tissue damage caused by severe burns, the
process of recovery can cause problems resulting from the high
requirement of the body for energy and proteins. Patients with
severe burns experience an initial ebb phase over the first two
days post-burn, where metabolism decreases. A period of flow occurs
during hypermetabolism that can last for many months post-burn
(4). This process involves multiple
factors; plasma catecholamines, cortisol and inflammatory cells are
elevated, leading to whole-body catabolism, elevated resting energy
expenditure and multi-organ dysfunction (4). If this process is left unchecked it can
prevent a patient's full rehabilitation and delay wound healing
(5). Modulation of the response by
early treatment, thermoregulation, early and continuous enteral
feeding with high protein-high carbohydrate diets and pharmacologic
treatments markedly decrease morbidity (4). Pharmacologic treatments include anabolic
agents, β-adrenergic receptor antagonists and anti-hyperglycemic
agents (6).
One of the pharmacologic treatments used to treat
severe burns involves recombinant human growth hormone (rhGH).
Growth hormone (GH) is an important anabolic hormone that has
multiple physiological functions. Human GH is composed of 191 amino
acid residues and is secreted by the pituitary gland for anabolism,
to promote the growth of different tissues. Since the 1950s,
purified bovine and human GH has been administered to patients
during clinical practice, but these are limited sources of GH, and
there have been some concerns about contamination (7). With the emergence of synthetic rhGH
produced commercially in bacteria, GH is now more widely used in
clinical therapeutic strategies (7).
For children, GH can be used to provide catch-up growth treatment
in children born small for their gestational age (8). At present, GH is widely used for many
different therapies including GH replacement, severe brain trauma,
and wound healing (9–11). Since most severely burned patients have
an associated dysfunction in protein synthesis, rhGH can promote
protein utilization and synthesis, and its curative effects have
been widely recognized by clinicians. In addition, rhGH has also
been recommended in the nutritional supporting guideline for burn
patients in China (12), and a recent
Cochrane review suggested that there was some evidence the rhGH
could result in more rapid healing of severe burns (13).
The commercially available forms of rhGH include
freeze-dried powders, but recently liquid formulas are available,
with similar pharmacokinetic and safety profiles to the powdered
form (14,15). A liquid form of rhGH may have
advantages in terms of there being no need to reconstitute the
powder, and so the risk of dilution or contamination problems may
be decreased (14), in addition
powdered rhGH may undergo aggregation during reconstitution
resulting in misfolded protein (16).
Therefore, liquid rhGH may be more biologically active, faster
acting, more convenient to use and more effective (17) than the powdered form. However, few
previous studies have compared the clinical effects of both forms
of rhGH, and for burn patients, it is unclear which form is most
beneficial. We hypothesized that liquid rhGH may be beneficial to
patients with severe burns when compared to the powdered form. Thus
the aim of the present study was to evaluate the effects of liquid
and powder rhGH in improving visceral protein synthesis in severely
burned patients, and to provide evidence for the rational use of
rhGH in burns patients.
Materials and methods
The present randomized controlled trial was approved
by the Medical Ethics Committee of the Second Affiliated Hospital
of Zhejiang University School of Medicine and informed consent was
obtained from all patients.
Study inclusion and exclusion
criteria
The inclusion criteria for the present study were as
follows: i) Patient was between 18–65 years-old; ii) total burn
area ≥20% total body surface area (TBSA) classed as a major burn
that requires nutritional therapy (18), of which the deep burn degree II and III
area was ≥1% TBSA; iii) the patient was admitted to hospital within
1 week following burn injury; and iv) the patient agreed to
participate in the current study. The exclusion criteria were as
follows: i) Patient aged <18 years or >65 years; ii) burn
area <20% TBSA; iii) superficial second degree burn patients;
iv) complicating factors such as unstable glycemic control in
diabetic patients, those with malignant tumors, and those with a
brain injury; v) pregnancy or lactation; and (5) a life expectancy of <15 days. The
elimination criteria of patients enrolled on the trial were as
follows: i) Those receiving a therapeutic dosage not consistent
with that of our study protocol; ii) those not completing a 10 day
rhGH treatment.
Patient grouping and treatments
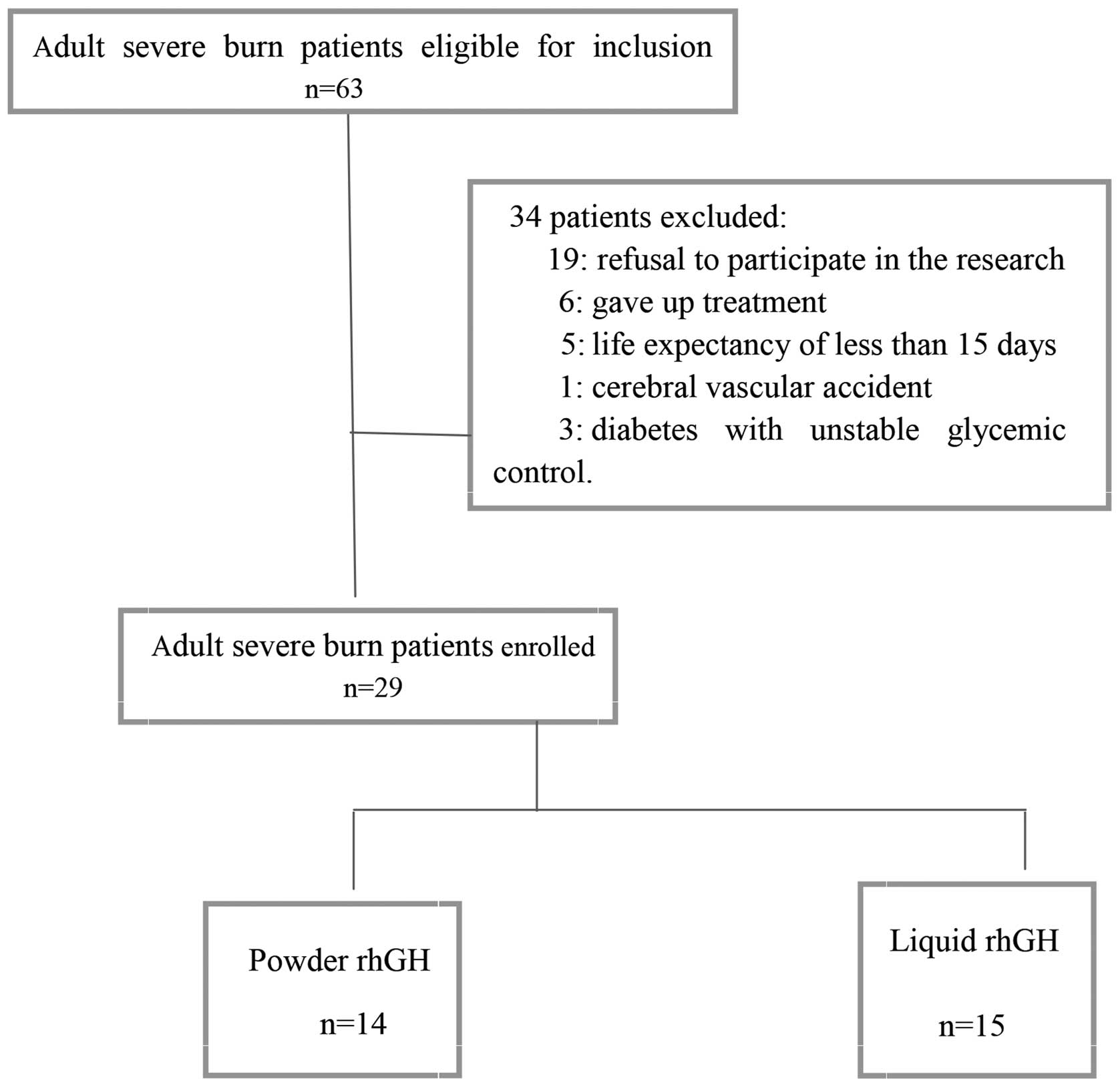
Between February 2009 and November 2011, 63 patients
eligible for inclusion in the current study were treated in the
Burns Department of the Second Affiliated Hospital of Zhejiang
University School of Medicine, Hangzhou, China. Of these, 34
patients were not enrolled, including 19 cases of refusal of
patient consent to participate in the research, six patients who
did not complete treatment, five cases with life expectancy of
<15 days, one case with a cerebral vascular accident, and three
cases of diabetes with unstable glycemic control. Finally, a total
of 29 patients were enrolled in the present study (Fig. 1). All enrolled patients completed the
study, without any cases being eliminated following their
inclusion. At ~1 week following burn injury, the patients were
randomly assigned by block randomization method (with each block
containing 4 cases) to either the liquid rhGH group (15 cases) or
powder rhGH group (14 cases). We defined burn index (BI) as the sum
of full thickness (III degree) burn area, 2/3 deep partial
thickness (II degree) burn area and 1/2 superficial partial
thickness burn area.
The two patient groups were given routine fluid
resuscitation, anti-infective treatment, wound management, and
nutritional supports. The patients' heat supply was calculated
according to the heat supply equation for burns provided by the
Third Military Medical University (19). From the 5–7th day after burn injury,
rhGH was administered (subcutaneously injected at 7 a.m.) once
daily for 10 days. For the liquid rhGH group, liquid rhGH (batch
number 081001, 081205, 090702, 20101202 and 20101204; GeneScience
Pharmaceuticals Co., Ltd., Changchun, China) was used while for the
powder rhGH group, powder rhGH (batch nos. 080902, 081102, 090702,
20100102 and 20100605; Shanghai United Cell Biotechnology Co.,
Ltd., Shanghai, China) was used. Both groups were the same in the
initial timing of drug administration, dosage (0.067 mg/kg/d,
depending on the patient's specific conditions) and course of
treatment. When the patient's blood glucose level was >10
mmol/l, a routine dosage of insulin was pumped using micropump to
control the blood glucose level under the target of 10 mmol/l.
Observational indexes
At 6 a.m., prior to rhGH injection and on days 3, 5
and 10 of rhGH injection, 3 ml fasting venous blood were drawn and
sent to the clinical laboratory for examination of pre-albumin
(PALB). In addition C-reactive protein (CRP) and fasting blood
glucose levels were examined at baseline and on days 5 and 10. PALB
and CRP were detected by an AU 5400 chemistry analyzer (Beckman
Coulter, Inc., Brea, CA, USA) through immunoturbidimetry.
In the morning, prior to rhGH injection, and on days
5 and 10 of rhGH injection, when the dressings were changed, the
patient was weighed without burn dressings and topical treatments.
Additionally, the patient's liver and renal functions [alanine
aminotransferase (ALT) and blood urea nitrogen (BUN)] were also
detected before rhGH injection and on day 10 of rhGH injection.
Based on the reference range of our hospital during the testing
period, we defined serum ALT >50 U/l and BUN >7.2 mmol/l as
abnormal.
The wound healing time was recorded as the time from
the burn occurrence until the residual burn area <1% TBSA or on
the 5th day following the last skin graft.
Statistical analysis
All statistical analyses were conducted using SPSS
version 17.0 (SPSS Inc., Chicago, IL, USA). The data in the present
study were expressed as median (min, max), and compared using
Mann-Whitney U test for independent samples. Count data were
analyzed by Fisher's exact test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Baseline characteristics
There was no significant difference in the median
values of general characteristics such as gender, age, deep II
degree burn area, deep III degree burn area and BI between the 15
subjects in the liquid rhGH and the 14 subjects in the powder rhGH
groups (all P>0.05), except for body weight (P=0.046), as shown
in Table I.
 | Table I.Baseline characteristics of severe
burn patients treated with powder or liquid forms of rhGH. |
Table I.
Baseline characteristics of severe
burn patients treated with powder or liquid forms of rhGH.
|
| Powdered rhGH
(n=14) | Liquid rhGH
(n=15) | P-value |
|---|
| Gender
(male/female) | 14/0 | 13/2 | 0.480 |
| Age (years) | 42 (25–64) | 41(20–64) | 0.813 |
| Body weight (kg) | 72.25 (62.5–89) | 69 (47–115) | 0.046 |
| Deep second degree
burn area (TBSA), % | 9 (0–35) | 19 (1–61) | 0.290 |
| Third degree burn
area (TBSA), % | 0 (0–32) | 3 (0–45) | 0.377 |
| BI | 26.7 (14.8–56.8) | 37.5 (12.0–53.3) | 0.290 |
PALB level
Over the 10 days of observation, PALB levels rose in
both groups after treatment, as shown in Table II. Following an initial fall of 3.1
(−55.5,60) mg/l on day 3 in the powder rhGH group compared to an
increase of 16.5 (−15.9,120) mg/l in the liquid rhGH group both
increased on day 5 by 24.5 (−265.4,107.6) mg/l in the powder rhGH
group and 42.7 (−30.6,123.2) mg/l in the liquid group compared to
baseline, and further increased by 65 (−259.3,122) mg/l in the
powder rhGH group and 88 (−41.7,23.5) mg/l in the liquid rhGH
group. Although the levels were not statistically different between
the groups, the differences from baseline were significant on day
10 (P=0.046).
 | Table II.Serum pre-albumin (PALB) in severe
burn patients treated with powder or liquid forms of recombinant
human growth factor (rhGH). |
Table II.
Serum pre-albumin (PALB) in severe
burn patients treated with powder or liquid forms of recombinant
human growth factor (rhGH).
|
| Baseline | Day 3 | Day 5 | Day 10 | Δ1 | Δ2 | Δ3 |
|---|
| PALB (mg/l) |
|
|
|
|
|
|
|
| Powder rhGH | 81.8 (55.4,364) | 92.6 (41,311) | 111.25
(67.2,189) | 145 (73,227) | −3.1 (−55.5,60) | 24.5
(−265.4,107.6) | 65 (−259.3,122) |
| Liquid rhGH | 72.5 (44.2,163) | 89.5 (58,283) | 97 (42.4,284) | 139 (53,336) | 16.5 (−15.9,120) | 42.7
(−30.6,123.2) | 88 (−41.7,235) |
| P-valuea | 0.077 | 0.880 | 0.591 | 0.505 | 0.063 | 0.477 | 0.046 |
CRP, fasting blood glucose level and
body weight
The CRP levels fell in both groups after treatment
(Table III). However, the levels and
the change from baseline did not reveal any significant differences
between the groups (all P>0.05). The fasting blood glucose
levels also fell in both groups following treatment, but not
significantly from baseline, nor between groups (all P>0.05;
Table III). Body weight was
significantly lower in the liquid rhGH group compared to the powder
rhGH group before treatment, and on days 5 and 10 (P=0.046, P=0.018
and P=0.006, respectively). However, the weight change from before
treatment was not significantly different between the 2 groups (day
5 difference, P=0.591; day 10 difference, P=0.400; Table III).
 | Table III.Clinical variables in severe burn
patients treated with powder or liquid forms of rhGH. |
Table III.
Clinical variables in severe burn
patients treated with powder or liquid forms of rhGH.
| Variable | Baseline | Day 5 | Day 10 | Δ1 | Δ2 |
|---|
| CRP (mg/l) |
|
|
|
|
|
| Powder
rhGH | 133.5 (61, 175) | 103 (21.8, 217) | 57.75 (17.9,
147) | −46 (−88, 155.8) | −58.5 (−133.7,
85.8) |
| Liquid
rhGH | 156 (39, 266) | 102 (5.2, 276) | 56.5 (1.9, 217) | −42 (−125, 21) | −91 (−218.6,
134) |
|
P-valuea | 0.201 | 0.880 | 0.914 | 0.715 | 0.400 |
| FPG (mmol/l) |
|
|
|
|
|
| Powder
rhGH | 146.5 (101, 245) | 123 (67, 217) | 115 (71, 228) | −24 (−76, 44) | −10.5 (−137, 99) |
| Liquid
rhGH | 137 (81, 255) | 143 (60, 312) | 110 (60, 333) | 18 (−181, 151) | −30 (−132, 194) |
|
P-valuea | 0.377 | 0.477 | 0.561 | 0.093 | 0.715 |
| BW (kg) |
|
|
|
|
|
| Powder
rhGH | 75.25 (63, 89) | 71.5 (59, 91) | 71.5 (61, 87) | −1.25 (−7, 4) | −2.25 (−9, 6) |
| Liquid
rhGH | 69 (47, 115) | 66 (51, 105.5) | 65 (48, 89) | −3 (−10, 4.5) | −3.5 (−26, 4) |
|
P-valuea | 0.046 | 0.018 | 0.006 | 0.591 | 0.400 |
Liver and renal function indexes
Among the 15 cases in the liquid rhGH group, there
were 7 cases of liver dysfunction prior to treatment and 6
following treatment; by contrast, among the 14 cases of powder rhGH
group, there were 6 cases of liver dysfunction prior to treatment
and 7 following treatment as shown in Table IV. The comparison of liver dysfunction
between the 2 groups before and after treatment did not demonstrate
any significant difference (both P>0.05). All 15 cases in the
liquid rhGH group had abnormal renal function prior to and
following treatment; for the 14 cases in the powder rhGH group, 14
had abnormal renal function prior to treatment and 13 following
treatment, as shown in Table IV.
 | Table IV.Liver and kidney function in severe
burn patients, treated with powdered or liquid forms of rhGH. |
Table IV.
Liver and kidney function in severe
burn patients, treated with powdered or liquid forms of rhGH.
|
| Baseline | Day 10 |
|---|
|
|
|
|
|---|
|
| Powder rhGH
n=14 | Liquid rhGH
n=15 | P-value | Powder rhGH
n=14 | Liquid rhGH
n=15 | P-value |
|---|
| Liver function, n
(%) |
|
|
|
|
|
|
|
Normal | 8 (53.3) | 8 (57.1) | 1.000 | 7 (50.0) | 9 (60.0) | 0.715 |
|
Abnormal | 6 (46.7) | 7 (42.9) |
| 7 (50.0) | 6 (40.0) |
|
| Kidney function, n
(%) |
|
|
|
|
|
|
|
Normal | 0 | 0 | – | 1 (7.1) | 0 | 0.483 |
|
Abnormal | 14 (100) | 15 (100) |
| 13 (92.9) | 15 (100) |
|
Wound healing time
Would healing time was 38.0 (19.0, 48.0) days in the
liquid rhGH group and 30.5 (15.0, 60.0) days in the powder rhGH
group, without any significant difference observed (P=0.270)
(Fig. 2).
Discussion
Clinically rhGH reduces hypercatabolism following
burn injuries, speeding up protein synthesis, improving negative
nitrogen balance, promoting burn wound and skin donor site healing,
and shortens length of hospital stay, and its wide use in treating
burn patients has been commonly recognized by physicians (12,13). Thus,
rhGH is one of the options available for treatment of severe burns
(20). However, it is unclear which
form of rhGH, liquid or powder, has better effects in patients with
severe burns. Despite the evidence that confirm the effects of
powder rhGH on burned patients (21).
Hence the present study evaluated the clinical effects of both
liquid and powder forms of rhGH on burned patients who needed
nutritional support.
Serum albumin, PALB and transferrin are directly
linked to nutritional status and have been regarded as traditional
indexes for evaluating nutritional status as well as nutritional
therapeutic effects (22). However,
these indexes are negatively associated with inflammation and
stress level. Albumin has a half-life up to 20 days, but is poorly
sensitive for short-term nutritional evaluation and predisposed to
exogenous protein input (22). Thus,
the present study aimed to observe the short-term effects of rhGH,
and albumin was not used as one of evaluation indexes. Instead,
PALB with half-life of 48 h was the main observational index since
it is more sensitive to the change of nutritional status (22). Statistically, when comparing between 2
groups, PALB change is more sensitive than the raw data of
different points (21). In the present
study, liquid rhGH took less time and was better at improving PALB
levels compared with powdered rhGH, supporting the suggestion that
rehydration of powdered rhGH may risk causing protein aggregation
(16), resulting in a lower ratio of
biologically active protein than those that have been stored in
solution. The two groups were given the same dosage of rhGH
calculated according to mg/kg/d, however, the liquid rhGH was
actually more potent. In other words, the liquid rhGH was more
clinically biologically active and more effective. This may be seen
as contradictory to previous studies that found liquid and powdered
rhGH to be bioequivalent (14,15). But those studies were more concerned
with pharmacokinetics and not the levels of PALB. In addition,
since the changes of inflammatory and stress indexes such as CRP
and blood glucose levels did not differ significantly between the
two groups, the results of the present study suggest that
inflammation and stress levels were not different in the two groups
and so should not have influenced the detection of PALB.
One factor that may be considered to be less
beneficial is the significantly lower total body weight in the
patients receiving liquid rhGH; however, this difference was clear
before treatment started and the loss in body weight from baseline
was not significant between the two groups. As the issue of
hypermetabolism lasts several months after the occurrence of the
severe burn, overall the body weight measurements would have more
value if they were investigated over a longer period of time as
rhGH has been shown in children with severe burns to improve growth
and body mass in studies that have lasted 12 months (23,24). It
would have been interesting to observe any significant difference
between the groups after many months.
Theoretically, since liquid rhGH is more spatially
stable and biologically active, it should be more effective in
promoting wound healing than powdered rhGH. As studies have
indicated that rhGH promotes wound healing including speeding up
burn wound and skin graft donor/recipient site healing, compared to
placebo for the control group (13,21).
Nevertheless, the results of the present study showed no
significant difference in wound healing time between the two
groups, and did not confirm the advantages of rhGH injection in
promoting wound healing. This may be due to the small sample size,
as both groups were receiving the benefit of rhGH, but if in the
case of the powdered form there was slightly less benefit, the
differences may have been too small to see in a study of this
kind.
The present study has some limitations. A limited
number of cases were included, and some of the indexes especially
wound healing time were not significantly different between the two
groups. After a comprehensive analysis of our data, we primarily
draw on the conclusion that rhGH injection is faster acting and
more effective in improving the nutritional status of burn
patients, but it still should be supported by a large-sample,
multicenter clinical trial in the future.
In conclusion, the liquid form of rhGH may be more
beneficial to patients with severe burns requiring nutritional
support than the powdered form.
Acknowledgements
The present study was supported by special clinical
research fund of Zhejiang Medical Association (grant no.
2008ZYC13).
References
|
1
|
Palmieri TL, Przkora R, Meyer WJ III and
Carrougher GJ: Measuring burn injury outcomes. Surg Clin North Am.
94:909–916. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Spanholtz TA, Theodorou P, Amini P and
Spilker G: Severe burn injuries: Acute and long-term treatment.
Dtsch Arztebl Int. 106:607–613. 2009.PubMed/NCBI
|
|
3
|
Al-Mousawi AM, Mecott-Rivera GA, Jeschke
MG and Herndon DN: Burn teams and burn centers: The importance of a
comprehensive team approach to burn care. Clin Plast Surg.
36:547–554. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Williams FN, Herndon DN and Jeschke MG:
The hypermetabolic response to burn injury and interventions to
modify this response. Clin Plast Surg. 36:583–596. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Atiyeh BS, Gunn SW and Dibo SA:
Nutritional and pharmacological modulation of the metabolic
response of severely burned patients: Review of the literature
(Part II)*. Ann Burns Fire Disasters. 21:119–123. 2008.PubMed/NCBI
|
|
6
|
Rojas Y, Finnerty CC, Radhakrishnan RS and
Herndon DN: Burns: An update on current pharmacotherapy. Expert
Opin Pharmacother. 13:2485–2494. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Blizzard RM: History of growth hormone
therapy. Indian J Pediatr. 79:87–91. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hwang IT: Efficacy and safety of growth
hormone treatment for children born small for gestational age.
Korean J Pediatr. 57:379–383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fukuda I, Hizuka N, Muraoka T and Ichihara
A: Adult growth hormone deficiency: Current concepts. Neurol Med
Chir (Tokyo). 54:599–605. 2014. View Article : Google Scholar
|
|
10
|
Arce VM, Devesa P and Devesa J: Role of
growth hormone (GH) in the treatment on neural diseases: From
neuroprotection to neural repair. Neurosci Res. 76:179–186. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kemp SF and Frindik JP: Emerging options
in growth hormone therapy: An update. Drug Des Devel Ther.
5:411–419. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen GX and Han CM: Influence of
recombinant human growth hormone on the prognosis of patients with
severe burns a prospective multi-center clinical trial. Zhonghua
Shao Shang Za Zhi. 21(347): 92005.(In Chinese). PubMed/NCBI
|
|
13
|
Breederveld RS and Tuinebreijer WE:
Recombinant human growth hormone for treating burns and donor
sites. Cochrane Database Syst Rev. 15:CD0089902012.
|
|
14
|
Liedert B, Forssmann U, Wolna P, Golob M
and Kovar A: Comparison of the pharmacokinetics, safety and
tolerability of two concentrations of a new liquid recombinant
human growth hormone formulation versus the freeze-dried
formulation. BMC Clin Pharmacol. 10:142010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fuhr U, Tuculanu D, Berghout A, Balser S,
Schwebig A and Saenger P: Bioequivalence between novel ready-to-use
liquid formulations of the recombinant human GH Omnitrope and the
original lyophilized formulations for reconstitution of Omnitrope
and Genotropin. Eur J Endocrinol. 162:1051–1058. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wiesbauer J, Prassl R and Nidetzky B:
Renewal of the air-water interface as a critical system parameter
of protein stability: Aggregation of the human growth hormone and
its prevention by surface-active compounds. Langmuir.
29:15240–15250. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Iyoda K, Moriwake T, Seino Y and Niimi H:
The clinical usefulness of liquid human growth hormone (hGH)
(Norditropin SimpleXx in the treatment of GH deficiency. Horm Res.
51(Suppl 3): S113–S115. 1999. View Article : Google Scholar
|
|
18
|
Rousseau AF, Losser MR, Ichai C and Berger
MM: ESPEN endorsed recommendations: Nutritional therapy in major
burns. Clin Nutr. 32:497–502. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jie W and Wang S: A nutritional formula
suitable for adults' burns in China. Chinese Journal of Surgery.
5:262–266. 1992.
|
|
20
|
Gauglitz GG, Williams FN, Herndon DN and
Jeschke MG: Burns: Where are we standing with propranolol,
oxandrolone, recombinant human growth hormone, and the new incretin
analogues? Curr Opin Clin Nutr Metab Care. 14:176–181. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Herndon DN, Barrow RE, Kunkel KR,
Broemeling L and Rutan RL: Effects of recombinant human growth
hormone on donor-site healing in severely burned children. Ann
Surg. 212:424–429; discussion 430–421. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cynober L: Laboratory measurements in
clinical nutrition. In: Basics in clinical nutrition (4th edition).
Sobotka L (eds) and Wei C (translator). Shanghai Jiaotong
University Press. (Shanghai). 398–402. 2013.(In Chinese).
|
|
23
|
Branski LK, Herndon DN, Barrow RE, Kulp
GA, Klein GL, Suman OE, Przkora R, Meyer W III, Huang T, Lee JO, et
al: Randomized controlled trial to determine the efficacy of
long-term growth hormone treatment in severely burned children. Ann
Surg. 250:514–523. 2009.PubMed/NCBI
|
|
24
|
Przkora R, Herndon DN, Suman OE, Jeschke
MG, Meyer WJ, Chinkes DL, Mlcak RP, Huang T and Barrow RE:
Beneficial effects of extended growth hormone treatment after
hospital discharge in pediatric burn patients. Ann Surg.
243:796–801; discussion 801–793. 2006. View Article : Google Scholar : PubMed/NCBI
|