Introduction
The thyroid follicle, which is the fundamental unit
of the thyroid, consists of polarized thyroid cells. The synthesis
and secretion of thyroid hormone depends on the integrity of the
thyroid follicular structure, as well as the uptake of iodine
(1,2).
The follicular lumen is an enclosed cavity, where the intermediate
processes of thyroid hormone synthesis, such as iodine activation
and iodination of thyroglobulin (TG) take place. However, under
anaerobic conditions, synthesis is completed outside the cells.
Currently, the energy source supporting follicular hormone
synthesis is unknown.
In the present study, the three dimensional
structure of the follicle was constructed in vitro using a
primary culture of swine thyroid cells and the protein was
extracted from the follicular lumen (3). Proteomics analysis was subsequently
conducted to identify the unknown follicular lumen proteins that
were associated with energy metabolism. The aim of the study was to
improve the understanding of the mechanism of energy metabolism in
the follicular lumen.
Materials and methods
Preparation of the thyroid monolayer
and three-dimensional cell culture
Adult swine were maintained in Qingyuan Farm
(Quanzhou, China). The present study was approved by the ethics
committee of the Second Affiliated Hospital of Fujian Medical
University (Fujian, China). Two adult swine were sacrificed by
carotid artery bleeding and the thyroid gland was removed
immediately. Subsequent to serial washing with milli-Q water, 75%
ethanol, and sterilized phosphate-buffered saline (PBS; Thermo
Fisher Scientific, Inc., Shanghai, China), the capsule and
overlaying tissues were peeled away. The remaining thyroid tissue
was minced into 1-mm3 sections. Following digestion with
0.125% trypsin (Thermo Fisher Scientific, Inc.) for 30 min at room
temperature and filtering through a mesh sieve (size, 200; BD
Biosciences, Shanghai, China), the cell suspension was inoculated
in 6-well plates, and maintained in a 37°C incubator (5%
CO2) in Dulbecco's modified Eagle's medium and Ham's
F-12 medium (Thermo Fisher Scientific, Inc.) consisting of 1 mU/ml
thyroid-stimulating hormone, 0.05% NaI and 10% fetal bovine serum
(Thermo Fisher Scientific, Inc.). For construction of the
three-dimensional thyroid follicle, the cells were seeded at a
density of 2×106 cells/ml, and the thyroid follicle was
formed three days after inoculation (Fig.
1A-D).
Extraction of thyroid follicular lumen
and intracellular proteins
The follicular lumen protein was prepared as
follows: After aspiration of the culture medium from the culture
plate, the three-dimensional thyroid cells were gently washed,
twice, with pre-cooled PBS, followed by incubation with 0.02% EDTA
for 5 min at room temperature. EDTA loosened the thyroid structure.
After removing the EDTA solution, the thyroid structure was
mechanically deconstructed using 100 µl PBS and the protein was
released from the follicular lumen. The follicular lumen protein
concentration was diluted to a similar concentration to thyroid
intracellular protein using bovine serum albumin (BSA). For
preparing the intracellular protein, the solution was centrifuged
at 12,000 × g for 5 min at 4°C and the supernatant was aliquoted.
The cell pellet was lysed using RIPA buffer (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and centrifuged at 12,000 × g
for 10 min at 4°C to harvest the intracellular protein. The protein
concentration was determined using a Micro BCA Protein Assay kit
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions.
SDS-PAGE electrophoresis and matrix
assisted laser desorption/ionization time-of-flight mass
spectrometry (MALDI-TOF) analysis
For SDS-PAGE electrophoresis, 20 µg protein was
loaded and separated on 4 or 12% polyacrylamide gels according to
the standard protocol. After running for 1 h at 120 V, the gel was
stained with Coomassie Brilliant Blue. The protein bands that were
differentially expressed and highly overexpressed in the follicular
lumen were sliced from the gel and digested using 12.5 ng/µl
trypsin in 50 mmol/l ammonium bicarbonate (pH 8.0). Following
digestion, the samples were eluted with 2 µl matrix solution
containing 10 mg/m α cyano-4-hydroxycinnamic acid, and submitted to
Bruker III MALDI-TOF mass spectrometry. The trypsin autolysis
products were used for calibration by flexAnalysis software
(version 2.4; Bruker, Coventry, UK) and searched against the
SWISS-PROT (http://www.uniprot.org/) and NCBI
database (http://www.ncbi.nlm.nih.gov/) using the MASCOT tool
from Matrix Science (http://www.matrixscience.com/) with a 50 ppm mass
tolerance.
Imaging of monolayer thyroid cells and
follicular lumen
Primary thyroid cells were isolated as described
above. Then 6×106 cells per well were inoculated into
6-well plates for reconstructing the three dimensional structure
and 6×105 cells per well for monolayer cells. After
continuous cultivation, cells growing in a monolayer fashion were
visualized and photographed under a normal inverted optical
microscope. For laser confocal microscopy, the cells forming the
follicular lumen were gently rinsed with PBS twice followed by
incubation with fluorophore-conjugated anti-TG (Novus Biologicals
LLC, Littleton, CO, USA) for 1 h at room temperature. After three
washes with PBS, the cells were visualized under a laser confocal
microscope.
Statistical analysis
Statistical significance was determined using the
one-way analysis of variance (SPSS 18.0; SPSS, Inc., Chicago, IL,
USA). The results were expressed as means ± standard error of the
mean and P<0.05 was considered to indicate a statistically
significant difference.
Results
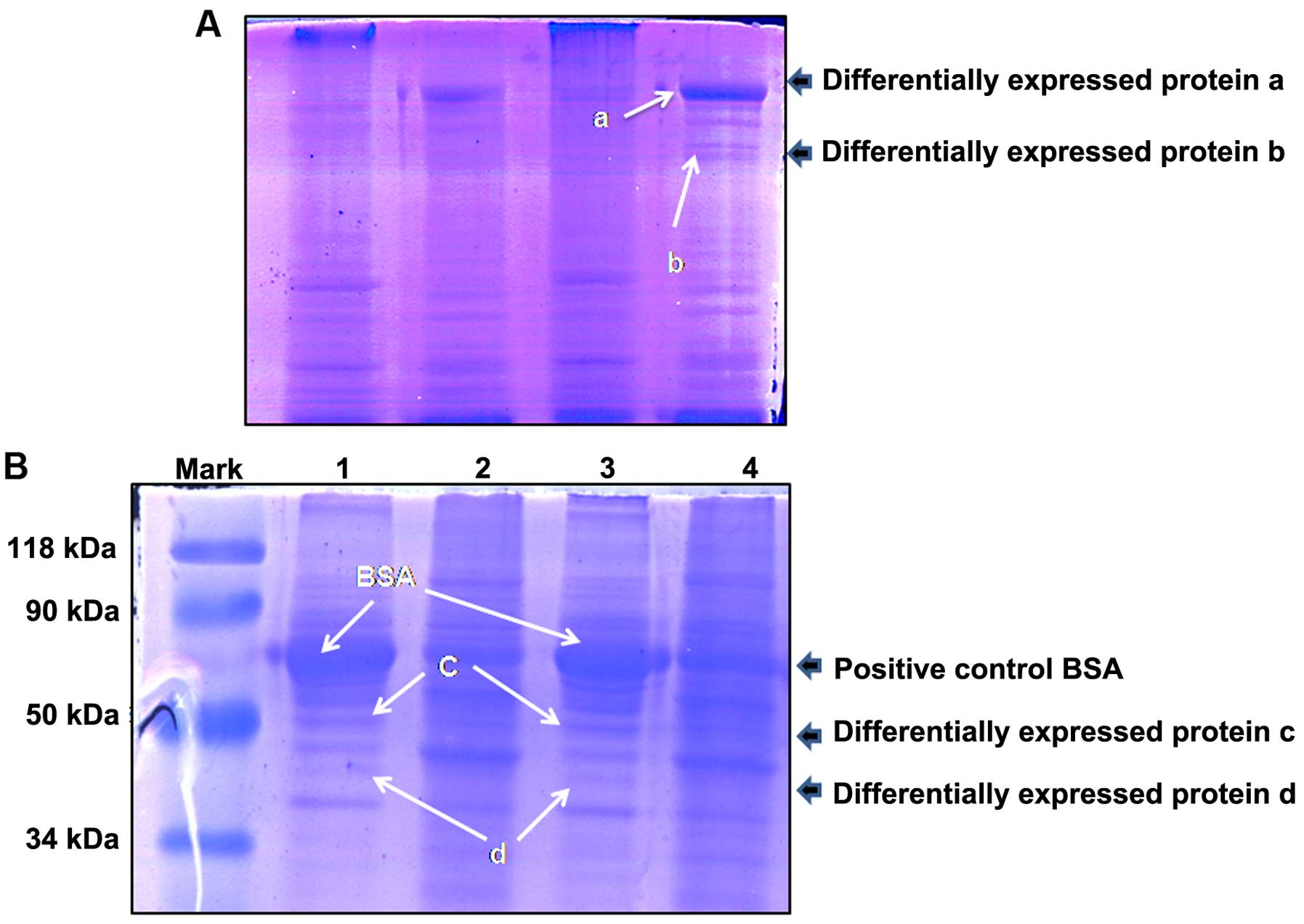
Gel analysis of the thyroid
intracellular and follicular lumen proteins
In order to visualize the abundance of proteins, the
intracellular protein and follicular lumen proteins were separated
by SDS-PAGE (4 and 12%) according to standard procedures, using the
total proteins that were extracted from the thyroid epithelial
cells with RIPA buffer. Equal quantities of total protein were
loaded into each lane. After staining with Coomassie Brilliant
Blue, certain follicular lumen proteins were observed to be
differentially expressed compared with the control intracellular
proteins. These are demonstrated in Fig.
2A and B.
Identification of differentially
expressed proteins by mass spectrometry
Five protein bands were excised and digested with
trypsin, then identified by mass spectrometry. The identified
proteins, TG, enolase, pyruvate kinase and phosphoglyceraldehyde
dehydrogenase are presented in Table
I.
 | Table I.Differentially expressed follicular
proteins as identified by mass spectrometry. |
Table I.
Differentially expressed follicular
proteins as identified by mass spectrometry.
| Band | Protein | Accession no. | Molar mass
calculation (Da) | Score |
|---|
| Protein a | Thyroglobulin (Sus
scrofa) | gi|270289746 | 299851 | 302 |
| Protein b | Enolase | gi|34789 |
47285 | 111 |
| Protein c | Pyruvate kinase | gi|206205 |
58314 | 292 |
| Protein d | Phosphoglyceraldehyde
dehydrogenase | gi|65987 |
35914 | 172 |
Discussion
The thyroid follicle is the fundamental unit of the
thyroid gland with round or elliptical shaped cells that are 20–900
µm in diameter. Monolayer follicular endothelial cells aggregate to
form a lumen, which is filled with TG (2,4). TG is
considered to be the carrier for thyroid hormone synthesis. The
biosynthesis of thyroid hormone is a process of chemical
modification of TG (5,6). Follicular endothelial cells transport TG
and iodine into the follicular lumen and partial tyrosine in TG is
catalyzed to 3-monoiodotyrosine (MIT) and 3,5-diiodotyrosine (DIT)
in the apical membrane surface in the lumen by thyroid peroxidase.
MIT or DIT further couple with DIT to create triiodothyronine (T3)
and thyroxine (T4). TG resides in the lumen carrying the newly
synthesized T3 and T4, which must be trafficked back to thyroid
follicular epithelial cells for further processing prior to the
release of T3 and T4 (7). The process
includes the uptake of TG, degradation of iodinated TG and release
of thyroid hormones. Therefore, the physiological features of
thyroid follicular epithelial cells are that the synthesis and
storage of thyroid hormones are conducted within the follicular
lumen and are absorbed into cells for further processing before
being released into the blood (8–10), which is
distinct from other endocrine cells that preserve the hormone
intracellularly. Thus, the follicular lumen is where thyroid
hormone synthesis is performed. All these steps, including TG
synthesis and transportation, and iodine uptake, consume ATP
(11,12); however, the mechanism by which ATP is
generated in the follicular lumen remains unknown.
As an extracellular structure, the thyroid
follicular lumen is isolated from the blood and cannot access
oxygen support; therefore, it is proposed that the energy supply in
the lumen is via anaerobic glycolysis, and that anaerobic
glycolysis-associated enzymes must exist in the lumen. To analyze
this hypothesis, the follicular lumen was constructed using a
primary culture of swine thyroid cells in the present study. In
addition, the soluble protein was extracted from the lumen and
SDS-PAGE was performed to separate the follicular lumen proteins.
It must be noted that, as the follicular lumen proteins are not
abundant, each band in the gel may have represented a single
protein. Furthermore, as a result of the method that was used to
isolate the thyroid follicular lumen proteins, intracellular
proteins may have been mixed into the samples. Equal quantities of
total protein (thyroid intracellular or follicular lumen proteins)
were loaded into each lane for SDS-PAGE gel electrophoresis and
those protein bands that were highly overexpressed in the
follicular lumen or were absent in the thyroid intracellular
protein represented follicle-specific proteins. Following
electrophoresis, five follicle-specific proteins were identified by
MALDI-TOF mass spectrometry. The band with 60 kDa MW was BSA, and
the other bands were swine TG, enolase, pyruvate kinase and
phosphoglyceraldehyde dehydrogenase. Among these five proteins, BSA
served as a positive control protein that was artificially
supplemented, swine TG was previously shown to be present in the
follicular lumen, and the other three proteins were key
glycolysis-associated enzymes, which have previously been
identified to be critical in anaerobic glycolysis of glucose
(13–15).
In conclusion, anaerobic glycolysis of glucose
commonly occurs in the cytoplasm and is the major energy source
when cells are subjected to hypoxia (16–18). The
present study demonstrated that these glycolysis-associated
enzymes, enolase, pyruvate kinase and phosphoglyceraldehyde
dehydrogenase exist in the follicular lumen; however, their roles
in the lumen remain undefined, and further investigation is
required to establish whether anaerobic glycolysis of glucose also
occurs in the follicular lumen and supports the energy consumption
for hormone synthesis.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Fujian (grant no. 2012J01332), the Natural
Science Foundation of China (grant no. 81370886), the Key
Scientific Project of Fujian Province (grant no. 2014Y0017) and the
Innovative Medical Research Project of Fujian Province (grant no.
2012-CXB-24).
References
|
1
|
Susarla R, Gonzalez AM, Watkinson JC and
Eggo MC: Expression of receptors for VEGFs on normal human thyroid
follicular cells and their role in follicle formation. J Cell
Physiol. 227:1992–2002. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bernier-Valentin F, Trouttet-Masson S,
Rabilloud R, Selmi-Ruby S and Rousset B: Three-dimensional
organization of thyroid cells into follicle structures is a pivotal
factor in the control of sodium/iodide symporter expression.
Endocrinology. 147:2035–2042. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang H, Shi Y, Lin L, Li L, Lin X, Li X
and Xu D: Inhibition of thyroid-restricted genes by follicular
thyroglobulin involves iodinated degree. J Cell Biochem.
112:971–977. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tonoli H, Flachon V, Audebet C, Callé A,
Jarry-Guichard T, Statuto M, Rousset B and Munari-Silem Y:
Formation of three-dimensional thyroid follicle-like structures by
polarized FRT cells made communication competent by transfection
and stable expression of the connexin-32 gene. Endocrinology.
141:1403–1413. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Di Jeso B and Arvan P: Thyroglobulin from
molecular and cellular biology to clinical endocrinology. Endocr
Rev. 37:2–36. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ishido Y, Luo Y, Yoshihara A, Hayashi M,
Yoshida A, Hisatome I and Suzuki K: Follicular thyroglobulin
enhances gene expression necessary for thyroid hormone secretion.
Endocr J. 62:1007–1015. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hennemann G, Vos RA, de Jong M, Krenning
EP and Docter R: Decreased peripheral 3,5,3′-triiodothyronine (T3)
production from thyroxine (T4): A syndrome of impaired thyroid
hormone activation due to transport inhibition of T4- into
T3-producing tissues. J Clin Endocrinol Metab. 77:1431–1435. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miot F, Dupuy C, Dumont J and Rousset B:
Thyroid hormone synthesis and secretion. Endotext. De Groot LJ,
Beck-Peccoz P, Chrousos G, Dungan K, Grossman A, Hershman JM, Koch
C, McLachlan R, New M, Rebar R, Singer F, Vinik A and Weickert MO:
MDText.com, Inc. (South Dartmouth, MA). 2000.
|
|
9
|
Koibuchi N: Molecular mechanisms of
thyroid hormone synthesis and secretion. Nihon Rinsho.
70:1844–1848. 2012.(In Japanese). PubMed/NCBI
|
|
10
|
Vickers AE, Heale J, Sinclair JR, Morris
S, Rowe JM and Fisher RL: Thyroid organotypic rat and human
cultures used to investigate drug effects on thyroid function,
hormone synthesis and release pathways. Toxicol Appl Pharmacol.
260:81–88. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Massart C, Hoste C, Virion A, Ruf J,
Dumont JE and Van Sande J: Cell biology of
H2O2 generation in the thyroid: investigation
of the control of dual oxidases (DUOX) activity in intact ex vivo
thyroid tissue and cell lines. Mol Cell Endocrinol. 343:32–44.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Corvilain B, Laurent E, Lecomte M,
Vansande J and Dumont JE: Role of the cyclic adenosine
3′,3′-monophosphate and the phosphatidylinositol-Ca2+
cascades in mediating the effects of thyrotropin and iodide on
hormone synthesis and secretion in human thyroid slices. J Clin
Endocrinol Metab. 79:152–159. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li W, Xu Z, Hong J and Xu Y: Expression
patterns of three regulation enzymes in glycolysis in esophageal
squamous cell carcinoma: association with survival. Med Oncol.
31:1182014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Slamovits CH and Keeling PJ:
Pyruvate-phosphate dikinase of oxymonads and parabasalia and the
evolution of pyrophosphate-dependent glycolysis in anaerobic
eukaryotes. Eukaryot Cell. 5:148–154. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Atlante A, Giannattasio S, Bobba A,
Gagliardi S, Petragallo V, Calissano P, Marra E and Passarella S:
An increase in the ATP levels occurs in cerebellar granule cells en
route to apoptosis in which ATP derives from both oxidative
phosphorylation and anaerobic glycolysis. Biochim Biophys Acta.
1708:50–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pietsch J, Sickmann A, Weber G, Bauer J,
Egli M, Wildgruber R, Infanger M and Grimm D: Metabolic enzyme
diversity in different human thyroid cell lines and their
sensitivity to gravitational forces. Proteomics. 12:2539–2546.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou L, Huang H, McElfresh TA, Prosdocimo
DA and Stanley WC: Impact of anaerobic glycolysis and oxidative
substrate selection on contractile function and mechanical
efficiency during moderate severity ischemia. Am J Physiol Heart
Circ Physiol. 295:H939–H945. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
von Kleist-Retzow JC, Hornig-Do HT,
Schauen M, Eckertz S, Dinh TA, Stassen F, Lottmann N, Bust M,
Galunska B and Wielckens K: Impaired mitochondrial Ca2+
homeostasis in respiratory chain-deficient cells but efficient
compensation of energetic disadvantage by enhanced anaerobic
glycolysis due to low ATP steady state levels. Exp Cell Res.
313:3076–3089. 2007. View Article : Google Scholar : PubMed/NCBI
|