Introduction
Soyasaponin is classified among the sugar conjugates
of triterpenes, and can take various chemical and sugar-chain
structures, such as soyasapogenol. Numerous saponins have
demonstrated antimutagenic, anticarcinogenic and antimetastatic
effects against multiple cell lines (1). Triterpenoid saponins are natural sugar
conjugates of triterpenes that possess various biological effects.
Soyasaponin exerts antiviral effects on herpes simplex virus, human
cytomegalovirus, influenza virus, and human immunodeficiency virus
through its suppression of gene and viral protein synthesis
(2,3).
Soyasaponin was reported to suppress cancer cell growth in the
colon cancer cell lines (4–6), and reduced colon cancer rates were
identified in individuals who consume large amounts of soy legumes
(7), suggesting that soyasaponins may
have a protective effect against colon cancer. In addition,
soyasaponin showed hypotensive action in spontaneous hypertension
(8).
Soyasapogenol, an aglycon of soyasaponin, extracted
from Glycine max Merr, 22β-methoxyolean-12-ene-3β, 24(4β)-diol
(ME3738) is a derivative of soyasapogenol B. ME3738 has been
reported to ameliorate liver damage in several experimental models
of acute and chronic liver injury induced by concanavalin A (Con
A), ethanol, lithocholate, and bile duct ligation (9–13).
ME3738 induces interleukin-6 (IL-6) expression, and
serum amyloid A and α1 acid glycoprotein as downstream targets of
the IL-6 signal protecting against Con A-induced liver injury.
Hepatocellular carcinoma (HCC) is one of the most
common cancer types worldwide and a leading cause of cancer
mortality, especially in countries with a high prevalence of
chronic infections with hepatitis B virus and hepatitis C virus
(HCV). ME3738 suppresses HCV replication by inhibiting mRNA in HCV
replicon cells, along with NS3 and core proteins (14). Saibara et al treated chronic
hepatitis C patients for 48 weeks with a combination of PEG
interferon (IFN)-α-2b and ME3738 (15). Authors of that study reported that the
subjects became HCV RNA-negative during the administration, and
that combination treatment was very safe with no side effects other
than those seen with PEG IFN-α-2b alone. ME3738 is effective in
treating chronic hepatitis C, however, to the best of our
knowledge, there are no reports available on the effect of ME3738
on HCC. In the present study, we investigated the antiproliferative
effects of ME3738 on HCC cell lines.
Materials and methods
Cell lines and cell culture
The present study used 11 HCC cell lines (KIM-1,
KYN-1, KYN-2, KYN-3, HAK-1A, HAK-1B, HAK-2, HAK-3, HAK-4, HAK-5 and
HAK-6). The HCC cell lines were originally established in our
laboratory, and each cell line retained the morphological and
functional features of the original tumor as described elsewhere
(16–22). The cells were grown in Dulbecco's
modified Eagle's medium (Nissui Pharmaceutical, Tokyo, Japan) and
supplemented with 2.5% heat-inactivated (56°C, 30 min) fetal bovine
serum (Bioserum, Victoria, Australia), 100 U/ml penicillin, 100
µg/ml streptomycin (Gibco BRL, Gaithersburg, MD, USA) and 12 mmol/l
sodium bicarbonate, in a humidified atmosphere of 5% CO2
in air at 37°C.
Effects of ME3738 on the proliferation
of HCC cell lines in vitro
The cells (1.5–6.5×103 cells/well) were
seeded 96-well plates (Thermo Fisher Scientific, Roskilde,
Denmark), cultured for 24 h and the medium was replaced with ME3738
(Meiji Seika Pharm Co., Ltd., Tokyo, Japan; 0, 0.08, 0.16, 0.32,
0.63, 1.25, 2.5, 5 and 10 µM). After culturing for 24, 48 or 72 h,
the number of viable cells was examined using MTT cell growth assay
kits (Chemicon International, Inc., Temecula, CA, USA). The 50%
inhibitory concentration (IC50) of each cell line was
estimated at 24 h of culture with ME3738.
Quantitative analysis of
ME3738-induced apoptosis in vitro
Cells cultured with or without ME3738 (1 µM) for 72
h were stained with the Annexin V-enhanced green fluorescent
protein (EGFP) Apoptosis Detection kits (Medical and Biological
Laboratories Co., Ltd., Nagoya, Japan) according to the
manufacturer's protocol. After staining, the cells were analyzed
using a FACScan (BD Biosciences, San Jose, CA, USA), and the
Annexin V-EGFP-positive apoptotic cell rate was determined.
Effects of ME3738 on cell cycle
HAK-1B and HAK-4 were cultured with ME3738 (0.63 or
2.5 µM) for 12, 24 or 48 h, labeled with 10 µM BrdU for 30 min,
fixed in 70% cold ethanol at 4°C overnight, stained with anti-BrdU
and propidium iodide, and then analyzed using a FACScan. Staining
was performed using the modified technique described elsewhere
(23).
Effects of ME3738 with or without
PEG-IFN-α-2b on the proliferation of HCC cell lines in vitro
The cells (1.5–6.5×103 cells/well) were
seeded in 96-well plates (Thermo Fisher Scientific), cultured for
24 h and the medium was replaced with ME3738 (0, 0.1 or 0.5 µM)
with or without PEG-IFN-α-2b (PEGIntron®; MSD K.K.,
Tokyo, Japan; 0 or 1,000 IU/ml). After culturing for 72 h, the
number of viable cells was examined using MTT cell growth assay
kits (Chemicon International, Inc.).
Effects of ME3738 with or without
PEG-IFN-α-2b on the proliferation of HCC cell lines in BALB/c
mice
HAK-1B cells (1×107 cells/mouse) were
transplanted subcutaneously into the backs of 4-week-old female
BALB/c mice. After tumor formation was confirmed, the mice were
divided into four groups (n=7 in each group), i.e., control group,
ME3738 alone group, PEG-IFN-α-2b alone group and
ME3738+PEG-IFN-α-2b (combination) group. ME3738 was mixed with food
(1.5 mg/g) and was taken orally for 15 days. PEG-IFN-α-2b (1,920
IU/mouse) was subcutaneously injected twice a week for two
consecutive weeks. The clinical dose of PEG-IFN-α-2b in chronic
hepatitis C treatment was 9.6×104 IU/kg and was 3-fold
that of the lowest dose (3.2×104 IU/kg) in the
experiment. The tumor size was measured in two directions using
calipers (until day 15), and the tumor volume (mm3) was
estimated using the equation ‘length × (width)2 × 0.5′.
On day 15, the mice were sacrificed by cervical dislocation and the
resected tumor was fixed in formalin and prepared into paraffin
sections.
In addition, immunostaining was performed with
anti-mouse endothelial cell antibody (anti-mouse CD34 monoclonal
antibody, clone MEC14.7, final dilution, 1/100, cat. no. MCA1825;
AbD Serotec, Raleigh, NC, USA) and anti-human Ki-67 monoclonal
antibody (clone MIB-1, final dilution, 1/50, cat. no. M724029;
Dako, Carpinteria, CA, USA).
The number of blood vessels and MIB-1 index in a
unit area (mm2) of every section was calculated and its
mean was obtained.
The present study was approved by the Ethics
Committee of Kurume University (approval no. 10248). Animal
experiments for the current study were approved by the Ethics
Review Committee for Animal Experimentation of Kurume University
School of Medicine.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Comparisons between groups were performed using Student's t-test
and two-factor factorial ANOVA. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of ME3738 on the proliferation
of HCC cell lines in vitro
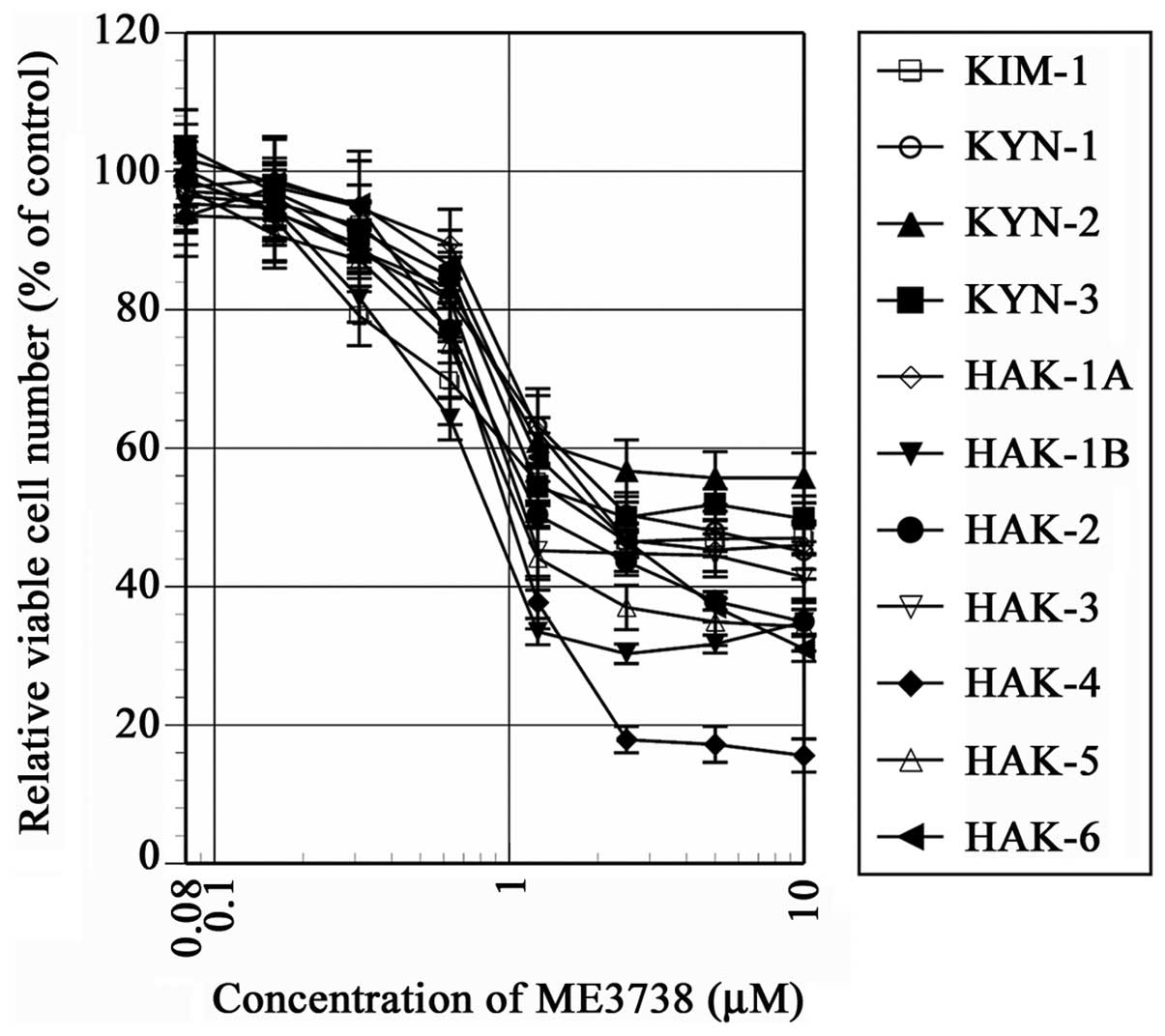
In all the cell lines, a dose-dependent
antiproliferative effect was observed at various degrees in 24, 48
and 72 h cultures with ME3738. The cell number was suppressed in 10
cell lines to <50% of the control at 24 h (Fig. 1) and the suppression was statistically
significant in all the cell lines with a dose range of 0.08–10 µM
(P<0.001). The IC50 of ME3738 cultured for 24 h was
0.8–2.4 µM, with the exception of KYN-2 (Table I).
 | Table I.IC50 of ME3738 in the cell
lines. |
Table I.
IC50 of ME3738 in the cell
lines.
| Cell line | IC50
(µM) |
|---|
| KIM-1 | 1.8 |
| KYN-1 | 2.0 |
| KYN-2 | ND |
| KYN-3 | 2.0 |
| HAK-1A | 2.0 |
| HAK-1B | 0.8 |
| HAK-2 | 1.9 |
| HAK-3 | 1.2 |
| HAK-4 | 1.4 |
| HAK-5 | 1.1 |
| HAK-6 | 2.4 |
Quantitative analysis of
ME3738-induced apoptosis in vitro
Quantitative analysis of apoptosis revealed ME3738
did not induce a significant increase in the amount of apoptosis
(Table II).
 | Table II.Quantitative analysis of apoptosis
induced by ME3738 in 11 liver cancer cell lines. |
Table II.
Quantitative analysis of apoptosis
induced by ME3738 in 11 liver cancer cell lines.
|
| Annexin
V-EGFP-positive apoptotic cells (%) |
|---|
|
|
|
|---|
| Cell line | Control | ME3738 |
|---|
| KIM-1 | 6.4±1.3 | 9.1±2.1 |
| KYN-1 | 9.6±1.1 | 10.7±0.8 |
| KYN-2 | 13.6±3.2 | 16.8±1.5 |
| KYN-3 | 6.9±1.3 | 6.9±1.3 |
| HAK-1A | 7.9±1.6 | 7.3±3.5 |
| HAK-1B | 12.9±1.8 | 12.8±2.1 |
| HAK-2 | 8.6±2.4 | 7.9±2.0 |
| HAK-3 | 9.2±1.9 | 9.9±0.8 |
| HAK-4 | 6.3±2.1 | 5.3±1.8 |
| HAK-5 | 7.9±1.2 | 7.1±0.8 |
| HAK-6 | 5.4±3.4 | 5.6±2.9 |
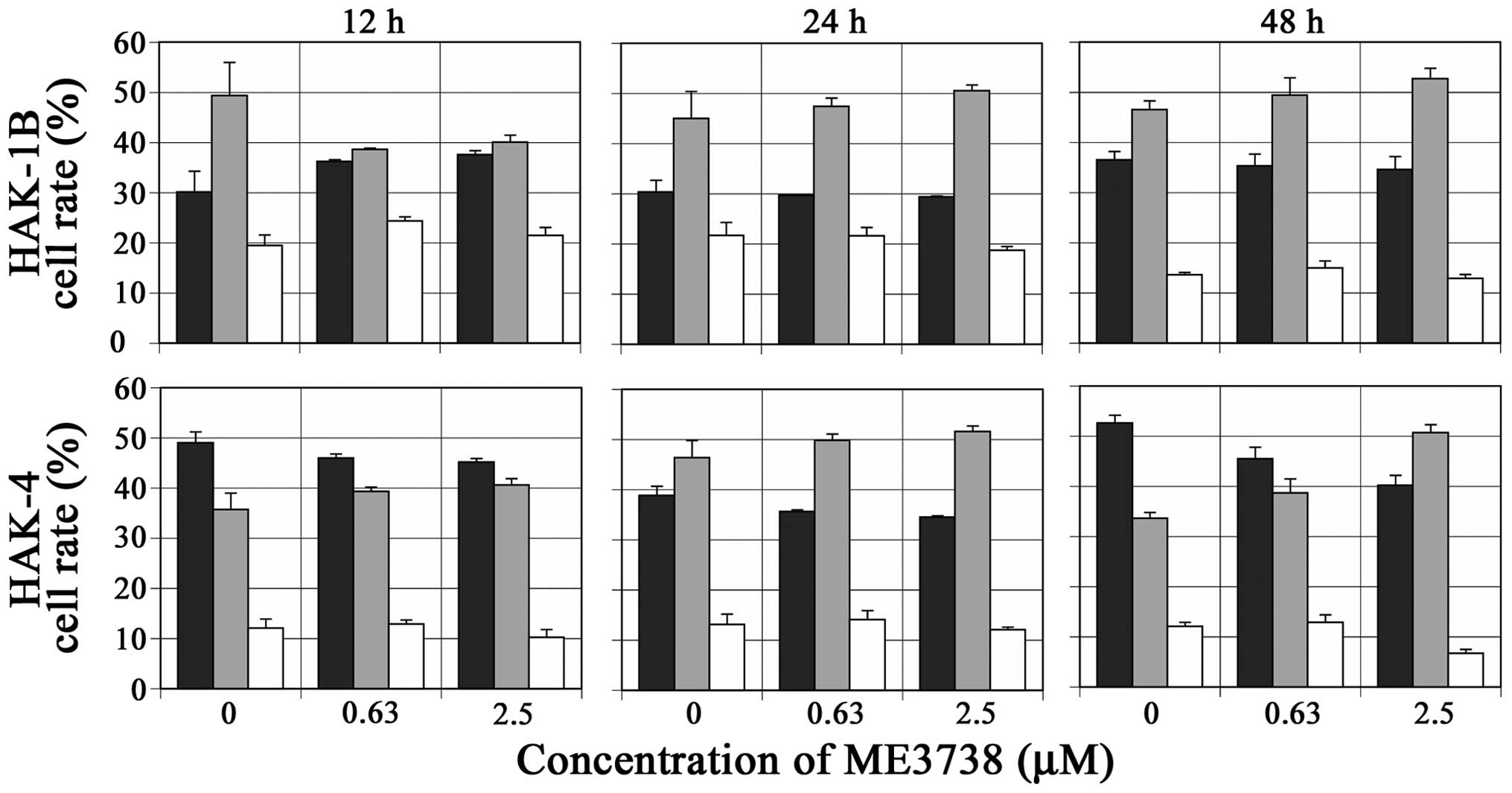
Effects of ME3738 on cell cycle
In HAK-1B and HAK-4, the ratio of the cells in the
S-phase increased, and ratio of the cells in the G2/M phase
slightly decreased or did not change, compared with those in the
control cells at any time-point 12–48 h after the addition of
ME3738 (Fig. 2).
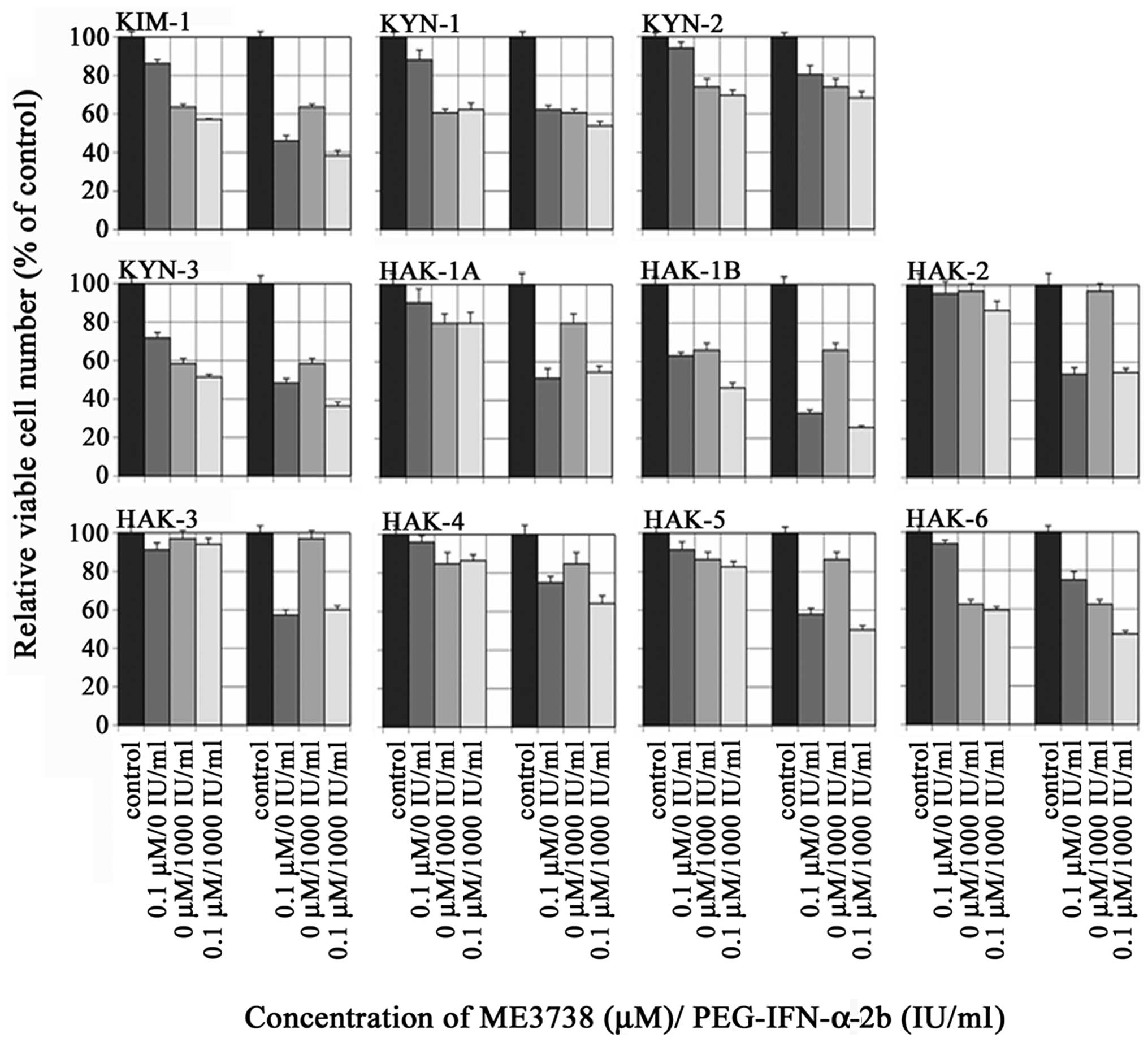
Effects of ME3738 with or without
PEG-IFN-α-2b on the proliferation of HCC cell lines in vitro
A total of 72 h after the addition of PEG-IFN-α-2b
alone, the relative viable cell number was suppressed in all the
cell lines. The combination treatment of ME3738 and PEG-IFN-α-2b
did not exert synergistic antiproliferative effects in any cell
lines (Fig. 3).
Effects of ME3738 with or without
PEG-IFN-α-2b on the proliferation of HCC cell lines in nude
mice
On day 15, the tumor volume of mice receiving ME3738
(P<0.05), PEG-IFN-α-2b (P<0.001), and ME3738+PEG-IFN-α-2b
(P<0.001) was 69.3, 30.9, and 33.5%, respectively, of the
control tumor volume (Fig. 4). The
MIB-1 index showed >70% positive cells in all the groups, and
there were no significant differences in the mean number of blood
vessels per unit area among the groups (data not shown).
Discussion
We have previously reported that PEG-IFN-α-2b and
IFN-β, which are widely used in the treatment of HCV, suppress cell
proliferation by inducing apoptosis and/or cell cycle arrest in
many HCC cell lines (23,24). However, some cell lines showed low
sensitivity for PEG-IFN-α-2b and IFN-β on proliferation. By
contrast, ME3738 was able to suppress cell proliferation at various
concentrations in all the cell lines we studied, including those
with low sensitivity to IFN. Even KYN-2, which had the lowest
degree of cell suppression, showed a 40% inhibition of cell
proliferation at doses of ≥1.25 µM.
The findings of the present study suggest that the
suppression of HCC cell proliferation by ME3738 was due, not to
apoptosis, but rather to the inhibition of cell cycle progression.
ME3738 upregulates the production of IFN-β in non-neoplastic liver
cells (25). We examined the effect of
ME3738 on IFN-β expression in HAK-1B cells by microarray, but no
change in IFN-β expression was observed (data not shown). Our
previous studies have reported that the suppression of cell
proliferation by IFN-β in HCC cell lines was primarily due to
apoptosis (24). However, the present
study was unable to confirm the induction of apoptosis by ME3738,
suggesting that the mechanism of induction of IFN-β by ME3738 may
differ between non-neoplastic and neoplastic cells.
ME3738 was reported to increase the expression of
IL-6 in the liver (26). From the
point of view of host defense, IL-6 is a multifunctional cytokine
that, not only plays an important role in the immune reaction, but
is also involved in acute phase response and cell growth and
differentiation. ME3738 is known to induce IL-6 by increasing the
expression of Bcl-2 and Bcl-xL, which have anti-apoptotic effects,
thereby protecting the cells from Fas-mediated death. Furthermore,
the anti-apoptotic effects of IL-6 are reported to be mediated by
downstream STAT3 signaling (27).
Therefore, although it is not direct, ME3738 has an anti-apoptotic
effect. We did not observe anti-proliferative effects of HCC cells
mediated by apoptosis in the present study. Thus studies should be
conducted. On the other hand, S-phase cell cycle arrest was
observed in two of the HCC cell lines we studied. Ellington et
al similarly reported that soyasaponin B inhibited the
activation of cyclin-dependent kinase-2 and induced S-phase arrest
in a colon cancer cell line (28).
However, a different study found that saponins such as ginsenoside
induced G0/G1 arrest in multiple cell lines
(1), suggesting that cell-cycle
regulation by saponin differs according to cell type or saponin
source.
With regard to the suppression of tumor formation in
nude mice, ME3738 alone significantly inhibited tumorigenicity
compared with the control, but we found with PEG-IFN-α-2b alone
that tumor diameters at day 15 of administration were approximately
the same as that at the start of the experiment, and combination
treatment with ME3738 and PEG-IFN-α-2b produced no additive or
synergistic effects on tumor suppression, similar to the in
vitro results. CD34 immunostaining revealed no differences in
the number of blood vessels among the groups, indicating that
ME3738 had no antiangiogenic effect. There was no significant
difference on MIB-1, which is known to be a marker of cell
proliferation in many tumors, among the groups. Tumor volume
decreased significantly in the treatment groups with ME3738 as
compared with the control. MIB-1 is detected in all the phases
except G0/G1, and one of the reasons that no
differences in MIB-1 expression were seen in the mouse tissue may
be that, as ME3738 showed S-phase arrest in vitro, ME3738
treatment in HAK-1B-transplanted mice may have induced S-phase
arrest, with the result that similar levels of MIB-1-positive cells
were observed in the ME3738-treated mice and in the controls. In
addition, S-phase arrest in vitro, while not remarkable, may
help account for the fact that MIB-1 expression showed no change
after ME3738 administration in vivo, regardless of the
number of doses or length of observation period.
IFN has been widely used for therapies for chronic
hepatitis C. ME3738 promotes the secretion of INF-β and phase II
trials of the combination treatment with ME3738 and PEG-IFN-α-2a
were conducted to determine whether the combination treatment would
increase the rate of patients achieving a sustained virological
response (SVR). However, a clear additional effect on SVR was not
confirmed in phase II trials (15).
Furthermore, direct acting antivirals (DAA) radically altered the
treatment of hepatitis C (29). Oral
administration of DAAs, which acts directly to suppress HCV
replication, is able by itself to achieve high rates of SVR, and as
a result the use of IFN to treat chronic hepatitis C no longer
constituted an issue.
The introduction of DAAs was expected to further
improve SVR rates and reduce the incidence of HCC associated with
HCV (30–36). Nevertheless, it has been reported that
even among patients who achieved SVR, some still develop HCC, and
risk factors of HCC are known to affect the elderly, male gender,
advanced liver fibrosis, and high AFP levels (30–36). In a
rat bile duct ligation model, ME3738 was shown to inhibit hepatic
stellate cell activation and collagen synthesis, resulting in the
suppression of liver fibrosis (13).
Furthermore, no significant adverse effects on the combination
treatment of ME3738 and Peg-IFN-α2a in the clinical trials have
been reported thus far, and the safety of ME3738 treatment in
chronic hepatitis patients has been confirmed (15). ME3738 may exert a protective effect
against hepatocarcinogenesis through the inhibition of hepatic
fibrosis. Our current study shows that, ME3738 suppressed cell
proliferation to varying degrees in all the HCC cell lines we
tested. In conclusion, our findings suggest that ME3738 may exert
antitumorigenic effects in a wide range of HCC patients, and that
when used in combination with current therapeutic medications it
may function as a modulator to increase the antitumorigenic effect
in HCC.
Acknowledgements
We would like to thank Ms. Akemi Fujiyoshi for her
assistance in our experiment.
References
|
1
|
Rao AV and Gurfinkel DM: The bioactivity
of saponins: Triterpenoid and steroidal glycosides. Drug Metabol
Drug Interact. 17:211–235. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hayashi K, Hayashi H, Hiraoka N and
Ikeshiro Y: Inhibitory activity of soyasaponin II on virus
replication in vitro. Planta Med. 63:102–105. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Simões CM, Amoros M and Girre L: Mechanism
of antiviral activity of triterpenoid saponins. Phytother Res.
13:323–328. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gurfinkel DM and Rao AV: Soyasaponins: The
relationship between chemical structure and colon anticarcinogenic
activity. Nutr Cancer. 47:24–33. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim HY, Yu R, Kim JS, Kim YK and Sung MK:
Antiproliferative crude soy saponin extract modulates the
expression of IkappaBalpha, protein kinase C, and cyclooxygenase-2
in human colon cancer cells. Cancer Lett. 210:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sung MK, Kendall CW, Koo MM and Rao AV:
Effect of soybean saponins and gypsophilla saponin on growth and
viability of colon carcinoma cells in culture. Nutr Cancer.
23:259–270. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Spector D, Anthony M, Alexander D and Arab
L: Soy consumption and colorectal cancer. Nutr Cancer. 47:1–12.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hiwatashi K, Shirakawa H, Hori K, Yoshiki
Y, Suzuki N, Hokari M, Komai M and Takahashi S: Reduction of blood
pressure by soybean saponins, renin inhibitors from soybean, in
spontaneously hypertensive rats. Biosci Biotechnol Biochem.
74:2310–2312. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Klein C, Wüstefeld T, Heinrich PC, Streetz
KL, Manns MP and Trautwein C: ME3738 protects from concanavalin
A-induced liver failure via an IL-6-dependent mechanism. Eur J
Immunol. 33:2251–2261. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kuzuhara H, Nakano Y, Yamashita N, Imai M,
Kawamura Y, Kurosawa T and Nishiyama S: Protective effects of
alpha1-acid glycoprotein and serum amyloid A on concanavalin
A-induced liver failure via interleukin-6 induction by ME3738. Eur
J Pharmacol. 541:205–210. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fukumura A, Tsutsumi M, Tsuchishima M,
Hayashi N, Fukura M, Yano H, Ozaki K and Takase S: Effect of the
inducer of interleukin-6 (ME3738) on rat liver treated with
ethanol. Alcohol Clin Exp Res (Suppl 1). 31:S49–S53. 2007.
View Article : Google Scholar
|
|
12
|
Nomoto M, Miyata M, Shimada M, Yoshinari
K, Gonzalez FJ, Shibasaki S, Kurosawa T, Shindo Y and Yamazoe Y:
ME3738 protects against lithocholic acid-induced hepatotoxicity,
which is associated with enhancement of biliary bile acid and
cholesterol output. Eur J Pharmacol. 574:192–200. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maeda K, Koda M, Matono T, Sugihara T,
Yamamoto S, Ueki M, Murawaki Y, Yamashita N and Nishiyama S:
Preventive effects of ME3738 on hepatic fibrosis induced by bile
duct ligation in rats. Hepatol Res. 38:727–735. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abe H, Imamura M, Hiraga N, Tsuge M,
Mitsui F, Kawaoka T, Takahashi S, Ochi H, Maekawa T, Hayes CN, et
al: ME3738 enhances the effect of interferon and inhibits hepatitis
C virus replication both in vitro and in vivo. J Hepatol. 55:11–18.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saibara T, Enomoto N, Kaneko S, Chayama K,
Sata M, Imawari M, Onishi S and Okita K: Clinical efficacy of
combination therapy with ME3738 and pegylated interferon-alpha-2a
in patients with hepatitis C virus genotype 1. Hepatol Res.
44:410–419. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Murakami T: Establishment and
characterization of human hepatoma cell line (KIM-1). Act Hepatol
Jpn. 25:532–539. 1984. View Article : Google Scholar
|
|
17
|
Yano H, Kojiro M and Nakashima T: A new
human hepatocellular carcinoma cell line (KYN-1) with a
transformation to adenocarcinoma. In Vitro Cell Dev Biol.
22:637–646. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yano H, Maruiwa M, Murakami T, Fukuda K,
Ito Y, Sugihara S and Kojiro M: A new human pleomorphic
hepatocellular carcinoma cell line, KYN-2. Acta Pathol Jpn.
38:953–966. 1988.PubMed/NCBI
|
|
19
|
Murakami T, Maruiwa M, Fukuda K, Kojiro M,
Tanaka M and Tanikawa K: Characterization of a new human hepatoma
cell line (KYN-3) derived from the ascites of the hepatoma patient.
Proceedings of the Japanese Cancer Association. Jpn J Cancer Res.
Tokyo. pp. 2921988;
|
|
20
|
Yano H, Iemura A, Fukuda K, Mizoguchi A,
Haramaki M and Kojiro M: Establishment of two distinct human
hepatocellular carcinoma cell lines from a single nodule showing
clonal dedifferentiation of cancer cells. Hepatology. 18:320–327.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Haramaki M, Yano H, Iemura A, Momosaki S,
Ogasawara S, Inoue M, Yamaguchi R, Kusaba A, Utsunomiya I and
Kojiro M: A new human hepatocellular carcinoma cell line (HAK-2)
forms various structures in collagen gel matrices. Hum Cell.
10:183–192. 1997.PubMed/NCBI
|
|
22
|
Utsunomiya I, Iemura A, Yano H, Akiba J
and Kojiro M: Establishment and characterization of a new human
hepatocellular carcinoma cell line, HAK-3, and its response to
growth factors. Int J Oncol. 15:669–675. 1999.PubMed/NCBI
|
|
23
|
Yano H, Iemura A, Haramaki M, Ogasawara S,
Takayama A, Akiba J and Kojiro M: Interferon alfa receptor
expression and growth inhibition by interferon alfa in human liver
cancer cell lines. Hepatology. 29:1708–1717. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ogasawara S, Yano H, Momosaki S, Akiba J,
Nishida N, Kojiro S, Moriya F, Ishizaki H, Kuratomi K and Kojiro M:
Growth inhibitory effects of IFN-beta on human liver cancer cells
in vitro and in vivo. J Interferon Cytokine Res. 27:507–516. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hiasa Y, Kuzuhara H, Tokumoto Y, Konishi
I, Yamashita N, Matsuura B, Michitaka K, Chung RT and Onji M:
Hepatitis C virus replication is inhibited by
22beta-methoxyolean-12-ene-3beta, 24(4beta)-diol (ME3738) through
enhancing interferon-beta. Hepatology. 48:59–69. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kovalovich K, Li W, DeAngelis R, Greenbaum
LE, Ciliberto G and Taub R: Interleukin-6 protects against
Fas-mediated death by establishing a critical level of
anti-apoptotic hepatic proteins FLIP, Bcl-2, and Bcl-xL. J Biol
Chem. 276:26605–26613. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Haga S, Ogawa W, Inoue H, Terui K, Ogino
T, Igarashi R, Takeda K, Akira S, Enosawa S, Furukawa H, et al:
Compensatory recovery of liver mass by Akt-mediated hepatocellular
hypertrophy in liver-specific STAT3-deficient mice. J Hepatol.
43:799–807. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ellington AA, Berhow M and Singletary KW:
Induction of macroautophagy in human colon cancer cells by soybean
B-group triterpenoid saponins. Carcinogenesis. 26:159–167. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kumada H, Suzuki Y, Ikeda K, Toyota J,
Karino Y, Chayama K, Kawakami Y, Ido A, Yamamoto K, Takaguchi K, et
al: Daclatasvir plus asunaprevir for chronic HCV genotype 1b
infection. Hepatology. 59:2083–2091. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Asahina Y, Tsuchiya K, Nishimura T,
Muraoka M, Suzuki Y, Tamaki N, Yasui Y, Hosokawa T, Ueda K,
Nakanishi H, et al: α-fetoprotein levels after interferon therapy
and risk of hepatocarcinogenesis in chronic hepatitis C.
Hepatology. 58:1253–1262. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ikeda M, Fujiyama S, Tanaka M, Sata M, Ide
T, Yatsuhashi H and Watanabe H: Risk factors for development of
hepatocellular carcinoma in patients with chronic hepatitis C after
sustained response to interferon. J Gastroenterol. 40:148–156.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Oze T, Hiramatsu N, Yakushijin T, Miyazaki
M, Yamada A, Oshita M, Hagiwara H, Mita E, Ito T, Fukui H, et al:
Osaka Liver Forum: Post-treatment levels of α-fetoprotein predict
incidence of hepatocellular carcinoma after interferon therapy.
Clin Gastroenterol Hepatol. 12:1186–1195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nagaoki Y, Aikata H, Nakano N, Shinohara
F, Nakamura Y, Hatooka M, Morio K, Kan H, Fujino H, Kobayashi T, et
al: Hiroshima Liver Study Group: Development of hepatocellular
carcinoma in patients with hepatitis C virus infection who achieved
sustained virological response following interferon therapy: A
large-scale, long-term cohort study. J Gastroenterol Hepatol.
31:1009–1015. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nagaoki Y, Aikata H, Miyaki D, Murakami E,
Hashimoto Y, Katamura Y, Azakami T, Kawaoka T, Takaki S, Hiramatsu
A, et al: Clinical features and prognosis in patients with
hepatocellular carcinoma that developed after hepatitis C virus
eradication with interferon therapy. J Gastroenterol. 46:799–808.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tokita H, Fukui H, Tanaka A, Kamitsukasa
H, Yagura M, Harada H and Okamoto H: Risk factors for the
development of hepatocellular carcinoma among patients with chronic
hepatitis C who achieved a sustained virological response to
interferon therapy. J Gastroenterol Hepatol. 20:752–758. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Makiyama A, Itoh Y, Kasahara A, Imai Y,
Kawata S, Yoshioka K, Tsubouchi H, Kiyosawa K, Kakumu S, Okita K,
et al: Characteristics of patients with chronic hepatitis C who
develop hepatocellular carcinoma after a sustained response to
interferon therapy. Cancer. 101:1616–1622. 2004. View Article : Google Scholar : PubMed/NCBI
|