Introduction
Gallstone disease is one of the most common
gastrointestinal disorders encountered in clinical practice
(1,2).
Gallstones are often associated with the bile duct or intrahepatic
stones, which have difficulty clearing the biliary system and are
associated with a high rate of recurrence (3). Thus, prevention of the formation and
recurrence of gallstones is necessary (4–7).
Gallstones are formed for many reasons. One cause is
inflammation, which often changes the bile acid composition and
accelerates stone formation (8,9). Previous
findings have shown that inflammatory cytokines such as
lipopolysaccharide (LPS), tumor necrosis factor (TNF), and
interleukin-1 (IL-1) can inhibit the production of bile acid
through classic and bypass pathways (9–11).
Inflammatory cytokines can disturb cholate transport in the
hepatocytes and reduce the secretion of bile salts and
phospholipids (9).
Apart from inflammation, an imbalance in bile
components such as cholesterol, bile acids, and lecithin, also
contributes to gallstone formation. As the concentration of
cholesterol in bile increases, the bile begins to be saturated with
cholesterol, and at a certain level, the cholesterol begins to
crystallize. McNeilly et al (12) found that bile acids are involved in the
regulation of glucocorticoid metabolism within the liver of female
patients (12,13). The increased level of bile acids during
cholestasis may induce the downregulation of the
hypothalamic-pituitary-adrenal (HPA) axis. The HPA axis plays a
role in the body's response to stress by mediating the secretion of
the adrenocorticotropic hormone (ACTH). Clinical studies have shown
that gallstone disease is associated with dysregulation of the HPA
axis with abnormal secretion of serum corticosterone (14). Thus, homeostasis of the HPA axis is
important for the prevention of pigment gallstones.
In traditional Chinese medicine, gallstone refers to
‘rib pain’ or ‘liver bilges’, and the treatment of gallstones is
based on the principle of ‘liver-dispersing and bile discharging,’
i.e., stone clearance and restoration of liver function. Lidan
Granule (LDG) is formulated on the basis of these principles and
consists of 15 types of Chinese herbs. It has been used for many
years in clinical practice to treat and prevent gallstones
(4,15,16). The aim
of the present study was to investigate the role of LDG in the
treatment and prevention of pigment gallstones and to explore the
underlying mechanisms using a guinea pig model.
Materials and methods
Drugs and reagents
LDG was provided by the Department of the
Integrative Medicine, Huashan Hospital, Fudan University (Shanghai,
China), and the components and efficacy of LDG are described in
Table I. Ursodeoxycholic acid (UDCA)
was purchased from Sanwei Changjiang Biochemical Pharmaceutical
Factory (Shanghai, China), and the enzyme-linked immunosorbent
assay (ELISA) kits were obtained from eBioscience, Inc. (San Diego,
CA, USA). The lithogenic diet food (the components of which are
listed in Table II) was purchased
from Trophic Animal Feed High-tech Co., Ltd. (Jiangsu, China). LDG
and UDCA were diluted in sterile saline. Animals in the LDG-H (2
g/kg/day, determined by the dosage used for humans clinically),
LDG-L (1 g/kg/day), and UDCA (50 mg/kg/day) groups were fed twice
per day.
 | Table I.Components and efficacy of LDG. |
Table I.
Components and efficacy of LDG.
| English | Latin | Species | Effect |
|---|
| Oriental
wormwood | Artemisia
capillaris Thunb | Origanum
L. | Clear away heat and
promote diuresis |
| Hawthorn fruit | Crataegus
pinnatifida Bunge | Crataegus
L. | Aid digestion,
increase appetite |
| Rice sprout | Setaria
italica | Rice | Aid digestion,
increase appetite |
| Germinated
barley | Hordeurn
vulgare L. | Gramineae
L. | Aid digestion,
increase appetite |
| Green orange
peel | Citrus
reticulata Blanco | Citrus L. | Relieve food
retention |
| Tangerine peel | Citrus
reticulata Blanco | Citrus L. | Dispel moisture and
eliminate phlegm |
| Medicated leaven | Massa Medicata
Fermentata | Powder | Digestion |
| Cyperus tuber | Cyperus
rotundus L. | Cyperus
Linn. | Relieve chest and
abdominal pain |
| Radish seed | Semen Raphani | R. sativus
L. | Relieve abdominal
distention |
| Caulis perillae | Perilla
frutescens (L.) Britton. | P. frutescens
L. Britt. | Relieve nausea |
| Turmeric root | Curcuma longa
L. | Curcuma
L. | Relieve abdominal
pain |
| Rhubarb | Rheum
palmatum L. | Rhubarb
L. | Clear
heat-fire |
| Pinellia tube | Pinellia
ternata (Thunb.) Breit. | Pinellia
Ten. | Antiemetic
effects |
| Chinese honeylocust
fruit | Gleditsia
sinensis Lam. | Gleditsia
Linn | Aid digestion;
increase appetite |
 | Table II.Components of lithogenic diet
food. |
Table II.
Components of lithogenic diet
food.
| Name | Dosage (g/kg) | Name | Dosage (g/kg) | Name | Dosage (g/kg) |
|---|
| Corn flour | 136.3 | Alfalfa meal | 416.5 | Cellulose | 20 |
| Salt | 10 | Whole wheat |
90.9 | Cholesterol | 1 |
| Flour |
90.7 | Yeast powder | 10 | Vitamin C |
0.05 |
| Soy bean flour |
90.9 | Lard oil | 20 | Cholic acid |
0.4 |
| Fish meal |
63.6 | Sucrose | 20 | Casein | 20 |
Animals and treatments
One hundred male guinea pigs (weighing 230–250 g)
were obtained from the Shanghai SLAC Laboratory Animal Co., Ltd.
(Shanghai, China). Animals were maintained in a temperature-
(20±2°C) and humidity- (50–60%) controlled facility upon arrival on
a 12-h light/dark cycle (lights on from 7:00 a.m. to 7:00 p.m.) and
given access to food and water ad libitum. Animals were
randomly divided into five groups. The control group mice were fed
a chow diet, whereas mice in the other groups were fed a high
cholesterol lithogenic diet (17,18). The
LDG-H group was fed with a lithogenic diet and LDG (2 g/kg/day),
given orally, and the LDG-L group was fed the lithogenic diet and
LDG (1 g/kg/day), given orally (Fig.
1). The UDCA group was fed UDCA (50 mg/kg/day), and the model
group was fed with lithogenic diet and saline. Experiment animals
were housed for a minimum of 7 days prior to the start of the
experiment to adapt to the environment. Experiments were conducted
in accordance with the guidelines of the Animal Care and Use
Committee of Fudan University.
Serum, bile, and histological sample
preparation
The animals were sacrificed by an overdose of sodium
pentobarbital (50 mg/kg body weight, i.p.), and blood samples were
obtained from the aorta abdominal and clotted for 2 h at room
temperature before centrifugation (1,006.2 × g at 4°C for 10 min).
The supernatant was subsequently collected and stored at −80°C for
measurement of the levels of IL-6, IL-1, TNF-α, and cortisol (CORT)
in the peripheral blood. The guinea pigs were decapitated, and the
brain tissues were removed and stored on ice. The bilateral
hippocampus and hypophysis were rapidly and carefully removed using
curved tweezers. Once snap frozen in liquid nitrogen, the brain
tissue samples were stored at −80°C until use. The adrenal glands
of guinea pigs were also removed and weighed carefully.
Bile samples were obtained from dissected gall
bladders, stored at −20°C, and diluted 1:5 with distilled water
prior to analysis by ultra-performance liquid chromatography-mass
spectrometry (UPLC-MS).
Physical state score (PSS)
The physical states of the guinea pigs in the
different groups were assessed as previously described (19). The physical states of guinea pigs were
evaluated weekly until the end of the experiment. The coat state
was recorded on a scale from 1 to 3 as follows: guinea pigs in a
good state were scored as 3 (the fur was smooth with no tousling),
animals in a bad state were scored as 1 (dirty fur on most of the
body), and those with a coat state between 1 and 3 were scored as
2. Each measurement was scored by another experimenter blinded to
the treatment group.
Serum CORT, corticotrophin-releasing
hormone (CRH), IL-6, and ACTH ELISA
Serum levels of CORT, IL-6, IL-1, and TNF-α were
measured using a commercially available enzyme competitive ELISA
test kit following the manufacturer's instructions (eBioscience,
Inc.).
Statistical analysis
Data were presented as mean ± standard error of the
mean and analyzed with SPSS 17.0 software (SPSS, Inc., Chicago, IL,
USA). The statistical significance of differences between the means
of groups was determinant by one-way analysis of variance (ANOVA)
followed by the least significant difference (LSD) for post-hoc
comparisons. The rate of different groups were compared with the
Chi-square test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect on weight, PSS change, and the
rate of gallstone formation
During 6 weeks of exposure to a lithogenic diet,
guinea pigs in the model group showed a mild increase in weight,
whereas those on low concentration (1 g/kg) or high concentration
(2 g/kg) of LDG showed an obvious increase in weight after 2 weeks
(Fig. 2A). Six weeks later, the
differences in weights between the model and LDG groups were
significant (both P<0.01). The PSS score of the model group
guinea pigs decreased during the 6-week experiment, and the PSS
score was statistically different between the control and model
groups (P<0.01) beyond the second week. The PSS scores for the
treatment groups were reduced significantly during the fourth and
fifth weeks (Fig. 2B).
Six weeks later, the rate of gallstone formation was
5.56% among 18 guinea pigs in the control group (Table III). For the model group, the stone
formation rate was 81.25%, which was significantly higher than that
in the control group (P<0.01; Table
III). Four of 13 guinea pigs developed gallstones in the UDCA
group (P<0.05), and the stone formation rates in the LDG-H and
LDG-L groups were 14.29% (P<0.01) and 21.43% (P<0.01),
respectively. The rates were lower than the stone formation rate
observed in the model group (Table
III).
 | Table III.Rates of gallstone formation in the
different groups. |
Table III.
Rates of gallstone formation in the
different groups.
| Group | No. of animals | No. of
gallstones | Rate of gallstone
formation (%) |
|---|
| Control | 18 | 1 | 5.56b |
| Model | 16 | 13 | 81.25 |
| LDG-H | 14 | 2 | 14.29b |
| LDG-L | 14 | 3 | 21.43b |
| UDCA | 13 | 4 | 30.77a |
Effect on histopathological assessment
of liver tissues
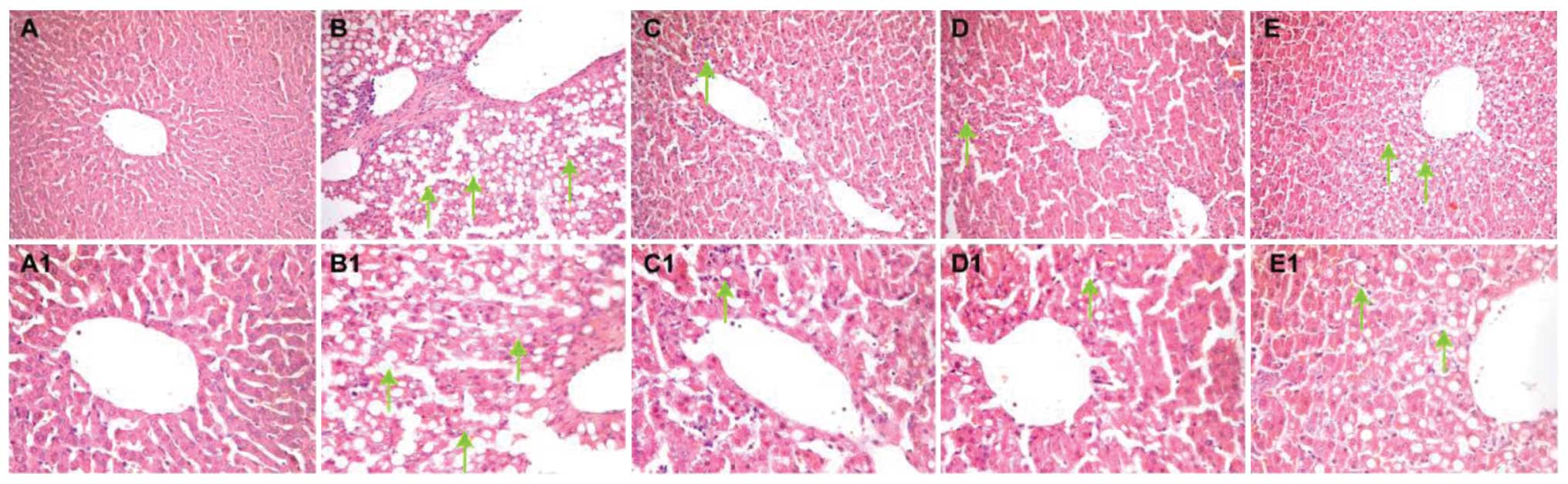
After 6 weeks of treatment, normal hepatocytes with
blue-stained nuclei located in the center of the cell and arranged
in cord-like patterns were evident in the control group (Fig. 3A and A1). In the pigment gallstone
model group, hepatic steatosis in the form of fat globules in the
hepatocytes with an eccentric nucleus and loose cytoplasm was
observed. Many inflammatory cells were dispersed around the central
vein, and small Mallory bodies were identified at higher
magnification (Fig. 3B and B1).
Compared to the control group, the LDG-H (2 g/kg/day; Fig. 3C and C1) and LDG-L (1 g/kg/day;
Fig. 3D and D1) groups showed fewer
inflammatory cells, and no obvious ballooning degeneration. Similar
findings were observed in the UDCA group (Fig. 3E and E1), except for the presence of
ballooning degeneration in a few areas around the central vein.
Effect of LDG on HPA axis
activation
As shown in Fig. 4, 6
weeks of the lithogenic diet increased the CORT concentration in
serum (P<0.01) compared to that in the control group and
decreased the level of CRH in the hypothalamus (P<0.01) and
expression of ACTH in the hypophysis (P<0.01). The lithogenic
diet also had some effect on the weight of the adrenal gland
(P<0.01). Six weeks of treatment with LDG (2 and 1 g/kg/day)
significantly improved the disturbance in the HPA axis caused by
the lithogenic diet. Similarly, UDCA had a positive effect on the
level of CORT in the peripheral blood (P<0.01) and the weight of
the adrenal gland (P<0.01).
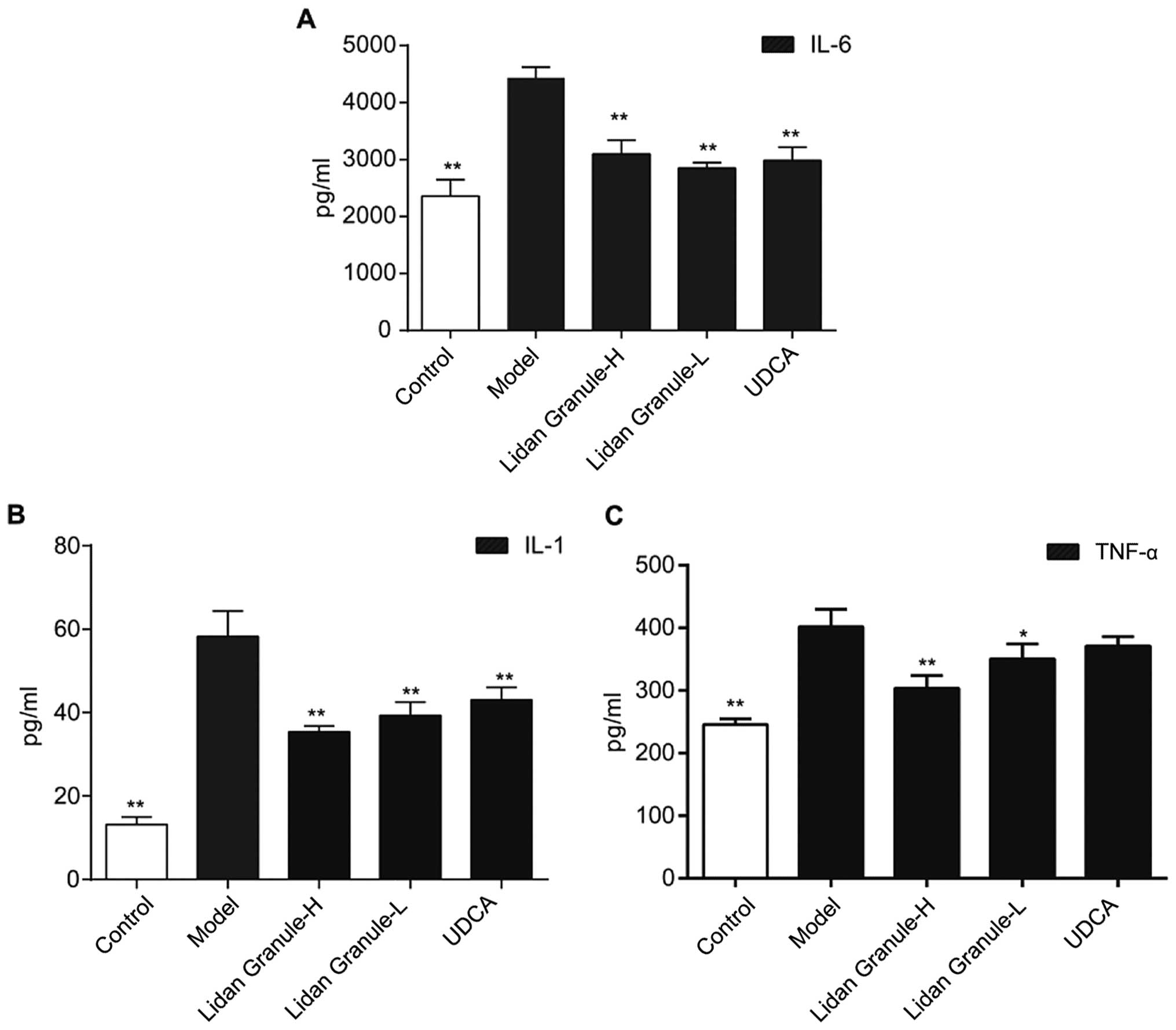
Effect of LDG on IL-6, IL-1, and TNF-α
levels in peripheral blood
The levels of IL-6, IL-1, and TNF-α in the
peripheral blood were increased after 6 weeks on a lithogenic diet.
Following treatment with high- or low-dose LDG, decreases in the
serum concentration of IL-6 (Fig. 5A;
P<0.01), IL-1 (Fig. 5B; P<0.01),
and TNF-α (Fig. 5C; P<0.01) were
observed.
Effect of LDG on the bile
components
As expected, the lithogenic diet increased the
concentration of cholesterol, direct bilirubin (DBIL), and indirect
bilirubin (IBIL) in the bile and reduced the bile concentrations of
bile acids and phospholipids (Fig. 6A and
D). However, LDG significantly reduced the cholesterol, IBIL,
and DBIL concentrations in the bile (Fig.
6B; P<0.01). At the same time, LDG increased the acid
concentration in the bile (Fig. 6A;
P<0.01) after 6 weeks of treatment.
Discussion
The results of the present study suggest that a
lithogenic diet can induce the formation of gallstones and disturb
the HPA axis in guinea pigs, whereas LDG can ameliorate the effect
of a lithogenic diet on the HPA axis and bile components.
Most gallstones are formed in the bile duct system,
where they can block the bile duct and lead to cholestasis
(20). During the disease process,
cholestasis results in a decrease in liver function, and also,
endocrine function (21). The diet is
one of the most common pathogenic factors for gallstone formation.
Thus, in our study, a lithogenic diet was given to guinea pigs for
6 weeks to induce the formation of pigment gallstones (22). After 6 weeks, serum IL-6, IL-1, and
TNF-α levels in the model group were found to be elevated, while
the histopathological examination of the livers showed hepatic
steatosis and loose cytoplasm, with nuclei pushed to the edge of
cells and inflammatory cell infiltration occurring. These findings
confirm that the pigment gallstone model is suitable for studying
the pathophysiology of pigment stone formation.
LDG has been used as a routine treatment for
gallstones for many years, and clinical studies have demonstrated
that LDG can promote biliary excretion and litholysis (15). LDG is composed of 15 types of Chinese
herbs, namely, oriental wormwood, hawthorn fruit, rice sprout,
germinated barley, green orange peel, tangerine peel, medicated
leaven, cyperus tuber, radish seed, perilla stem, turmeric root,
bitter orange, pinellia tube, and Chinese honey locust fruit, which
is based on the Chinese medicinal principle of smoothening liver
function, dredging the gallbladder, harmonizing the stomach, and
strengthening the spleen (23).
The neuroendocrine system plays an important role in
maintaining homeostasis (14). A
clinical study has shown that gallstone formation in patients is
always followed by endocrine disturbance, and changes in the
corticosterone levels may indicate the presence of gallstone
disease (24). In our study, after 6
weeks on a lithogenic diet, the CORT level in the model group was
increased along with disruption of the HPA axis and a change in the
weight of the adrenal gland. LDG treatment can ameliorate the
secretion of proteins in the HPA axis, which means that LDG can
regulate the neuroendocrine system during the process of gallstone
formation.
IL-6 is one of the most important inflammatory
biomarkers of cholelith disease (9,25). In our
study, serum IL-6 levels in the model group were elevated. Previous
findings have shown that IL-6 in combination with other
inflammatory cytokines or alone can influence the action of the HPA
axis at different levels (26), which
may explain the reason HPA axis dysfunction being considered in
cholelith disease. LDG can improve the inflammatory state of guinea
pigs by reducing the amounts of serum IL-6, IL-1, and TNF-α, and
improving the function of the neuroendocrine system at the same
time.
Bile components are an important factor for the
clinical diagnosis and differential diagnosis of gallstone disease
(27,28). In our study, the DBIL of the model
group was higher than that of other groups, and it was in agreement
with the clinical characteristics of silt-type cholesterol
gallstones. After 6 weeks of LDG treatment, the DBIL was reduced
significantly in the study group, which proves the dredging action
of LDG.
In conclusion, the present study confirmed that
cholelith disease is always accompanied by dysregulation of the HPA
axis. LDG cannot only regulate the excretion of inflammatory
factors but also improve the function of the neuroendocrine system,
and improve the composition of the bile. LDG may influence the
lithogenic character of bile via multiple pathways and has a
definite role in the prevention and treatment of
cholelithiasis.
Acknowledgements
The present study was funded by grants from the
Development Project of Shanghai Peak Disciplines-Integrated Chinese
and Western Medicine, the Doctoral Program of Higher Education of
China (no. 20120071120076), the National Science Foundation for
Distinguished Young Scholars of China (grant no. 81403148), and the
National Science Foundation for Distinguished Young Scholars of
China (grant no. 81501180).
References
|
1
|
Erichsen R, Frøslev T, Lash TL, Pedersen L
and Sørensen HT: Long-term statin use and the risk of gallstone
disease: a population-based case-control study. Am J Epidemiol.
173:162–170. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stinton LM and Shaffer EA: Epidemiology of
gallbladder disease: cholelithiasis and cancer. Gut Liver.
6:172–187. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Markar SR, Karthikesalingam A, Thrumurthy
S, Muirhead L, Kinross J and Paraskeva P: Single-incision
laparoscopic surgery (SILS) vs. conventional multiport
cholecystectomy: systematic review and meta-analysis. Surg Endosc.
26:1205–1213. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang SB and Fang BJ: Effects of Yanggan
Lidan Granule on rate of gallstone formation and content of plasma
cholecystokinin in guinea pigs with induced cholesterol gallstones.
Zhong Xi Yi Jie He Xue Bao. 6:405–408. 2008.(In Chinese).
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mann KS, Welfare E, Hughes D, et al:
Significant service reconfiguration is required to definitively
treat gallstone pancreatitis and prevent serious complications. Br
J Surg. 99:164–165. 2012.PubMed/NCBI
|
|
6
|
Smelt AH: Triglycerides and gallstone
formation. Clin Chim Acta. 411:1625–1631. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
You SF, Zheng PY, Ji G, Wei HF, Zhao J and
Zhu PT: Protective effects of yanggan lidan granules on carbon
tetrachloride-induced liver damage in mice. Zhong Xi Yi Jie He Xue
Bao. 3:470–472. 2005.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang SN, Yeh YT, Wang ST, Chuang SC, Wang
CL, Yu ML and Lee KT: Visfatin - a proinflammatory adipokine-in
gallstone disease. Am J Surg. 199:459–465. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maurer KJ, Carey MC and Fox JG: Roles of
infection, inflammation, and the immune system in cholesterol
gallstone formation. Gastroenterology. 136:425–440. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kasprzak A, Szmyt M, Malkowski W,
Przybyszewska W, Helak-Łapaj C, Seraszek-Jaros A, Surdacka A,
Małkowska-Lanzafame A and Kaczmarek E: Analysis of
immunohistochemical expression of proinflammatory cytokines (IL-1α,
IL-6, and TNF-α) in gallbladder mucosa: Comparative study in acute
and chronic calculous cholecystitis. Folia Morphol (Warsz).
74:65–72. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chai J, He Y, Cai SY, Jiang Z, Wang H, Li
Q, Chen L, Peng Z, He X, Wu X, et al: Elevated hepatic multidrug
resistance-associated protein 3/ATP-binding cassette subfamily C 3
expression in human obstructive cholestasis is mediated through
tumor necrosis factor alpha and c-Jun NH2-terminal
kinase/stress-activated protein kinase-signaling pathway.
Hepatology. 55:1485–1494. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McNeilly AD, Macfarlane DP, O'Flaherty E,
Livingstone DE, Mitić T, McConnell KM, McKenzie SM, Davies E,
Reynolds RM, Thiesson HC, et al: Bile acids modulate glucocorticoid
metabolism and the hypothalamic-pituitary-adrenal axis in
obstructive jaundice. J Hepatol. 52:705–711. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Soliman HM, Abd El-Haleem MR and El
Tarhouny SA: Histomorphometrical and Electron Microscopic Study of
Adrenocorticocytes Following Surgically Induced Extrahepatic
Biliary Obstruction in Adult Female Albino Rats:
Histomorphometrical and electron microscopic study of
adrenocorticocytes following surgically induced extrahepatic
biliary obstruction in adult female albino rats. Folia Biol
(Praha). 61:14–25. 2015.PubMed/NCBI
|
|
14
|
Rose AJ, Berriel Díaz M, Reimann A,
Klement J, Walcher T, Krones-Herzig A, Strobel O, Werner J, Peters
A, Kleyman A, et al: Molecular control of systemic bile acid
homeostasis by the liver glucocorticoid receptor. Cell Metab.
14:123–130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiang Y, Chen JH, Cai D and Ma BJ: Effect
of Lidan Granule on bile lithogenesis in patients with
choledocholithiasis combined with cholecystolithiasis. Chin J
Integr Med. 14:142–144. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zuo YT, Gao WY, Jia W, Duan HQ and Xiao
PG: Prevention and treatment of cholelithiasis by traditional
Chinese medicine. Zhongguo Zhong Yao Za Zhi. 29:831–833, 910.
2004.(In Chinese). PubMed/NCBI
|
|
17
|
Wang HH, Portincasa P, Mendez-Sanchez N,
Uribe M and Wang DQ: Effect of ezetimibe on the prevention and
dissolution of cholesterol gallstones. Gastroenterology.
134:2101–2110. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song XY, Xu S, Hu JF, Tang J, Chu SF, Liu
H, Han N, Li JW, Zhang DM, Li YT, et al: Piperine prevents
cholesterol gallstones formation in mice. Eur J Pharmacol.
751:112–117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alonso R, Griebel G, Pavone G, Stemmelin
J, Le Fur G and Soubrié P: Blockade of CRF(1) or V(1b) receptors
reverses stress-induced suppression of neurogenesis in a mouse
model of depression. Mol Psychiatry. 9:278–286, 224. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu L, Aili A, Zhang C, Saiding A and
Abudureyimu K: Prevalence of and risk factors for gallstones in
Uighur and Han Chinese. World J Gastroenterol. 20:14942–14949.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wittenburg H: Hereditary liver disease:
gallstones. Best Pract Res Clin Gastroenterol. 24:747–756. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Méndez-Sánchez N, Zamora-Valdés D,
Chávez-Tapia NC and Uribe M: Role of diet in cholesterol gallstone
formation. Clin Chim Acta. 376:1–8. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fang BJ, Zhou S, Pei XJ, Huang JY, Chen
BJ, Geng Y and Yang LK: Effects of Yanggan Lidan Granule on insulin
resistance in guinea pigs with induced cholesterol gallstones.
Zhong Xi Yi Jie He Xue Bao. 7:1159–1163. 2009.(In Chinese).
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
No authors listed: Current bibliographies
of neuropeptides prepared by the University of Sheffield Biomedical
Information Service. Neuropeptides. 29:B37–B49. 1995.PubMed/NCBI
|
|
25
|
Yun JW, Kim JS, Choi WS, et al: Inhibition
of sphingolipid pathway affects the formation of cholesterol
gallstone by the modulation of Il-6/Stat3 pathway in mice model. J
Gastroenterol Hepatol. 27:85–86. 2012.PubMed/NCBI
|
|
26
|
Rosenblat JD, Cha DS, Mansur RB and
McIntyre RS: Inflamed moods: a review of the interactions between
inflammation and mood disorders. Prog Neuropsychopharmacol Biol
Psychiatry. 53:23–34. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peng Y, Yang Y, Liu Y, Nie Y, Xu P, Xia B,
Tian F and Sun Q: Cholesterol gallstones and bile host diverse
bacterial communities with potential to promote the formation of
gallstones. Microb Pathog. 83–84:57–63. 2015. View Article : Google Scholar
|
|
28
|
Halilbasic E, Claudel T and Trauner M:
Bile acid transporters and regulatory nuclear receptors in the
liver and beyond. J Hepatol. 58:155–168. 2013. View Article : Google Scholar : PubMed/NCBI
|