Introduction
Plasma microparticles (MPs) are small extracellular
vesicles that originate from the membrane of cells undergoing
activation or apoptosis (1). In
addition to phosphatidylserine (PS), a phospholipid membrane
component that is exposed on the surface of MPs of different cell
origins, circulating MPs exhibit cell-specific proteins that
reflect their parent cells (2). For
example, cluster of differentiation (CD)41-, CD14-, CD235a- and
CD31-expressing MPs are considered to be platelet, monocyte,
erythrocyte and endothelial cell-derived MPs, respectively
(3). Additionally, plasma MPs have
been found to carry other parental molecules, including nucleic
acids and lipids (2). As revealed by
Arraud et al (4), plasma MPs
detected by electron microscopy were reported to be heterogeneous
in size and the majority of them were detected to be in the size
range, 0.03–0.9 µm in diameter.
Plasma is most abundant in platelet MPs, which were
initially described in 1967 as ‘platelet dust’, representing the
sediment of plasma ultracentrifugation (5). The function of these platelet-derived
particles was not completely understood, however, they were
believed to possess pro-coagulant properties (5). Subsequently, MPs were identified to be
involved in various biological processes, including inflammation,
blood coagulation and cell signaling; functions, which are mediated
by their unique structure and expression of certain cell-specific
molecules (6). Furthermore, MPs are
overproduced in various pathological conditions where inflammation
and vascular dysfunction are implicated, indicating that MPs may
serve as surrogate biomarkers for these diseases (6). Among these, endothelial MPs are usually
elevated in diseases that are associated with endothelial
dysfunction, including diabetes mellitus and atherosclerosis
(7,8).
Additionally, increased generation of specific subgroups of plasma
MPs may be associated with certain pathological conditions, such as
CD36-positive MPs, which were found to be elevated in patients with
type 2 diabetes mellitus (9).
Notably, increasing evidence indicates that plasma
MPs are involved in intercellular communication (10). Plasma MPs may act as vehicles of
parental proteins, nucleic acids, lipids and other molecules, which
are delivered to recipient cells where they may influence various
physiological and pathological processes (11). For example, MPs may initiate certain
signaling pathways in the target cell by direct ligand-surface
receptor binding or by being internalized into the target cell
(2). The exact mechanism by which MPs
target other cells has not been sufficiently determined; however,
it is proposed to be mediated by ligand-receptor binding (12). This was investigated in previous
research using endothelial MPs generated in vitro to target
endothelial cells (12) or other cell
types, such as the human proximal tubular HK2 cells
(13).
A lack of methodology for isolating specific MP
subsets from plasma has resulted in limited research in this area.
Specifically, to the best of our knowledge, there have been no
previous studies investigating the significance of MP size on its
cell binding. In addition, these heterogeneously sized MPs pose
many technical challenges in their analysis and functional
evaluation. Flow cytometry is a mainstay for MP analysis, however
the application of flow cytometric sorting is limited as a method
to analyze the whole MP population, which is heterogeneous in size.
Rather, for bulk MP preparation, ultracentrifugation is the
mainstay approach (12,13). However, the coalescence of membranous
material in a pellet requires considerable mechanical disruption to
resuspend and there are questions as to whether this is a true MP
suspension. In the current study, the aim was to use size exclusion
chromatography to isolate plasma MPs (14) as an alternative technique, which
purifies MPs in a native state and uses these MPs in cell binding
assays. An additional aim was to refine this technique to allow
further fractionation of MPs according to their size and to test
the ability of these different sized MPs to bind to cells in
vitro. The human proximal tubular HK2 cell line was
used, as it was successfully targeted by endothelial MPs, as
described by Fernandez-Martinez et al (13) and because renal tubular cells are
involved in the development of diabetic nephropathy. Furthermore,
the present study hypothesized that the diabetic marker, CD36, may
contribute to the pathophysiology of the disease by mediating MP
binding to cellular targets. Thus, MP expression of CD36 was
investigated in the present study, as well as the more abundant
platelet marker, CD41, when characterizing different sized MP
fractions.
Materials and methods
Antibodies, immunoblotting and other
reagents
Bio-maleimide (Bodipy® FL
N-(2-aminoethyl) maleimide) was obtained from Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). Mouse monoclonal antibody
against CD36 (prepared by Dr Rick Thorne, as previously described
(15); clone long 9; used for western
blotting) and the immunoblotting method (enhanced
chemiluminescence) were performed as previously described (15). Briefly, a homemade enhanced
chemiluminescence solution (100 mM Tris hydrochloride (pH 8.8), 0.4
mM p-Coumaric acid, 2.5 mM luminol and 2.6 mM
H2O2; Sigma-Aldrich, Castle Hill, Australia)
was used and the chemiluminescence reaction was detected using an
LAS-4000 imaging system (Fuji Photo Film Co., Tokyo, Japan). The
polyclonal antibody against platelet CD41 was a gift from Dr Pidard
(Unite de Pharmacologie Cellulaire, Unite Associee IP/INSERM 285,
Institut Pasteur, Paris).
Blood sampling and plasma
processing
Fresh whole blood samples (5 ml each) were collected
from healthy volunteers by venipuncture with 21-gauge needles and
transferred into centrifuge tubes containing EDTA as the
anticoagulant at a final concentration of 2 mM. Blood samples were
immediately double centrifuged at 3,000 × g for 15 min at room
temperature to prepare the platelet free plasma (PFP). The
upper-third of the PFP supernatant was carefully collected by
pipetting, and filtered using a 1.2-µm filter to remove any
platelet contamination.
Preparation of bio-maleimide-labeled
plasma MPs
Bio-maleimide was used to stain MPs in PFP as
previously described (16) with
certain modifications. Briefly, a working solution of bio-maleimide
was prepared by adding 1 µl of 5 mM bio-maleimide stock solution in
dimethyl sulfoxide to 49 µl phosphate-buffered saline (PBS; 1:50).
Thereafter, the diluted bio-maleimide solution was added to 1 ml
PFP sample and incubated for 15 min in the dark at room
temperature.
Isolation of bio-maleimide stained
plasma MPs by size exclusion
Bio-maleimide-stained PFP (1 ml) was applied to a
Sephacryl S-500 HR size exclusion column (40×0.7 cm; GE Healthcare
Australia Pty., Ltd., Parramatta, Australia) connected to a
BioLogic liquid chromatography system (BioRad Laboratories PTY
Ltd., Gladesville, Australia). PBS (pH 7.4) was used as a mobile
phase at a flow rate of 0.5 ml/min. Twenty-one elution fractions of
1 ml each were collected and kept in the dark for further
experiments. The protein concentrations of all fractions were
measured using a BCA Protein Assay Reagent kit (Thermo Fisher
Scientific, Scoresby, Australia; lower limit of detection, 20
µg/ml). The void volume fractions (200 µl of each; 1–11) were
ultracentrifuged for 1 h at 4°C at 100,000 × g in a TLA 100 rotor
(Beckman Coulter Australia Pty Ltd., Gladesville, Australia) to
isolate MPs as pellets.
Flow cytometric analysis of MPs
MP analysis was performed as previously described
(17) and according to the guidelines
that were established by the International Society on Thrombosis
and Haemostasis, Inc. Vascular Biology Scientific and
Standardization Committee on the Standardization of
platelet-derived MP enumeration by flow cytometry (18) with modifications suggested for the BD
FACS Canto (BD Biosciences, San Jose, CA, USA), including
calibration to set MP detection limits (9). To detect Bio-maleimide-stained MPs, 10-µl
aliquots of bio-maleimide-treated PFP size exclusion fractions were
diluted in PBS that did not contain Ca+2 or
Mg+2 and were analyzed by flow cytometry. Absolute MP
numbers were determined using TruCount counting tubes (BD
Biosciences) and events were collected for 60 sec at a low flow
rate as previously described (9).
Cell culture and MP-cell binding
assay
The human kidney proximal tubular epithelial cell
line (HK2) was obtained from Dr. Carol Pollock
(Department of Medicine, Royal North Shore Hospital, St. Leonards,
Australia). This HK2 cell line was originally obtained
from American Type Culture Collection (Manassas, VA, USA). Cells
were allowed to attach to 13-mm circular glass coverslips in
24-well cell-culture plates and maintained in Dulbecco's modified
Eagle's medium (Sigma-Aldrich) that contained 2 mM L-glutamine
(Lonza Australia Pty., Ltd., Mount Waverley, Australia) and 4.5 g/l
glucose (Sigma-Aldrich) and supplied with 10% fetal bovine serum
(FBS; Sigma-Aldrich), 0.1% penicillin/streptomycin (Invitrogen;
Thermo Fisher Scientific, Inc.), 25 mM HEPES
(4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) and 24 mM
sodium hydrogen carbonate (Lonza Australia Pty., Ltd.). Cells were
incubated at 37°C in a humidified atmosphere of 5% CO2
in air (v/v). At ~50% confluence, cells were deprived of FBS for
2–3 h and incubated with 0.5 ml of each of the
bio-maleimide-stained size exclusion fractions for a further hour.
Cells were washed twice using PBS to remove unbound MPs and were
fixed with 4% formaldehyde for 5 min. Thereafter, cells were washed
twice using PBS before staining with 4′,6-diamidino-2-phenylindole
(DAPI; Sigma-Aldrich) (19). The
coverslips were mounted on microscope slides and cells were imaged
under a fluorescence microscope.
Results
Isolation of bio-maleimide stained
plasma MPs by size exclusion chromatography
Size exclusion chromatography is an effective method
for isolating MPs from other plasma components without
ultracentrifugation (14). As plasma
MPs are large in size (0.1–1 µm in diameter), MPs are expected to
be eluted from the size exclusion column before the plasma proteins
(14). In the current study,
bio-maleimide-stained PFP were fractionated by size exclusion
chromatography into 21 fractions and the protein concentration in
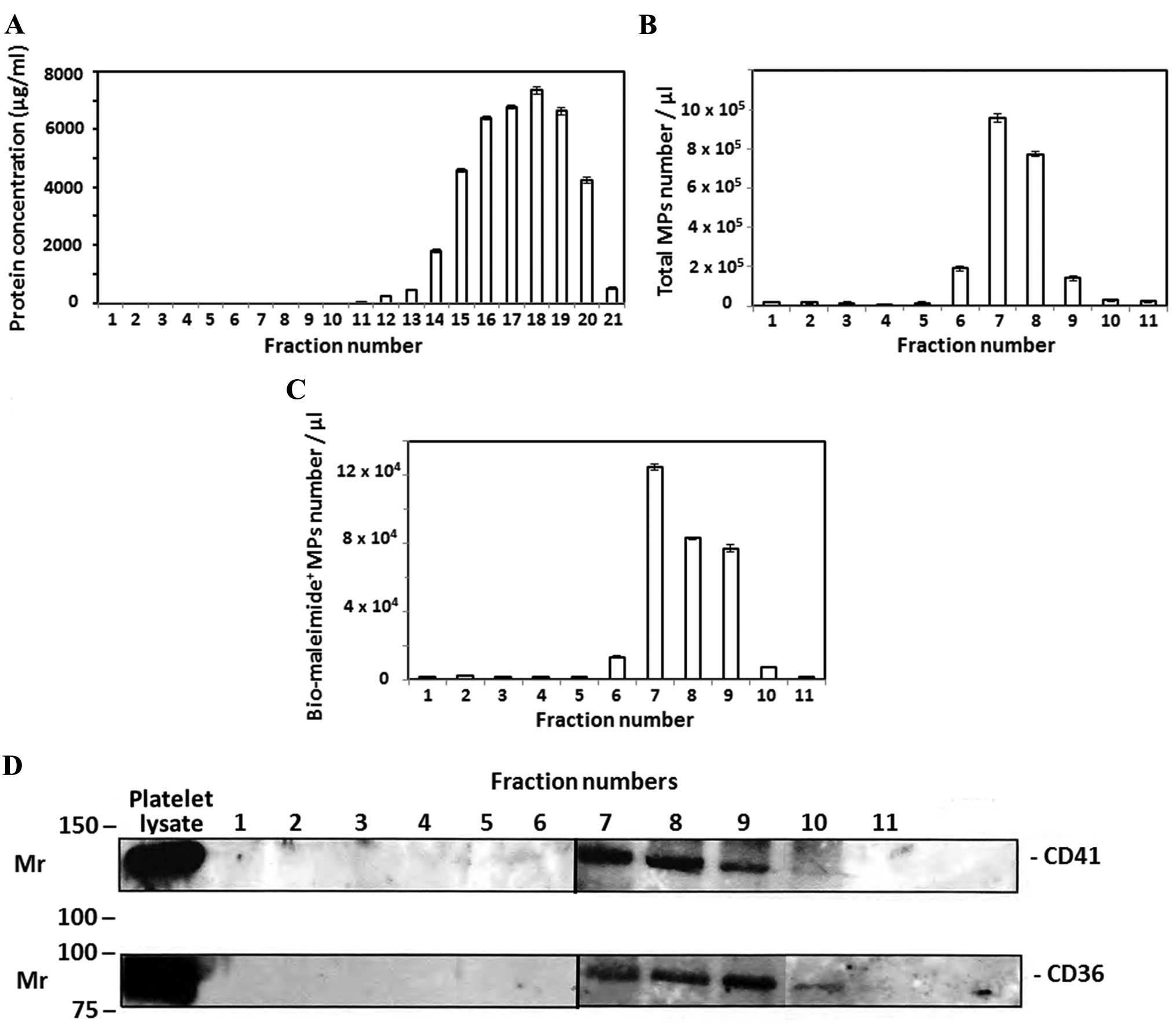
each fraction was determined. As shown in Fig. 1A, plasma proteins were detected in
fractions 12–21 without any protein detected in the first 11
fractions. By contrast, flow cytometric analysis of the same
fractions detected the majority plasma MPs in fractions 7–9
(Fig. 1B) without any MP detection in
fractions 12–21 (data not shown). In addition, plasma MPs were
successfully stained with bio-maleimide as a general MP stain as
previously described (16) and
enumerated in PFP size exclusion fractions 1–11 by flow cytometry
(Fig. 1C). Plasma MPs carry molecules
that are specific to their parent cells, such as cell-surface
receptors and proteins (2). PFP size
exclusion fractions 1–11 were ultracentrifuged to isolate the
suspended MPs. Western blot analysis was performed for CD36 and the
platelet-specific protein, CD41. As shown in Fig. 1D, MP pellets of size exclusion
fractions 7–9 were positive for CD36 and CD41. This indicates that
MPs isolated by size exclusion chromatography may originate from
platelets, as they were expressing CD41, and from other cells, as
CD36 is not only expressed by platelets, but also by other cell
types, such as red blood cells (9).
However, detecting plasma MPs in various size exclusion fractions
indicates that MPs in these fractions were different sizes and
bio-maleimide was able to stain the MPs regardless of their
size.
Plasma MPs isolated by size exclusion
chromatography target HK2 cells in vitro
To further investigate the function of plasma MPs
isolated by size exclusion chromatography, size exclusion fractions
that contained bio-maleimide-stained MPs (Fig. 1) were incubated with HK2
cells in vitro. MPs were previously reported to act as
vehicles that transport molecules from the cell of origin to other
target cells (11). As shown by
fluorescence microscopic images of HK2 cells treated
with the control, size exclusion fraction 1, and fraction 7
(Fig. 2); the isolated
bio-maleimide-stained MPs in fraction 7 were able to attach to
HK2 cells. By contrast, HK2 cells treated
with fractions number 8 and 9, that also contained
bio-maleimide-stained MPs, did not show any fluorescence indicative
of MP attachment (Fig. 2). As the
concentrations of bio-maleimide-stained MPs in fractions 7 and 8
were ~60–70% of the MPs concentration of fraction 7, which
contained the maximum MP concentration (Fig. 1C), this indicates that MP attachment to
HK2 cells was most likely to be dependent on MP size
rather than MP concentration.
 | Figure 2.Plasma MPs isolated by size exclusion
target HK2 cells in vitro. (A) Flow-cytometric analysis of
plasma MPs isolated from a healthy donor. Plasma was stained using
Bodipy® FL N-(2-aminoethyl) maleimide and fractionated
by size exclusion chromatography. To detect MPs, fractions were
analyzed by flow cytometry, demonstrating MPs enriched in the void
volume (fractions 6–10), but absent in the other fractions
(Fig. 1). The first representation
(fraction 1) is shown as the control, followed by fraction numbers
7, 8 and 9. Upper left quadrant, MPs stained with bio-maleimide;
lower left quadrant, >90% of MP events, which may be considered
as noise (i.e., not true MPs, as they did not show any MP
staining). (B) Fluorescence microscopic images of HK2
cells treated with the control (fraction 1), void volume fraction 7
stained with bio-maleimide (green), fraction numbers 8 and 9 (these
were bio-maleimide-stained, but did not show any MP binding to
HK2 cells). As highlighted, the small green dots are
consistent with the size of the MPs (<1 µm) and are attached to
the HK2 cells treated with void volume fraction 7. The
cell nuclei were counterstained with 4′,6-diamidino-2-phenylindole
(blue). |
Discussion
The current study demonstrates that size exclusion
chromatography using a Sephacryl S-500 HR column is a simple
technique to isolate circulating MPs from other plasma
constituents. Similarly, other studies have shown that size
exclusion chromatography using different matrices, including
Sepharose CL-2B (20), Sepharose CL-4B
and Sephacryl S-400 (21), was able to
purify plasma MPs. This indicates that MP isolation by size
exclusion chromatography may be performed using different matrices
as long as the size range of MPs is larger than the matrix pore
size range, which was originally designed to separate protein
molecules rather than larger components, such as MPs. Thus, plasma
MPs are expected to be eluted from the column in the void volume
without crossing the pores of size exclusion matrices (22). However, the aim from using size
exclusion chromatography in the present study was to fractionate
plasma MPs into various fractions, which are supposed to contain
MPs of different sizes. According to the theory of size exclusion
chromatography, larger plasma MPs are expected to be eluted from
the column prior to smaller sized MPs. As a result, the technique
used in the present study was able to fractionate plasma MPs into
three major fractions (7–9) in which the MP content was confirmed by
flow cytometric analysis (Fig. 1C) and
western blotting of proteins CD41 and CD36 in the MP pellet of size
exclusion fractions following ultracentrifugation (Fig. 1D). Consistently, Boing et al
(22) demonstrated that
platelet-derived vesicles were successfully isolated in early
consecutive fractions of Sepharose CL-2B size exclusion
chromatography, as confirmed by flow cytometric analysis and
western blotting of proteins specific to platelet-derived vesicles.
However, the column dimensions in the current study support better
MP isolation according to size, as it was longer and narrower than
that used in the study by Boing et al (22).
In addition, results from the current study have
confirmed the efficacy of using bio-maleimide as a general stain
for plasma MP detection and quantification by flow cytometry
(16). Bio-maleimide attaches to thiol
groups and cysteine residues of the MPs' membranes and it has a
fluorescent nature that can be detected by flow cytometry (13). Notably, bio-maleimide-treated PFP was
fractionated, to the best of our knowledge, for the first time by
size exclusion chromatography to isolate bio-maleimide-stained
plasma MPs from other plasma components. The purpose of
bio-maleimide staining was to detect MPs by flow cytometry, rather
than using Annexin V staining as an alternative method (16) and to enable fluorescent detection of
MPs when they were investigated for in vitro cell
targeting.
Although plasma MPs were previously reported to act
as vehicles for intercellular communication (10), factors that affect cell targeting were
not exactly determined. In the current study, the effect of plasma
MP size on HK2 cell targeting was investigated.
Unexpectedly, results demonstrated that plasma MPs isolated in
different size exclusion fractions possessed different binding
abilities to HK2 cells in vitro. MPs isolated in
fraction 7, which were presumably larger (as they were eluted from
the size exclusion column before other MPs in fractions 8 and 9),
were the only MPs that were able to attach to HK2 cells
(Fig. 2B). This suggests that MP
attachment to HK2 cells may be size dependent or that
the MP concentrations in fractions 8 and 9 were insufficient to
demonstrate cell binding by fluorescence microscopy. However, as
the content of bio-maleimide-stained MPs in fraction 8 and 9 was
>60% of the content of fraction 7 (Fig.
1C), this supports that MP concentration was less likely to
affect MP binding to HK2 cells.
Results from the present study indicate that plasma
MPs may be fractionated using size exclusion chromatography and
that MPs in these fractions may have different functions. However,
there were certain limitations of the current study that may affect
the generalization of its results. First, the exact size of plasma
MPs was not determinable in each fraction of size exclusion
chromatography. It is possible to determine MP sizes by
transmission electron microscopy, however, a lack of equipment and
funding were the main barriers for this determination. Furthermore,
although cell targeting appeared to be independent of MP expression
of CD36 and/or CD41, blocking studies are required to confirm this.
However, the effect of MP size on cell targeting requires further
investigation using multiple cell lines and animal models. In
addition, further studies are required to investigate differences
between plasma MPs of varying sizes in term of molecular content
and surface expression of proteins. This may explain why these MPs
may have different functions.
In conclusion, the current study has shown that
plasma MPs stained with bio-maleimide may be isolated using
Sephacryl S-500 HR size exclusion chromatography; a simple and
timesaving technique as compared with ultracentrifugation. Plasma
MPs were detected by flow cytometry and western blotting in three
consecutive fractions suggesting that these fractions contain MPs
of different sizes according to the theory of size exclusion
chromatography. Further investigations on the function of these MPs
have shown that MPs eluted in the earliest fraction were able to
target HK2 cells in vitro. This suggests that MP
attachment to HK2 cells may be size-dependent and, thus,
different sizes of MPs may have different functions.
Acknowledgements
The present study was supported by the HMRI Research
Grant (grant no. 10-08) funded by the Lions District 201 N3
Diabetes Foundation.
References
|
1
|
Hargett LA and Bauer NN: On the origin of
microparticles: From “platelet dust” to mediators of intercellular
communication. Pulm Circ. 3:329–340. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yáñez-Mó M, Siljander PR, Andreu Z, Zavec
AB, Borràs FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J,
et al: Biological properties of extracellular vesicles and their
physiological functions. J Extracell Vesicles. 4:270662015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nielsen MH, Beck-Nielsen H, Andersen MN
and Handberg A: A flow cytometric method for characterization of
circulating cell-derived microparticles in plasma. J Extracell
Vesicles. 3:Feb 4–2014.(Epub ahead of print). http://dx.doi.org/10.3402/jev.v3.20795PubMed/NCBI
|
|
4
|
Arraud N, Linares R, Tan S, Gounou C,
Pasquet JM, Mornet S and Brisson AR: Extracellular vesicles from
blood plasma: Determination of their morphology, size, phenotype
and concentration. J Thromb Haemost. 12:614–627. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wolf P: The nature and significance of
platelet products in human plasma. Br J Haematol. 13:269–288. 1967.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lynch SF and Ludlam CA: Plasma
microparticles and vascular disorders. Br J Haematol. 137:36–48.
2007.PubMed/NCBI
|
|
7
|
Horstman LL, Jy W, Jimenez JJ and Ahn YS:
Endothelial microparticles as markers of endothelial dysfunction.
Front Biosci. 9:1118–1135. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bernard S, Loffroy R, Sérusclat A, Boussel
L, Bonnefoy E, Thévenon C, Rabilloud M, Revel D, Moulin P and Douek
P: Increased levels of endothelial microparticles CD144
(VE-Cadherin) positives in type 2 diabetic patients with coronary
noncalcified plaques evaluated by multidetector computed tomography
(MDCT). Atherosclerosis. 203:429–435. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alkhatatbeh MJ, Enjeti AK, Acharya S,
Thorne RF and Lincz LF: The origin of circulating CD36 in type 2
diabetes. Nutr Diabetes. 3:e592013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jaiswal R, Grau GE Raymond and Bebawy M:
Cellular communication via microparticles: Role in transfer of
multidrug resistance in cancer. Future Oncol. 10:655–669. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Antonyak MA and Cerione RA: Microvesicles
as mediators of intercellular communication in cancer. Methods Mol
Biol. 1165:147–173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jansen F, Yang X, Hoyer FF, Paul K,
Heiermann N, Becher MU, Abu Hussein N, Kebschull M, Bedorf J,
Franklin BS, et al: Endothelial microparticle uptake in target
cells is annexin I/phosphatidylserine receptor dependent and
prevents apoptosis. Arterioscler Thromb Vasc Biol. 32:1925–1935.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fernandez-Martínez AB, Torija AV,
Carracedo J, Ramirez R and de Lucio-Cazaña FJ: Microparticles
released by vascular endothelial cells increase hypoxia inducible
factor expression in human proximal tubular HK-2 cells. Int J
Biochem Cell Biol. 53:334–342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alkhatatbeh MJ, Mhaidat NM, Enjeti AK,
Lincz LF and Thorne RF: The putative diabetic plasma marker,
soluble CD36, is non-cleaved, non-soluble and entirely associated
with microparticles. J Thromb Haemost. 9:844–851. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thorne RF, Zhang X, Song C, Jin B and
Burns GF: Novel immunoblotting monoclonal antibodies against human
and rat CD36/fat used to identify an isoform of CD36 in rat muscle.
DNA Cell Biol. 25:302–311. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Enjeti AK, Lincz L and Seldon M:
Bio-maleimide as a generic stain for detection and quantitation of
microparticles. Int J Lab Hematol. 30:196–199. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Robert S, Poncelet P, Lacroix R, Arnaud L,
Giraudo L, Hauchard A, Sampol J and Dignat-George F:
Standardization of platelet-derived microparticle counting using
calibrated beads and a Cytomics FC500 routine flow cytometer: A
first step towards multicenter studies? J Thromb Haemost.
7:190–197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lacroix R, Robert S, Poncelet P, Kasthuri
RS, Key NS and Dignat-George F: ISTH SSC Workshop: Standardization
of platelet-derived microparticle enumeration by flow cytometry
with calibrated beads: Results of the International Society on
Thrombosis and Haemostasis SSC Collaborative workshop. J Thromb
Haemost. 8:2571–2574. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sanchez-Niño MD, Fernandez-Fernandez B,
Perez-Gomez MV, Poveda J, Sanz AB, Cannata-Ortiz P, Ruiz-Ortega M,
Egido J, Selgas R and Ortiz A: Albumin-induced apoptosis of tubular
cells is modulated by BASP1. Cell Death Dis. 6:e16442015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
de Menezes-Neto A, Sáez MJ, Lozano-Ramos
I, Segui-Barber J, Martin-Jaular L, Ullate JM, Fernandez-Becerra C,
Borrás FE and Del Portillo HA: Size-exclusion chromatography as a
stand-alone methodology identifies novel markers in mass
spectrometry analyses of plasma-derived vesicles from healthy
individuals. J Extracell Vesicles. 4:273782015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Baranyai T, Herczeg K, Onódi Z, Voszka I,
Módos K, Marton N, Nagy G, Mäger I, Wood MJ, El Andaloussi S, et
al: Isolation of Exosomes from Blood Plasma: Qualitative and
Quantitative Comparison of Ultracentrifugation and Size Exclusion
Chromatography Methods. PLoS One. 10:e01456862015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Böing AN, van der Pol E, Grootemaat AE,
Coumans FA, Sturk A and Nieuwland R: Single-step isolation of
extracellular vesicles by size-exclusion chromatography. J
Extracell Vesicles. 3:Sep 8–2014.http://dx.doi.org/10.3402/jev.v3.23430
View Article : Google Scholar
|