Introduction
Tissue engineering has emerged as an
interdisciplinary field that seeks to restore, maintain or improve
organ function in vivo using artificial tissue or organs,
and is a promising approach for addressing transplantation organ
shortage (1,2). The general model of traditional tissue
engineering is based on the isolated cell and subsequent cell
seeding with exogenous scaffolds (3),
in order to obtain the matured tissue substitutes (4). However, the precise placement of a large
quantity of multicellular biomaterials in spatial and sequential
rapid prototyping remains a limitation of traditional tissue
engineering methods. Organs consist of extracellular matrix and
different cell types that require specific spatial organization
(5). In order to create a complex
artificial biosystem, a subtly arranged spatial mold should be
constructed, in which different cells, nutrients and biofactors are
precisely located to mimic the microenvironment in vivo and
to obtain the appropriate biological function (6). Another significant hurdle is providing
metabolically appropriate conditions inside of a three-dimensional
(3D) tissue construct with limited thickness, which may limit the
support for the metabolic demands of engineered cells, particularly
for certain metabolically active cells, including cardiomyocytes
and hepatocytes (7). In addition,
vascularization remains another big challenge for maintaining the
biological activity of engineered constructs. Out-branching blood
vessels usually require numerous days for vascularizing the
implanted tissue, while seeded cells are unable to obtain
sufficient nutrient support before they consume all the available
oxygen within a few hours (8).
Bioprinting, based on layer by layer deposition of
cells and/or cell aggregates into a thermo-reversible gel, is
defined as a new computer-aided 3D rapid prototyping technology
with sequential maturation of the printed structures into a living
tissue or organ (9). As a breakthrough
in regenerative medicine, this emerging technology is currently
adapted to produce a variety of tissue architectures and
biomaterials, providing a novel cell-based therapeutic approach
(7) for organ loss and failure, which
is economical and efficient (3,10,11). For instance, myocardial patches have
been successfully formed through the post-printing fusion (12), and fully biological scaffold-free
vascular tubular grafts have also been constructed using this
technology (4).
Although bioprinting is still a new technique
compared with existing traditional tissue engineering methods, this
novel approach has various advantages: i) With high-efficiency, the
tissue engineering project is simplified and can be performed
automatically (5,13); ii) a subtle spatial control of the cell
types, extracellular matrix, blends of polymers and other cell
inductive particles in well-defined 3D microenvironment can be
achieved with computer-aided design (CAD) software (11); iii) bioprinting can be applied in the
scalable generation of high-throughput cells (5,13); iv)
vascularization of complex constructs can be resolved (3); v) this method offers an effient approach
to realize the goal of repair and reconstruction in situ;
vi) the production cycle can be significantly shortened and the
process has higher repeatability than other techniques; and vii)
this method excels at producing a variety of tissue architectures
with high physical complexity (10).
Currently, widely used routes are available in
bioprinting, ranging from non-jet-based approaches, such as
laser-directed writing (LGDW) and photolithography, to jet-based
methods (14). LGDW is a
micro-patterning technique that can facilitate studies at the
single cell level. It utilizes a focused beam to confine and guide
cells by exploiting the differences in the refractive indexes of
the cells and cell media, and then depositing them onto
non-absorbing surfaces (8). However,
limited throughput and high complexity have restricted the
potential application of LGDW in tissue construction (15). Besides, exposure of excessive thermal
energy via laser light onto the cell biolayer and overheating of
the cell can be a potential challenges that must be overcome
(13). Jet-based technologies are the
most commonly used in bioprinting, and are promising for handling
various materials, including molecular polymers and micro/nanosized
compounds. There are numerous printing techniques based on
jet-based technology, such as inkjet printing, electrospraying and
electrospinning.
Inkjet printing, based on ink drop ejection
(5), was the first method to be used
for printing 3D architectures. During 3D inkjet printing, the ink
containing cells, culture medium and gel precursor solution is
expelled by the jet pens with specific drop volume, and the energy
supplied to the ink, from heating by a setting pulse, is
transformed into kinetic energy of drops and heating of the drops.
Subsequently, the bio-ink is printed layer-by-layer onto the
platform, resulting in 3D structures (5). However, nozzle blockage remains a problem
with this process (14), while
limitations in spatial resolution and control of the droplet size
result in coarse architectures (13).
Inkjet printers based on piezoelectric technology have numerous
applications in ceramics and polymer printing, and are also used in
bioprinting. However, due to problems with ink leakage and
formation of mist, more viscous ink is required, thus requiring
more power and higher vibration frequency that may cause damage to
the cells (15).
Electrospraying generates droplets between the
jetting needles, driven by electric fields created by the applied
potential difference between the jetting needle and a ground
electrode (16). This technique has
recently been developed to process living cellular organisms
(17), and previous studies have
elucidated the flexibility of using electrospraying technology in a
range of applications, including tissue engineering and
regenerative medicine with cell-based and molecular-based therapies
(18,19). Under the voltage driving effect, the
liquid is separated into microdroplets and spayed onto a
predesigned area. For instance, cell transmission based on
electrospraying principles, which is known as ‘bio-electrospraying’
(14,18), has being increasingly explored.
Bio-electrospraying directly uses cell suspensions as ejection
materials that form droplets at the microscale to nanoscale in an
electric field. This technique can produce single-cell scattering
and direct transmission. Thus, electrospraying is regarded as a
novel route to analyze cell biochemical characteristics and perform
3D printing in tissue engineering.
In the present study, the ability to directly
electrospray multiple types of human cell suspensions in
vivo was demonstrated. In addition, the study is the first to
verify that different human cells can maintain high cell viability
subsequent to electrospraying under the same set-up conditions.
This study demonstrates that electrospraying is a promising
technology for building human tissue substitutes in organ loss and
failure.
Materials and methods
Cell harvest and culture
All of the experimental procedures were approved by
the Ethics Committee of Shanghai 9th People's Hospital (Shanghai,
China). Informed consent was obtained from each patient prior to
participation in the study. Six different human cell types were
used for this cell printing experiment. Skin fibroblasts were
isolated from discarded prepuce tissue of 3 male patients (one aged
6 years and two aged 8 years), who underwent circumcision surgery.
The tissue was obtained following surgery and washed immediately
with saline solution containing gentamicin. Next, the tissue was
washed with antibiotic/antimycotic solution followed by
phosphate-buffered saline (PBS; Sigma-Aldrich, St. Louis, MO, USA).
The tissue was then cut into 1 mm sections and placed into a
culture dish with Gibco high glucose-Dulbecco's modified Eagle's
medium (H-DMEM; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing Gibco 10% fetal bovine serum (FBS; Thermo Fisher
Scientific, Inc.) and 1% antibiotic/antimycotic (Gibco; Thermo
Fisher Scientific, Inc.) solution.
Human adipose-derived stem cells (hADSCs) were
isolated from female patients (ages, 36, 42 and 45 years) who
underwent abdominal liposuction surgery, and these ADSCs were
maintained in Gibco low glucose-DMEM (L-DMEM; Thermo Fisher
Scientific, Inc.) containing 10% FBS and 1% antibiotic/antimycotic
solution. Human periodontal ligament cells (HPDLCs) were also
derived from the periodontal ligament of human third molars
extracted from 3 healthy donors (14-year-old male; 16-year-old
female; 15-year-old female) for orthodontic reasons, who had no
clinical signs of chronic periodontal disease. The isolated cells
were incubated with high H-DMEM containing 10% FBS and 1%
antibiotic/antimycotic solution. The abovementioned human tissue
separated cells were all obtained from patients undergoing surgery
at the Shanghai 9th People's Hospital. Up to three passages were
used for the experiments.
In addition, the adult human retinal pigment
epithelial (ARPE-19) cell line obtained from American Type Culture
Collection (ATCC; Manassas, VA, USA) was used in the present study
(passages 5–10), and cells were cultured in DMEM supplemented with
10% FBS and 1% antibiotic/antimycotic solution. Furthermore, human
umbilical vascular endothelial cells (HUVECs) were purchased from
ATCC (Manassas, VA, USA) and maintained in F-12 K (LGC Standards,
Barcelona, Spain) supplemented with 10% FBS, heparin and
endothelial cell growth supplement. GES-1, an immortalized human
gastric epithelial cell line, was also purchased from ATCC, and
cells (passages 5–10) were maintained in Gibco RPMI-1640 (Thermo
Fisher Scientific, Inc.) medium supplemented with 10% FBS, 2 mM
L-glutamine (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 100 mg/ml streptomycin.
All the cells were incubated in a 37°C, 5%
CO2, and 99% relative humidity chamber. The cells were
allowed to reach 80% confluence prior to passaging. The culture
medium was replenished with fresh medium every 2 or 3 days.
Cell preparation and cell print
suspension
When the cell reached 80% confluence, they were
detached with 0.25% trypsin-EDTA (Sigma-Aldrich) and then the cell
pellets were collected and centrifuged at 524 × g for 5 min.
Following aspiration of the supernatant, the cell pellets were
resuspended and each single cell suspension was diluted at a
density of 1×106/ml in the culture medium to obtain the
cell print suspension. The experimental and control groups were
treated under the same conditions, although there was no printing
in the control group. Prior to printing, the printer was prepared
within 1 h in sterile conditions.
Cell printing and cell culture
The cell suspension was resuspended at a final
concentration of 106 cells/ml and shaken vigorously
prior to printing. Next, approximately 1–2 ml of the cell
suspensions was sequentially conveyed to the cell electrospraying
printer each time with the parameters set as follows: Voltage, 15
kV; flow rate, 150 µl/min. The collecting Petri dish was placed 15
cm away from the sprayer nozzle (nozzle diameter, 0.5 mm). The
suspension of each cell group was electrosprayed directly onto the
petri dish for 30 min under sterile conditions and the cells were
collected in the culture medium similarly to the culture stage
mentioned previously. Subsequent to printing, the yielding cells
were centrifuged at 524 × g for 5 min and resuspended with culture
medium. The medium was changed every 1–2 days duing the 5-day
culture. The control groups were synchronously placed under the
same sterile conditions as the experimental groups, without
printing, they were subsequently treated the same as the
experimental group.
Cell viability assay
Following the identification of the required
parameters for the generation of printed cells under stable
electrospraying conditions (voltage, 15 kV; flow rate, 150 µl/min),
the present study attempted to determine whether the
electrospraying conditions affected the electrosprayed cells. The
changes of cells were evaluated using the cell viability assay. The
LIVE/DEAD Viability/Cytotoxicity Assay kit (Thermo Fisher
Scientific, Inc.,) was used to analyzed cell viability with two
fluorescent agents: Calcein acetoxymethyl (AM), which is retained
within live cells and produces an intense uniform green
fluorescence (ex/em ~495/~515 nm); and ethidium homodimer-1
(EthD-1), which enters cells with damaged membranes and produces a
bright red fluorescence in dead cells (ex/em ~495/~635 nm). First,
50 µM working solution of calcein AM was obtained by forming an
80-fold dilution of calcein AM in DMSO. In addition, single cell
suspensions at a density of 0.1–5×106 cells/Ml were
prepared. Subsequently, 2 µl calcein AM working solution and 4 µl
EthD-1 stock solution (2 mM) were added to each milliliter of
cells, and the sample was mixed. The cells were then incubated for
15–20 min at room temperature, in the dark. Following the
incubation, the samples were analyzed by flow cytometry at an
excitation wavelength of 488 nm, measuring green fluorescence
emission for calcein AM and red fluorescence emission for EthD-1.
The cell population was thus separated into two groups: Live cells
showing green fluorescence and dead cells showing red
fluorescence.
Morphological characteristic
assay
Subsequent to printing, the cells were at a
resuspended and diluted single cell suspension at a density of
0.5×106/ml in the culture medium, and then regularly
cultured for 5 days at 37°C in a humidified atmosphere containing
5% CO2 with the media changed twice a week. After 6 h of
incubation, the cells were allowed to adhere to the bottom of the
dishes. Cell morphological characteristics were then observed under
a phase-contrast microscope between the samples at 6 h of
incubation and up to 5 days of culturing. Within this period,
experimental and control groups were treated with same resuspension
and culture procedures.
Cell proliferation assay
A total of 100 µl cell suspensions (1,000
cells/well) of all types of cells were dispensed in a 96-well
plate, and the plate was incubated as mentioned above for a
scheduled time period (for 0–5 days). In order to determine the
cell proliferation, the cells were washed with PBS and incubated
with the Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc., Rockville, MD, USA) along with the culture
media at a ratio of 1:10. Subsequently, the plates were incubated
for 2–4 h [different incubation times were set to ensure the
optical density (OD) values were within a precise range (<3) and
the incubation time between the control and experimental group of
the same cell type was the same. The fibroblast and HPDLC groups
were incubated for 2.5 h; hADSCs groups were incubated for 4 h; the
ARPE-19, HUVECs and GES-1 groups were incubated for 2 h] in an
incubator at 37°C in a humidified atmosphere containing 5%
CO2. The absorbance in terms of the OD was then read at
450 nm using a plate reader. The OD values measured the quantity of
formazon dye, a product of CCK-8, and cell dehydrogenase, which was
proportional to the number of living cells. Thus, the cell
proliferation trend was revealed by the OD values at different
time-points.
Statistical analysis
All statistical analyses were performed using the
GraphPad Prism 6 software (GraphPad Software, Inc., La Jolla, CA,
USA). The cell survival rate data are presented as the mean ±
standard deviation (n=3). The CCK-8 assay data are expressed as the
mean ± standard error of the mean (n=9 in each group). Statistical
analyses were performed using a two-tailed Student's t-test in the
CCK-8 assay between the control group and experimental group of
each type of cell, with P<0.05 considered to indicate a
statistically significant difference. Each experiment was performed
in triplicate.
Results
Cell viability
Flow cytometry assay results of each cell type in
each cell type sample are presented in Fig. 1 and Table
I. With the exception of the hADSCs, the mean survival rate of
each type, including the fibroblasts, HPDLCs, ARPE-19, HUVECs and
GES-1 reached >90%, and there was no statistically significant
difference detected between the experimental and control groups
(P=0.1362 in the fibroblast groups; P=0.0698 in the HPDLC group;
P=0.0664 in the ARPE-19 group; P=0.1217 in the HUVEC group;
P=0.0869 in the GES-1 group). By contrast, evident differences
among the three samples of hADSCs were observed, and a
statistically significant difference was detected between the
printed and unprinted groups (P=0.0112). According to the results,
it was concluded that all experimental groups attained viability
>80%, while other groups excluding the hADSCs achieved a
viability of >90%. Although, there was significant difference in
the hADSC group, the difference in the mean survival rate between
the printed and unprinted groups was within 5% (P=83.20±5.10 in the
printed group; P=87.57±5.29 in the unprinted group). Thus the
results indicate that the electrospraying process scarcely affected
the cell viability.
 | Table I.Cytometry assay results of cell
survival rate following bioprinting of different cell types. |
Table I.
Cytometry assay results of cell
survival rate following bioprinting of different cell types.
|
| Survival rate
(%) |
|
|---|
|
|
|
|
|---|
|
| Sample 1 | Sample 2 | Sample 3 | Mean survival |
|
|---|
|
|
|
|
|
|
|
|---|
| Cell type | Exp | Ctrl | Exp | Ctrl | Exp | Ctrl | Exp | Ctrl | P-value |
|---|
| Fibroblasts | 96.0 | 96.5 | 94.7 | 98.0 | 94.1 | 96.2 | 94.93±0.97 | 96.90±0.96 | 0.1362 |
| hADSCs | 77.7 | 81.6 | 91.3 | 91.7 | 88.4 | 90.3 | 83.20±5.10 | 87.57±5.29 | 0.0112 |
| HPDLCs | 95.2 | 96.6 | 94.1 | 95.0 | 95.5 | 96.0 | 94.93±0.74 | 95.87±0.81 | 0.0698 |
| ARPE-19 | 91.8 | 92.8 | 89.2 | 91.5 | 90.0 | 92.9 | 90.33±1.33 | 92.40±0.78 | 0.0664 |
| HUVECs | 97.5 | 98.5 | 96.0 | 98.2 | 98.0 | 98.6 | 97.83±0.29 | 98.43±0.21 | 0.1217 |
| GES-1 | 93.3 | 94.0 | 92.6 | 93.1 | 91.1 | 91.7 | 91.77±1.50 | 93.23±0.71 | 0.0869 |
Morphological characteristic
assay
Fig. 2 shows the
results of the observation of cellular morphology under a
phase-contrast microscope for 5 days after electrospraying. All the
cells in the printed groups presented similar growth to the control
cells. In addition, there was no observable difference in the
morphology and cell density between the printed and unprinted cells
at any of the time-points investigated.
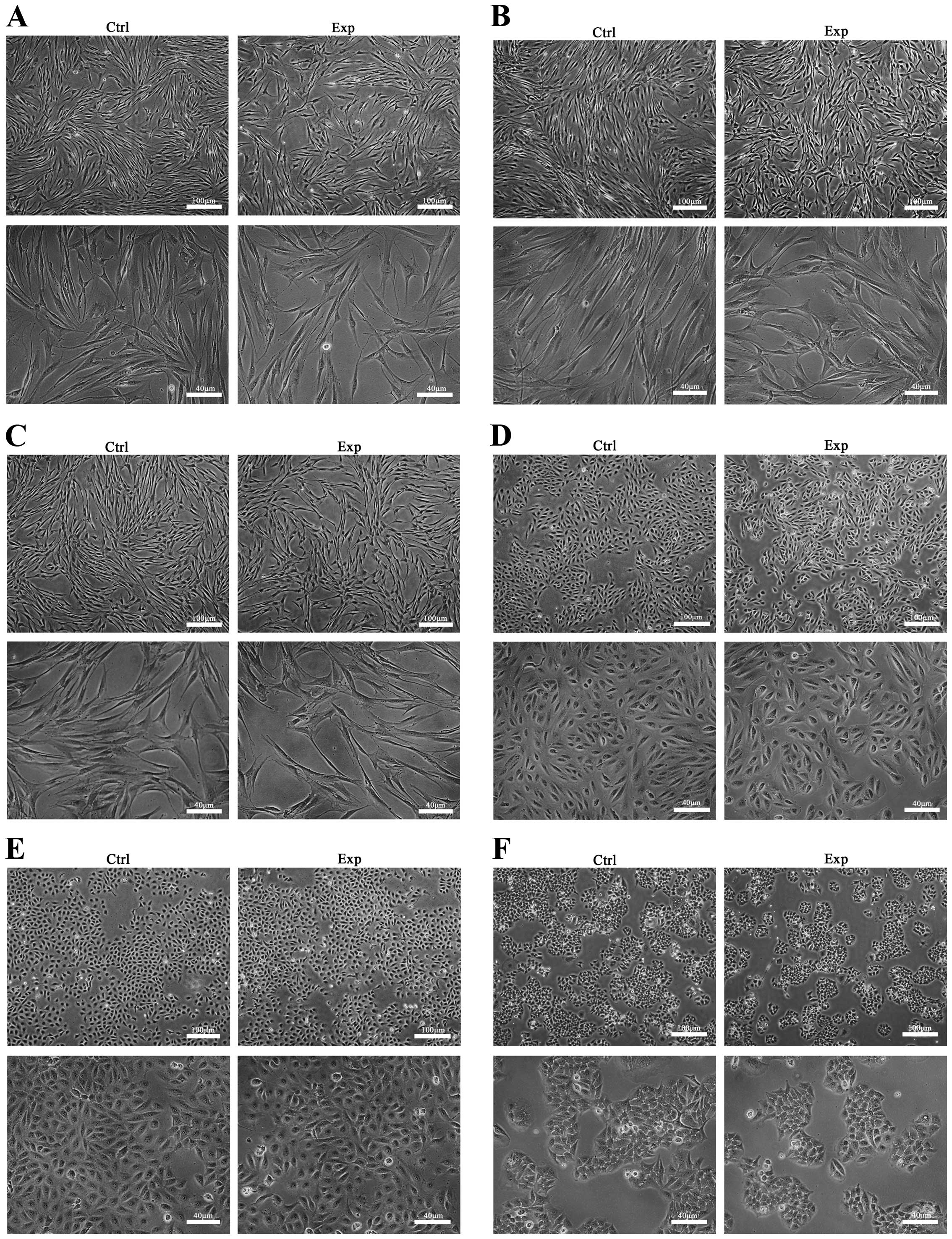 | Figure 2.Cellular morphology observation under
phase-contrast microscope at 5 days after electrospraying. The
observed cell morphology in the in vitro cultures of (A)
human fibroblasts, (B) hADSCs, (C) HPDLCs, (D) ARPE-19, (E) HUVECs
and (F) GES-1 exhibited similar shape and cell density between the
experimental and control groups. Images are shown at magnifications
of ×40 (top image) and ×100 (lower image) for each group/cell type.
Exp, experimental group; Ctrl, control group; hADSCs, human
adipose-derived stem cells; HPDLCs, human periodontal ligament
cells; ARPE-19, adult retinal pigment epithelial cells; HUVECs,
human umbilical vascular endothelial cells; GES-1, human gastric
epithelial cells. |
Cell proliferation after printing
The CCK-8 assay was used to detect OD values of the
experimental and control groups between 0 and 5 days of incubation.
As shown in Fig. 3, for all cell
types, the cells proliferated stably during the 5-day culture
period, and the cell growth trend was similar between the
experimental and control groups. As shown in Table II, the results indicated that there
was no significant difference in the OD values between the
experimental and control groups for all cells (P>0.05), with the
exception of the hADSCs (P=0.0155). This suggests that the
proliferation rate in the logarithmic growth period and plateau
period of the experimental groups was basically consistent with
that observed in the control group. Therefore, the printing process
did not have a significantly negative effect on the proliferation
of the majority of cell types.
 | Table II.Statistical difference of OD values
between the experimental and control groups (n=9). |
Table II.
Statistical difference of OD values
between the experimental and control groups (n=9).
|
|
OD
values |
|
|---|
|
|
|
|
|---|
|
| Day 0 | Day1 | Day 2 | Day 3 | Day 4 | Day 5 |
|---|
|
|
|
|
|
|
|
|
|---|
| Cell type | Ctrl | Exp | Ctrl | Exp | Ctrl | Exp | Ctl | Exp | Ctrl | Exp | Ctrl | Exp | P-value |
|---|
| Fibroblasts | 0.017±0.001 | 0.022±0.001 | 0.120±0.003 | 0.125±0.005 | 0.229±0.007 | 0.215±0.003 | 0.443±0.012 | 0.398±0.009 | 0.844±0.019 | 0.707±0.006 | 1.340±0.018 | 1.201±0.051 | 0.1044 |
| hADSCs | 0.034±0.003 | 0.019±0.002 | 0.300±0.005 | 0.232±0.020 | 0.862±0.043 | 0.682±0.031 | 1.366±0.045 | 1.059±0.053 | 1.686±0.030 | 1.354±0.029 | 1.814±0.067 | 1.499±0.044 | 0.0155 |
| HPDLCs | 0.033±0.002 | 0.041±0.005 | 0.090±0.010 | 0.098±0.004 | 0.308±0.030 | 0.277±0.023 | 0.635±0.030 | 0.560±0.021 | 0.980±0.048 | 0.804±0.045 | 1.264±0.050 | 0.981±0.056 | 0.1167 |
| ARPE-19 | 0.015±0.001 | 0.023±0.001 | 0.210±0.003 | 0.221±0.004 | 0.572±0.013 | 0.528±0.014 | 1.214±0.0176 | 1.042±0.028 | 1.835±0.0192 | 1.575±0.015 | 2.309±0.016 | 1.765±0.026 | 0.1137 |
| HUVECs | 0.022±0.002 | 0.023±0.001 | 0.280±0.008 | 0.318±0.006 | 0.726±0.011 | 0.699±0.018 | 1.466±0.014 | 1.197±0.033 | 2.296±0.050 | 1.987±0.024 | 2.443±0.037 | 2.339±0.022 | 0.1335 |
| GES-1 | 0.025±0.001 | 0.027±0.002 | 0.197±0.003 | 0.200±0.002 | 0.468±0.006 | 0.442±0.005 | 0.900±0.009 | 0.760±0.012 | 1.566±0.020 | 1.315±0.014 | 2.394±0.028 | 1.885±0.023 | 0.1198 |
As mentioned earlier, all these preliminary results
suggest that various types of human cells can be successfully
printed by the electrospraying technology. Furthermore,
electrosprayed cells were able to survive following printing, as
well as to maintain their cellular morphology and proliferation
capacity.
Discussion
In the present study, the cell electrospraying
technology was adopted to directly print multiple living cells, in
order to investigate its application as a simple and cost-effective
system to produce high-throughput biomaterials. During the
electrospraying process, droplets carry an electric charge that
inhibits droplet accumulation. Scattered droplets, with a size
within the micro- and nanoscale, are efficiently generated by
changing the electric field energy (16–19). Thus,
droplet movement tracks and gathering may be controlled by the
movement of the nozzle, and the distance between the nozzle and the
container. In addition, cell suspensions loaded into the printer
are precisely placed with the assistance of CAD software, while the
cell density can be controlled by the flow rate and the diameter of
the nozzle (18,20).
The present study indicated that multiple types of
human cells can be delivered successfully using an electrospraying
printer. A previous study observed that cell viability was retained
subsequent to electrospraying (19).
Consistent with this previous finding, the current study
demonstrated that bio-electrosprayed cells have a high cell
viability of >80% (Fig. 1). In
addition, the present study identified that these cells were able
to proliferate (Fig. 3) and retain
normal morphological characteristics (Fig.
2), indicating that damage, caused by heat and mechanical
stress during printing, was not observed. Although the present
study demonstrates that electrospray maintains satisfactory cell
viability with high-throughput printing, driven by the appropriate
voltage and flow rate, this technique continues to present
challenges in offering a stable environment via a high
concentration of ions (16,20).
Since the ultimate goal of bioprinting is to perform
in situ tissue repair with autologous cells, therapeutically
treating tissue loss and organ failure with cell substitutes in
vivo using extracorporeal devices remains significant (10). However, different cell types that are
derived from germ layers in the human body may demonstrate a
distinctly different biological performance when investigated in
vitro and in vivo, particularly considering that the
exogenous stimulation in environmental conditions can lead to cell
phenotype shifts (21). Besides,
mammalian cells strongly depend on the culture conditions and are
much more sensitive to heat and mechanical stress (15). In addition, the cell numbers and types,
spatial arrangement of cells, and interaction between the cells and
the extracellular microenvironment in a 3D structure remain largely
intractable, which affects the cell morphology, mechanical behavior
and adhesion ability more than planar substrates (10,22). Thus,
building human tissue analogues in the reconstructed
microenvironment remains a challenge.
Examining the feasibility of bioprinting multiple
cell types may help to rebuild the human tissue and cell therapy
in situ. In the present study, six types of human cells were
selected to be directly printed in the same printing setting, and
the cell survival and proliferation were analyzed subsequent to
printing. Five of the investigated cell types demonstrated no
significant difference in cell survival and proliferation when
comparing the experimental and control groups, suggesting the
feasibility of directly printing human cells in the printing
environment used in the current study. However, differences between
the experimental and control groups were observed for hADSCs in the
present study, with evident differences in the mean survival rates
after printing among the three samples examined (sample 1, 77.7%;
sample 2, 91.3%; sample 3, 88.4%). These differences may be due to
the printing process or may result from the sample source with
individual differences; therefore, the difference in cell survival
in hADSCs should be studied further. In addition, three types of
cells separated from human tissue and three cell lines were
selected in the current study, and the cell lines demonstrated
higher stability in the printing environment; therefore,
differences may appear in the autologous cell printing process.
Furthermore, it should be noted that only preliminary research was
conducted on the cell survival ability of these multiple cell types
after printing, while function tests still need to be further
explored. In a further study, we will verify the feasibility of
synchronous printing in a variety of human cells within
bio-scaffolds with a combination of bio-electrospraying and
electrospinning in a coaxial configuration. In this way, the cyclic
steps of traditional tissue engineering will be reduced, including
the cell seeding and incubation in the construction of scaffolds
(23), while vascularized functional
living tissues or organs suitable for clinical implantation will be
fabricated in a shorter period of time. Bio-electrospraying has
far-reaching implications and will enable significant advances in a
wide range of fields, from tissue engineering to regenerative
medicine.
In conclusion, the present study demonstrated the
feasibility of using electrospraying technology to directly print
living cells under appropriate conditions for biological and
biomedical applications. The ability of this technique to organize
multiple components at the appropriate time, position and amount in
the well-defined 3D architecture of their native organs requires
further exploration. The prospect of using the autologous cells to
build bioactive models of functional tissues and organ substitutes
offers a potentially revolutionary development to biomedical
research and health care in the future.
Acknowledgements
Financial support was provided by the National
Natural Science Foundation of China (grant no. 81372097), The
Shanghai Committee of Science and Technology, China (grant
nos.14441900800 and 14441900802), Project of Shanghai Jiao Tong
University Medical and Engineering Cross Fund (YG2014MS06) and
Shanghai Municipal Education Commission-Gaofeng Clinical Medicine
Grant Support (20161420).
References
|
1
|
Langer R and Vacanti JP: Tissue
engineering. Science. 260:920–926. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ikada Y: Challenges in tissue engineering.
J R Soc Interface. 3:589–601. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mironov V, Kasyanov V, Drake C and
Markwald RR: Organ printing: Promises and challenges. Regen Med.
3:93–103. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Norotte C, Marga FS, Niklason LE and
Forgacs G: Scaffold-free vascular tissue engineering using
bioprinting. Biomaterials. 30:5910–5917. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boland T, Xu T, Damon B and Cui X:
Application of inkjet printing to tissue engineering. Biotechnol J.
1:910–917. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hutmacher DW, Sittinger M and Risbud MV:
Scaffold-based tissue engineering: Rationale for computer-aided
design and solid free-form fabrication systems. Trends Biotechnol.
22:354–362. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Emmert MY, Hitchcock RW and Hoerstrup SP:
Cell therapy, 3D culture systems and tissue engineering for cardiac
regeneration. Adv Drug Deliv Rev 69–70. 254–269. 2014. View Article : Google Scholar
|
|
8
|
Nahmias Y, Schwartz RE, Verfaillie CM and
Odde DJ: Laser-guided direct writing for three-dimensional tissue
engineering. Biotechnol Bioeng. 92:129–136. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mironov V, Boland T, Trusk T, Forgacs G
and Markwald RR: Organ printing: Computer-aided jet-based 3D tissue
engineering. Trends Biotechnol. 21:157–161. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miller JS: The billion cell construct:
Will three-dimensional printing get us there? PLoS Biol.
12:e10018822014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moon S, Hasan SK, Song YS, Xu F, Keles HO,
Manzur F, Mikkilineni S, Hong JW, Nagatomi J, Haeggstrom E, et al:
Layer by layer three-dimensional tissue epitaxy by cell-laden
hydrogel droplets. Tissue Eng Part C Methods. 16:157–166. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Marga F, Neagu A, Kosztin I and Forgacs G:
Developmental biology and tissue engineering. Birth Defects Res C
Embryo Today. 81:320–328. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tasoglu S and Demirci U: Bioprinting for
stem cell research. Trends Biotechnol. 31:10–19. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jayasinghe SN, Eagles PA and Qureshi AN:
Electric field driven jetting: An emerging approach for processing
living cells. Biotechnol J. 1:86–94. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu T, Jin J, Gregory C, Hickman JJ and
Boland T: Inkjet printing of viable mammalian cells. Biomaterials.
26:93–99. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Townsend-Nicholson A and Jayasinghe SN:
Cell electrospinning: A unique biotechnique for encapsulating
living organisms for generating active biological
microthreads/scaffolds. Biomacromolecules. 7:3364–3369. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jayasinghe SN, Warnes G and Scotton CJ:
Bio-electrosprayed living composite matrix implanted into mouse
models. Macromol Biosci. 11:1364–1369. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jayasinghe SN, Qureshi AN and Eagles PA:
Electrohydrodynamic jet processing: an advanced
electric-field-driven jetting phenomenon for processing living
cells. Small. 2:216–219. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Clarke JD and Jayasinghe SN:
Bio-electrosprayed multicellular zebrafish embryos are viable and
develop normally. Biomed Mater. 3:0110012008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fenn JB, Mann M, Meng CK, Wong SF and
Whitehouse CM: Electrospray ionization for mass spectrometry of
large biomolecules. Science. 246:64–71. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stegemann JP and Nerem RM: Altered
response of vascular smooth muscle cells to exogenous biochemical
stimulation in two- and three-dimensional culture. Exp Cell Res.
283:146–155. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miron-Mendoza M, Koppaka V, Zhou C and
Petroll WM: Techniques for assessing 3-D cell-matrix mechanical
interactions in vitro and in vivo. Exp Cell Res. 319:2470–2480.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jayasinghe SN: Cell electrospinning: A
novel tool for functionalising fibres, scaffolds and membranes with
living cells and other advanced materials for regenerative biology
and medicine. Analyst (Lond). 138:2215–2223. 2013. View Article : Google Scholar
|

















