Introduction
Osteoporosis is a well-known disease and caused 9
million osteoporotic fractures worldwide in the year 2000 (1). Osteoporotic fractures occurring at the
spine and the forearm are associated with significant morbidity,
but the most serious consequences arise in patients with hip
fractures. The mortality rate is 20% in the first year following
hip fracture (2). Among those who
survive this period, 1 in 5 requires nursing home care (3). Thus, osteoporosis is associated with not
only morbidity, but also decline in the quality of life.
Osteoporosis is classified as primary osteoporosis induced by
menopause or aging and secondary osteoporosis. Well-known causes of
secondary osteoporosis include, endocrine disorders, such as
Cushing's syndrome, hypogonadism, hyperthyroidism,
hyperparathyroidism and diabetes mellitus (4). Gastrointestinal diseases, such as
inflammatory bowel disease (5),
rheumatoid arthritis (6) and myeloma
bone disease (7), also induce
osteoporosis. In addition, the incidence of osteoporosis following
gastrectomy has become a clinical issue. Although numerous studies
have examined bone metabolic disorders following gastrectomy since
it was first reported by Sarasin (8),
the pathophysiology and the treatment of these disorders have not
been fully elucidated. Based on experimental results using rat
models, general nutritional deficiencies (9), calcium malabsorption (10), vitamin D deficiency (11), loss of gastric acid (12) and secondary hyperparathyroidism
(13) have been suggested as possible
causes of bone metabolic disorder following gastrectomy. However,
fully understanding the morbidity and developing clinical therapies
for skeletal disorders is critical for improving patient quality of
life.
Recent epidemiological studies have shown that
long-term therapy with proton pump inhibitors (PPIs) significantly
increases the risk of osteoporosis and pathological hip fracture in
patients with gastroesophageal reflux disease (14). It is thought that PPIs reduce the
production and secretion of hydrochloric acid in stomach, increase
the pH in the stomach and inhibit absorption of insoluble calcium
in the small intestine, thus leading to malabsorption of calcium
phosphate and bone metabolism disorder (15,16). PPIs
also reportedly inhibit bone resorption by osteoclasts (17,18).
However, the irreversible PPI mediated by the PPIs requires a
specific pH environment. PPIs are all prodrugs that require two
sequential protonation steps for activation (19). In the first step, a pyridine radical is
activated, and in the second step, a benzimidazole radical is
activated. The first step is required for accumulation of the PPI
in the intracellular secretory canaliculus, and the second step is
necessary for binding of the PPI with the proton pump. The pKa in
the second step does not significantly differ (<1) among the
various PPIs (such as rabeprazole, lansoprazole and omeprazole),
whereas the pKa for the first step with rabeprazole (4.53) is
higher compared to that for lansoprazole (3.83) and omeprazole
(4.06) (20). This suggests that
rabeprazole can efficiently combine with the proton pump to produce
an immediate effect (21). For the two
sequential protonation steps, the proton pump inhibitory action of
the PPIs is extremely site-specific. In addition to the secretory
canaliculi of the gastric parietal cells, the osteoclastic
resorption vacuole may be the only other place in which proton pump
inhibition by PPIs is known to occur. Clinical studies have
supported the theory that the short-term use of a PPI reduces bone
resorption markers (18,22). There are conflicting data regarding the
effect of PPIs on bone metabolism, with little known concerning the
effects of PPIs on osteoclasts and bone resorption.
Therefore, the present study analyzed the effect of
PPIs on bone metabolism following total gastrectomy (TG) in a rat
model of osteoporosis. Using the rat TG model, poor calcium
absorption that is observed during gastric anacidity was reproduced
and the confounding antisecretory activity of the PPI was excluded
to determine the specific effect of a PPI on osteoclasts.
Materials and methods
Ethical approval
All the procedures performed in the studies
involving animals adhered to the Standard Guidelines for Animal
Experiments at Kanazawa University (Kanazawa, Ishikawa, Japan; date
of issue: May 18, 2012; registration number: AP-122484).
Animals
In total, 75 male Wistar rats (Charles River
Laboratories Japan, Inc., Kanagawa, Japan) that were 12 weeks of
age were used for the experiments. The rats were housed three to a
cage, and were maintained at a room temperature of 22±3°C and
humidity of 55±5% with a 12-h light-dark cycle. The rats were
provided a standard solid chow, CRF-1 (Charles River Laboratories
Japan, Inc.) and tap water. The animal welfare committee of
Kanazawa University approved the experiments.
Drugs
The effects of the PPI rabeprazole on bone
metabolism were compared with those of minodronic acid, a
bisphosphonate clinically administered in the treatment of patients
with osteoporosis. Minodronic acid was obtained from Astellas
Pharma, Inc. (Tokyo, Japan), and rabeprazple was obtained from
Eisai Co., Ltd. (Tokyo, Japan).
Experimental design
The rats were randomly divided into the following
four groups: i) Sham-surgery (n=15); ii) TG control (n=20); iii) TG
plus rabeprazole (30 mg/kg) administered three times per week
(n=20); iv) TG plus minodronic acid (0.04 mg/kg/day) (n=20).
Beginning 4 weeks after surgery, rabeprazole was administered
subcutaneously three times per week for 18 weeks to the rats in the
TG plus rabeprazole group, and minodronic acid was administered
subcutaneously daily for 18 weeks to the rats in the TG plus
minodronic acid group. The rationale for the dose of rabeprazole
used was based on prior studies, which showed that a subcutaneous
dose of rabeprazole at 30 mg/kg to rats reduced acid secretion by
100% within 4 h, with the return of acid secretion to normal levels
at 3 days (23,24).
Surgery
After 24 h of fasting, the rats were anesthetized
with intraperitoneal injections of medetomidine, midazolam and
butorphanol. TG using the reconstructed Roux-en-Y method was
performed through an upper middle incision. The duodenal stump was
closed with sutures. The jejunum was amputated ~6 cm distal to the
ligament of Treitz. The esophageal stump was anastomosed to the
anal side of amputated jejunum in an end-to-side manner. The
jejunojejunostomy was performed in a side-to-side manner.
Intestinal anastomosis was performed with interrupted
full-thickness stitches using 7-0 monofilament suture. The rats had
free access to water and food beginning 24 h after surgery.
Autopsy
Blood samples were obtained and the animals were
sacrificed by exsanguination under isoflurane anesthesia 22 weeks
after the surgery. The serum samples were immediately separated
from the blood by centrifugation at 1,000 × g for 10 min, and the
serum was frozen and stored at −80°C until used for analysis.
Femurs were isolated for evaluation, and the soft tissue was
removed. Femurs were wrapped in saline-soaked gauze and stored at
−80°C until used for analysis.
Bone morphometry and density
The right femur was fixed in 10% neutral buffered
formalin, degreased in 100% ethanol, re-fixed in cyanuric chloride
(Wako Pure Chemical Industries, Osaka, Japan) and decalcified with
formic acid. Thin sections of the right femur were made with a
sliding microtome (Leica SM-2000R; Leica Biosystems, Nussloch,
Germany). The sections were stained with hematoxylin and eosin
(H&E) stain. The amount and width of each trabecular bone,
correlated with bone strength, was traced distal to epiphyseal line
using a bio-imaging navigator (Biorevo BZ-9000; Keyence, Osaka,
Japan). The range of calcified bone was extracted. The area of the
calcified bone in the traced range was calculated using analytical
software (Hybrid Cell Count Software; Keyence).
Bone strength
The bending strength of the left femur was measured
with a three-point bending test using a mechanical testing machine
(AG-X; Shimadzu, Kyoto, Japan). The specimen was placed
horizontally in the loading section of the machine. The center of
the diaphysis was pressed with 50 kgf; the distance from the
support point was 15 mm. The breaking strength in Newtons (N) was
used as a measure of bone strength.
Serum biochemistry
Biochemical measurements, including serum calcium,
phosphorus, total protein and albumin were measured with an
auto-analyzer (Hitachi 7180; Hitachi High-Technologies, Tokyo,
Japan).
Bone metabolism
Serum tartrate-resistant acid phosphatase 5b
(TRACP-5b), a bone resorption marker, and bone-specific alkaline
phosphatase (BAP), a bone formation marker, were used as
biochemical markers of turnover and, measured using an enzyme
immunoassay (Rat TRACP-5b ELISA; Cusabio Biotech Co., Ltd., Wuhan,
China).
Statistical analysis
Continuous variables are expressed as mean ±
standard deviation. Comparisons between groups were made using the
Mann-Whitney U test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Final sample numbers
Subsequent to being assigned to the experimental
groups, 1 rat died in the TG plus rabeprazole group, and 3 rats
died in the TG plus minodronic acid group. All 4 rats succumbed to
ileus; none from drug toxicity. No rats died in the sham-surgery or
TG control groups. Of the 75 assigned animals, 71 rats survived 22
weeks post-surgery and were included in the study (15 in the sham
group, 20 in the TG control group, 19 in the TG plus rabeprazole
group, and 17 in the TG plus minodronic acid group).
Body weights
Changes in the body weights of the rats in each
experimental group are shown in Fig.
1. Body weights of the rats in the TG group were reduced in
weeks 1–2. The body weights of the rats in the sham group increased
gradually throughout the study period, whereas those in the TG
groups remained nearly constant after week 8. No significant
differences in the body weights were observed among the three TG
groups.
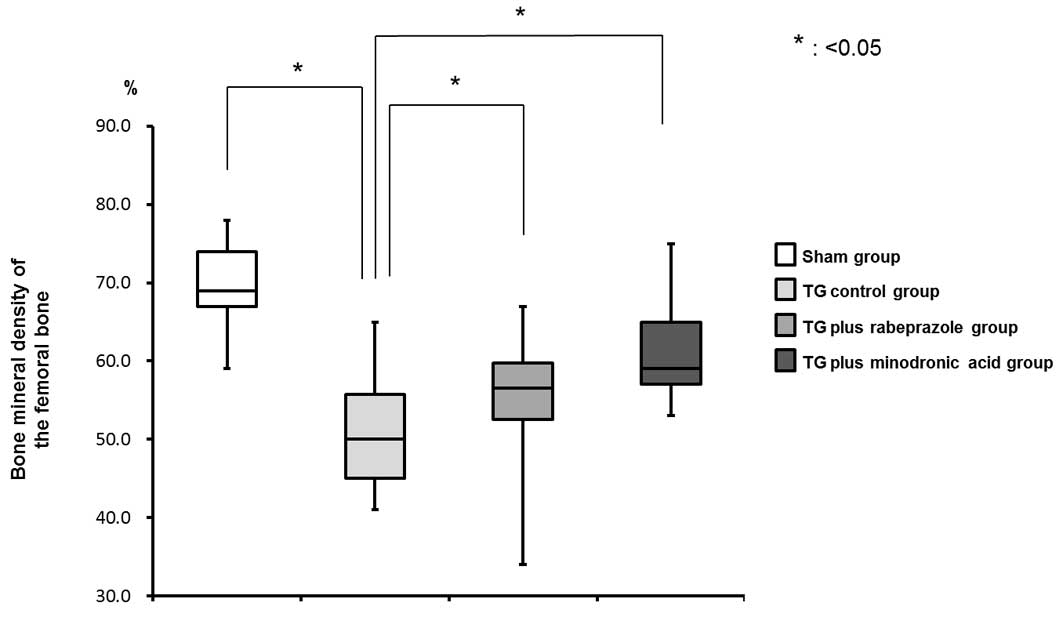
Bone mineral density (BMD) of the
femur
Twenty-two weeks after TG, the rate of the calcified
bone area in the traced are in the TG control group (50.0±8.1%) was
lower than that in the sham group (69.0±5.6%) (Fig. 2). The rates in the TG plus rabeprazole
(56.5±7.5%) and TG plus minodronic acid (59.0±6.0%) groups were
significantly higher than that in the TG control group (P<0.05).
These results indicated that rabeprazole inhibited the TG-induced
BMD decrease with an effect comparable to that of minodronic acid
(Fig. 2).
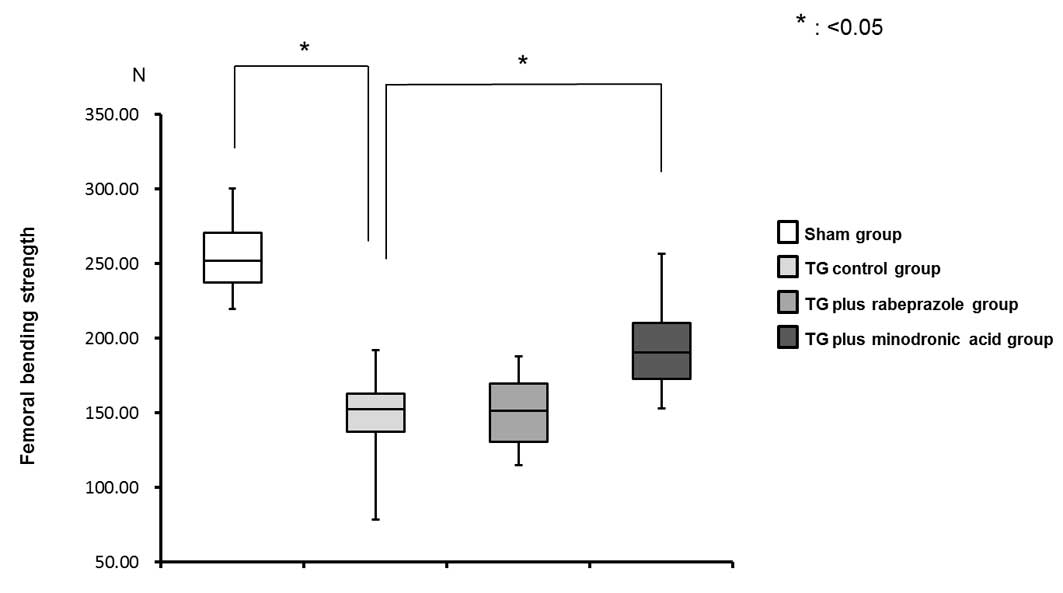
Bone strength
Femoral bending strength was markedly decreased in
the TG control group (152.34±24.01 N•m) compared with that in the
sham group (251.99±23.14 N•m). This effect was significantly
ameliorated and also counteracted with minodronic acid treatment
(190.50±26.95 N•m), but not by the rabeprazole treatment
(151.67±22.41 N•m) (Fig. 3).
Serum biochemistry
Compared with that in the sham group, the serum
calcium levels were reduced in rats with TG, while the serum
phosphorus levels remained unchanged by TG or by the administration
of either minodronic acid or rabeprazole. Minodronic acid
significantly decreased the serum calcium level compared with the
level in the TG control group (P<0.05). By contrast, rabeprazole
did not affect serum calcium levels (Fig.
4).
Bone metabolism
No significant differences in serum TRACP-5b levels
were observed between the four groups (Fig. 5A). The serum BAP level was reduced in
rats with TG compared with that in the sham group. The
administration of minodronic acid or rabeprazole did not ameliorate
this serum BAP decrease (Fig. 5B).
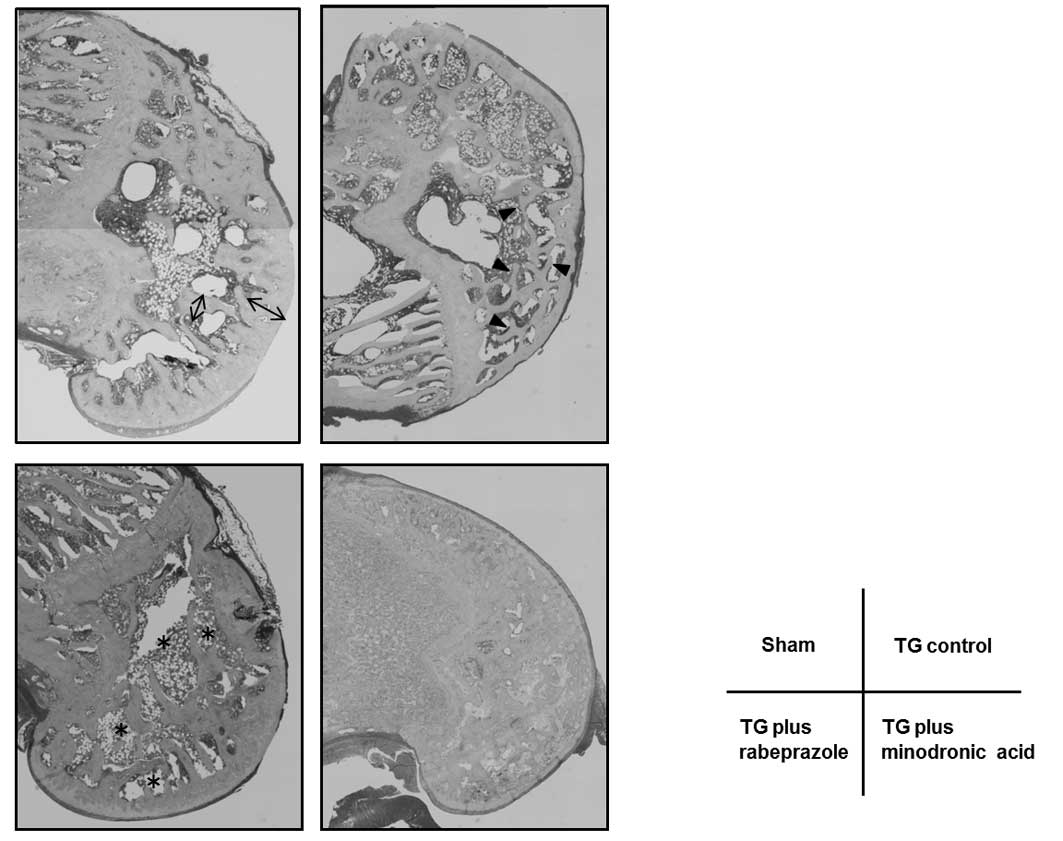
Bone morphology
The morphology of the femoral metaphysis stained
with H&E stain was observed microscopically (Fig. 6). The trabecular sponge-like network in
the metaphysis (arrowhead in Fig. 6)
and trabecular separation (asterisks in Fig. 6) were compared across the four groups.
The width (bidirectional arrow in Fig.
6) in the TG control group was thinner than that in the sham
group. By contrast, the width was wider in the rabeprazole and
minodronic acid-treated groups compared to the TG control
group.
Discussion
The present study demonstrated the effects of
rabeprazole on bone metabolic disorders in gastrectomized rats.
Minodronic acid almost completely blocked the TG-induced decreases
in bone density and bone strength. Rabeprazole also inhibited the
TG-induced decrease in bone density.
PPIs reduce gastric acid secretion, and are thus
widely used in conditions such as gastroesophageal reflux,
Zollinger-Ellison syndrome, dyspepsia and peptic ulcer disease
(25). For years, PPIs were considered
safe, without any major complications during long-term use
(26). However, Yang et al
(27) conducted a nested case-control
study using the General Practice Research Database and examined the
risk of hip fractures associated with PPI use. The study reported
that the risk of hip fracture was markedly increased among
long-term users of high-dose PPI therapy. PPIs were recently
identified as an independent risk factor for osteoporotic fracture
(14,27–31). PPIs
reportedly increase the risk of osteoporotic fracture by causing
hypochlorhydria, reducing intestinal calcium absorption and
subsequently inducing a negative calcium balance (26,30).
However, existing studies provide conflicting information regarding
the direct effects of PPIs on calcium absorption. Hansen et
al (32) administered omeprazole
(40 mg/day) to menopausal women for 30 days and reported that 30
days of continuous PPI therapy did not alter calcium absorption,
suggesting that PPI-associated hypochlorhydria does not reduce
calcium absorption.
However, several in vitro studies have
reported that PPIs inhibit the vacuolar-ATPase of osteoclasts and
reduce their activity (33,34). Sheraly et al (35) examined the potential of PPIs to prevent
osteoclast-mediated resorption of calcium phosphate cements in
vivo. The study reported that the PPIs (pantoprazole and
high-dose omeprazole) produced a delay in osteoclast resorption.
Ohta et al (22) administered
rabeprazole (10 mg/day for 8 weeks) to 22 non-osteoporotic patients
presenting with upper gastrointestinal symptoms and investigated
the effect of rabeprazole on bone metabolism. They reported that
rabeprazole did not affect BAP, but significantly decreased type I
collagen cross-linked N-telopeptides. However, in humans and rat
models, the effect of PPIs on bone metabolism appears complicated
as PPIs have contradictory effects: Inhibition of calcium
absorption by gastric acid suppression versus inhibition of
osteoclasts. The association between PPI-associated hypochlorhydria
and the decrease in calcium absorption remains controversial.
In the present study, the TG rat model was used to
exclude the PPI-induced effect on gastric acid secretion and
examine the PPI-induced inhibition of bone resorption mediated by
osteoclasts in skeletal metabolism. The results demonstrated that
rabeprazole ameliorated the reduction in bone density induced by TG
at the distal end of the femur, indicating that rabeprazole may
control osteoclastic bone resorption, similar to
bisphosphonate.
However, rabeprazole did not ameliorate the
reduction in bone strength at the femoral diaphysis. This may be
due to the improvement in bone density by rabeprazole, which was
milder than that of the bisphosphonate. The difference between
cancellous bone and cortical bone may also be one of the causes.
Iwamoto et al (36) examined
the influence of TG on cortical and cancellous bones in rats. The
study measured the bone mineral content and density and the
mechanical strength of the femoral distal metaphysis and diaphysis.
The TG-induced osteopenia and deterioration in bone strength were
more severe at skeletal sites rich in cancellous bone (distal
metaphysis) compared with those rich in cortical bone (diaphysis).
The present study measured bone density at the femoral distal
metaphysis, rich in cancellous bone. However, bone strength was
measured at the femoral diaphysis, rich in cortical bone and thus
less affected by gastrectomy or medication than cancellous bone.
This may be one reason that administration of rabeprazole did not
appear to affect bone strength in the present study.
The serum BAP level in the TG groups was
significantly lower than that in the sham group, whereas the serum
TRACP-5b levels in all groups were similar. This result indicates
that the bone metabolic disorder induced by TG is more dependent on
suppressing the bone formation compared to on increasing bone
resorption. Although in the group with TG plus the bisphosphonate
the serum TRACP-5b level appeared lower than those in the other TG
groups, the individual variability was large and this difference
was not statistically significant. It is plausible that feedback
occurred and altered the level of this marker, however, this change
could not be captured as the present study examined only chronic
associations. TRACP-5b is not considered a reflection of bone
metabolism in this particular model.
As aforementioned, osteoporosis following
gastrectomy has become a clinical issue. In patients receiving
proximal gastrectomy or pylorus preserving gastrectomy, which
preserves gastric acid secretion, reflux of gastric acid could be
another cause of esophagitis. PPIs are typically effective in these
patients (37,38). Certain epidemiological studies
suggested that PPIs induce skeletal metabolism disorders. However,
due to the effect of PPIs on osteoclasts, the administration of PPI
may improve TG-induced bone metabolic disorders. The present study
used the TG model to reproduce the poor calcium absorption that
occurs during gastric anacidity. As the effect of calcium
malabsorption due to rabeprazole-induced gastric acid suppression
could be excluded, the specific effects of rabeprazole on
osteoclasts could be examined. Rabeprazole inhibited the TG-induced
bone density decrease, suggesting that the administration of a PPI
is at least not an exacerbating factor in bone metabolic
disorders.
Acknowledgements
The authors thank Dr Seiji Naganuma, Department of
Pathology, Kochi University, for the expert advice on the
pathological bone density measurement method.
References
|
1
|
Johnell O and Kanis JA: An estimate of the
worldwide prevalence and disability associated with osteoporotic
fractures. Osteoporos Int. 17:1726–1733. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cumming RG, Nevitt MC and Cummings SR:
Epidemiology of hip fractures. Epidemiol Rev. 19:244–257. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leibson CL, Tosteson AN, Gabriel SE,
Ransom JE and Melton LJ III: Mortality, disability, and nursing
home use for persons with and without hip fracture: A
population-based study. J Am Geriatr Soc. 50:1644–1650. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hofbauer LC, Hamann C and Ebeling PR:
Approach to the patient with secondary osteoporosis. Eur J
Endocrinol. 162:1009–1020. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bernstein CN, Leslie WD and Leboff MS: AGA
technical review on osteoporosis in gastrointestinal diseases.
Gastroenterology. 124:795–841. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van Staa TP, Geusens P, Bijlsma JW,
Leufkens HG and Cooper C: Clinical assessment of the long-term risk
of fracture in patients with rheumatoid arthritis. Arthritis Rheum.
54:3104–3112. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Melton LJ III, Kyle RA, Achenbach SJ,
Oberg AL and Rajkumar SV: Fracture risk with multiple myeloma: A
population-based study. J Bone Miner Res. 20:487–493. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sarasin C: Osteomalacie und hypochrome
anaemie nach magenresektion. Gastroenterologia. 66:182–197. 1941.
View Article : Google Scholar
|
|
9
|
Klinge B, Lehto-Axtelius D, Akerman M and
Håkanson R: Structure of calvaria after gastrectomy. An
experimental study in the rat. Scand J Gastroenterol. 30:952–957.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lehto-Axtelius D, Surve VV, Johnell O and
Håkanson R: Effects of calcium deficiency and calcium
supplementation on gastrectomy-induced osteopenia in the young male
rat. Scand J Gastroenterol. 37:299–306. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Axelson J, Persson P, Gagnemo-Persson R
and Håkanson R: Importance of the stomach in maintaining calcium
homoeostasis in the rat. Gut. 32:1298–1302. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Persson P, Gagnemo-Persson R, Chen D,
Axelson J, Nylander AG, Johnell O and Häkanson R: Gastrectomy
causes bone loss in the rat: Is lack of gastric acid responsible?
Scand J Gastroenterol. 28:301–306. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mühlbauer RC, Schenk RK, Chen D,
Lehto-Axtelius D and Hâkanson R: Morphometric analysis of
gastrectomy-evoked osteopenia. Calcif Tissue Int. 62:323–326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Targownik LE, Lix LM, Metge CJ, Prior HJ,
Leung S and Leslie WD: Use of proton pump inhibitors and risk of
osteoporosis-related fractures. CMAJ. 179:319–326. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chonan O, Takahashi R, Yasui H and
Watanuki M: Effect of L-lactic acid on calcium absorption in rats
fed omeprazole. J Nutr Sci Vitaminol (Tokyo). 44:473–481. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
O'Connell MB, Madden DM, Murray AM, Heaney
RP and Kerzner LJ: Effects of proton pump inhibitors on calcium
carbonate absorption in women: A randomized crossover trial. Am J
Med. 118:778–781. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tuukkanen J and Väänänen HK: Omeprazole, a
specific inhibitor of H+-K+-ATPase, inhibits bone resorption in
vitro. Calcif Tissue Int. 38:123–125. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mizunashi K, Furukawa Y, Katano K and Abe
K: Effect of omeprazole, an inhibitor of H+,K(+)-ATPase, on bone
resorption in humans. Calcif Tissue Int. 53:21–25. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shin JM, Cho YM and Sachs G: Chemistry of
covalent inhibition of the gastric (H+, K+)-ATPase by proton pump
inhibitors. J Am Chem Soc. 126:7800–7811. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sachs G, Shin JM, Vagin O, Lambrecht N,
Yakubov I and Munson K: The gastric H,K ATPase as a drug target:
Past, present, and future. J Clin Gastroenterol. 41(Suppl 2):
S226–S242. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saitoh T, Fukushima Y, Otsuka H, Hirakawa
J, Mori H, Asano T, Ishikawa T, Katsube T, Ogawa K and Ohkawa S:
Effects of rabeprazole, lansoprazole and omeprazole on intragastric
pH in CYP2C19 extensive metabolizers. Aliment Pharmacol Ther.
16:1811–1817. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ohta T, Hashimoto T, Murai H and Kimura H:
Influence of proton pump inhibitor on bone metabolism marker. J N
Rem Clin. 57:1341–1345. 2008.
|
|
23
|
Kawai T, Ikeda H, Harada Y and Saitou T:
Changes in the rat stomach after long-term administration of proton
pump inhibitors (AG-1749 and E-3810). Nihon Rinsho. 50:188–193.
1992.(In Japanese). PubMed/NCBI
|
|
24
|
Miyashita T, Shah FA, Marti GP, Wang J,
Bonde P, Gibson MK, Ohta T, Montgomery EA, Duncan M and Harmon JW:
Rabeprazole impedes the development of reflux-induced esophageal
cancer in a surgical rat model. Dig Dis Sci. 56:1309–1314. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Savarino V, Di Mario F and Scarpignato C:
Proton pump inhibitors in GORD An overview of their pharmacology,
efficacy and safety. Pharmacol Res. 59:135–153. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jacobson BC, Ferris TG, Shea TL, Mahlis
EM, Lee TH and Wang TC: Who is using chronic acid suppression
therapy and why? Am J Gastroenterol. 98:51–58. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang YX, Lewis JD, Epstein S and Metz DC:
Long-term proton pump inhibitor therapy and risk of hip fracture.
JAMA. 296:2947–2953. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vestergaard P, Rejnmark L and Mosekilde L:
Proton pump inhibitors, histamine H2 receptor antagonists, and
other antacid medications and the risk of fracture. Calcif Tissue
Int. 79:76–83. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
de Vries F, Cooper AL, Cockle SM, van Staa
TP and Cooper C: Fracture risk in patients receiving
acid-suppressant medication alone and in combination with
bisphosphonates. Osteoporos Int. 20:1989–1998. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu EW, Blackwell T, Ensrud KE, Hillier TA,
Lane NE, Orwoll E and Bauer DC: Acid-suppressive medications and
risk of bone loss and fracture in older adults. Calcif Tissue Int.
83:251–259. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Roux C, Briot K, Gossec L, Kolta S, Blenk
T, Felsenberg D, Reid DM, Eastell R and Glüer CC: Increase in
vertebral fracture risk in postmenopausal women using omeprazole.
Calcif Tissue Int. 84:13–19. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hansen KE, Jones AN, Lindstrom MJ, Davis
LA, Ziegler TE, Penniston KL, Alvig AL and Shafer MM: Do proton
pump inhibitors decrease calcium absorption? J Bone Miner Res.
25:2786–2795. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Karsdal MA, Henriksen K, Sørensen MG, Gram
J, Schaller S, Dziegiel MH, Heegaard AM, Christophersen P, Martin
TJ, Christiansen C, et al: Acidification of the osteoclastic
resorption compartment provides insight into the coupling of bone
formation to bone resorption. Am J Pathol. 166:467–476. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Niikura K, Takeshita N and Takano M: A
vacuolar ATPase inhibitor, FR167356, prevents bone resorption in
ovariectomized rats with high potency and specificity: Potential
for clinical application. J Bone Miner Res. 20:1579–1588. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sheraly AR, Lickorish D, Sarraf F and
Davies JE: Use of gastrointestinal proton pump inhibitors to
regulate osteoclast-mediated resorption of calcium phosphate
cements in vivo. Curr Drug Deliv. 6:192–198. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Iwamoto J, Sato Y and Matsumoto H:
Influence of gastrectomy on cortical and cancellous bones in rats.
Gastroenterol Res Pract. 2013:3816162013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Someya S, Shibata C, Tanaka N, Kudoh K,
Naitoh T, Miura K and Unno M: Duodenal switch for intractable
reflux gastroesophagitis after proximal gastrectomy. Tohoku J Exp
Med. 230:129–132. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Imada T, Rino Y, Takahashi M, Suzuki M,
Tanaka J, Shiozawa M, Kabara K, Hatori S, Ito H, Yamamoto Y, et al:
Postoperative functional evaluation of pylorus-preserving
gastrectomy for early gastric cancer compared with conventional
distal gastrectomy. Surgery. 123:165–170. 1998. View Article : Google Scholar : PubMed/NCBI
|