Introduction
The role of microbiota in controlling the balance
between health and disease is a current topic of study due to its
potential to be used for novel therapeutic approaches (1). In particular, the local application of
bacteria to enhance wound healing has previously been reported
(2,3).
These favorable effects may be related, though not exclusively, to
certain anti-inflammatory and antibiotic actions of bacteria.
Previous experiments by our group have indicated
that the Italian calcium magnesium bicarbonate-based Comano spring
water (Comano, Italy) improves skin regeneration: In an in
vivo rabbit wound model, it was identified that the topical
administration of the Comano spring water increased keratinocyte
proliferation and migration, and favorably modulated the
regeneration of dermal collagen and elastic fibers (4); while in in vitro cultures of
human skin fibroblasts, it was observed that cells maintained in
conventional Dulbecco's modified Eagle's medium (DMEM) with 20%
Comano spring water exhibited a 31% higher vitality than control
cells maintained in conventional DMEM alone after 72 h (5).
Other studies have demonstrated the
anti-inflammatory effect of the spring waters of Avène and La
Roche-Posay in France, effective in activating toll-like receptors
due to the specific actions of the non-pathogenic bacteria
Aquaphilus dolomiae (6) and
Vitreoscilla filiformis (7),
respectively. The Comano spring water also exhibits a diverse
non-pathogenic bacterial flora (8,9). To
further investigate the regenerative effects of the native
bacterial flora of the Comano spring water, the present study
evaluated the efficacy of spring water treatment in a human ex
vivo model of physiological wound healing. The study was
performed at the Plastic Surgery Unit in the Department of
Clinical-Surgical, Diagnostic and Pediatric Sciences, in
cooperation with the Histology and Embryology Unit in the
Department of Public Health, Experimental and Forensic Medicine at
the University of Pavia (Pavia, Italy). The results of the current
research may aid the development of novel clinical approaches for
tissue regeneration and wound healing within the modern concept of
‘natural’ medicine. Additionally, they may provide novel scientific
data on thermalism and indicate its therapeutic use with a rational
basis.
Materials and methods
Spring water collection and
processing
Comano spring water was collected in January 2014
from the Comano spring by an aseptic procedure. Briefly, a single
operator wearing sterile surgical gloves collected 3,000 ml water
with a sterile 60-ml syringe.
The samples were poured into three sterile 1-L
containers, stored at 4°C and transported to the Histology and
Embryology Unit of the University of Pavia (Pavia, Italy). After 2
days, the spring water was filtered through 0.20-µm pore cellulose
nitrate membranes (Nalgene 0.2 Analytical Filter Units; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), stored at 4°C in
sterile 100 ml ampoules and used as described below. All ex
vivo experiments were performed within 3 months of water
filtration and storage.
Human skin specimen collection
Human skin samples were obtained from anatomical
specimens harvested during sessions of elective abdominoplasty or
reduction mammoplasty performed on 6 healthy female patients (age
range, 43–56 years). The specimens were sampled by a surgeon in
~6×6-cm segments and conserved in sterile containers filled with
saline solution enriched with 1% (10,000 U/ml) penicillin (Biowest,
Nuaillé, France), then transported to a partner laboratory for
further processing. The time delay between tissue harvesting and
the initiation of laboratory procedures was ~45 min. The study was
conducted in accordance with the 1975 Declaration of Helsinki,
informed consent was obtained from all patients and the protocol
was approved by the Ethics Committee of Salvatore Maugeri Research
and Care Institute, Pavia, Italy (project identification code,
2064).
Human skin specimen processing
The skin samples were sufficient for the harvesting
of 6 mm-punch biopsies that in turn were injured in their central
portion with a sterile 3-mm circular punch to establish skin loss
in each sample as described previously (10–12). The
injured specimens were placed into Transwell inserts for 24-well
multiwell plates (membrane pore size, 0.40 µm; Constar insert, 0.33
cm2; Corning Incorporated, Corning, NY, USA).
Study design
Each of the 6 skin specimens was used to harvest
paired control and experimental samples. The control samples were
cultured in DMEM with 1.0 g/l D-glucose (Biochrom, Ltd., Cambridge,
UK), 10% fetal bovine serum (FBS), 1% penicillin (10,000 U/ml) and
streptomycin (10 mg/ml), 1% gentamicin (all from Biowest) and 10
ml/l 200 mM L-glutamine (Eurobio Laboratoires, Les Ulis, France),
and the central skin loss region was treated with a constant volume
(200 µl) of sterile saline solution.
The samples treated with Comano spring water
(treated samples) were cultured with DMEM powder without
NaHCO3 with 1.0 g/l D-glucose (Biochrom, Ltd.) and 10
ml/l 200 mM L-glutamine, reconstituted with filtered Comano spring
water and enriched with 10% FBS, 1% penicillin (10,000 U/ml) and
streptomycin (10 mg/ml) and 1% gentamicin (all from Biowest). The
central skin loss region was treated with a constant volume (200
µl) of filtered Comano spring water. Following treatment, the
control and treated samples were incubated at 37°C for 24, 48 and
72 h.
Assessment modalities
Morphological analysis
Morphological analysis of the specimens was
performed by two independent operators at 0 h (T0) to
identify the histological features of the untreated skin (US) and
at 24, 48 and 72 h thereafter (T1–3) to identify the
features of the skin in the different experimental groups.
The skin specimens were fixed with 4%
paraformaldehyde in phosphate buffer (pH 7.4) for 8 h at 4°C,
dehydrated through graded concentrations of ethanol and embedded in
paraffin. Subsequently, 7.5-µm specimen sections were obtained with
a microtome, rehydrated and stained by different methods.
Hematoxylin and eosin (H&E) staining was
performed with Harry's hematoxylin for 5 min and eosin for 30 sec
(Bio-Optica SpA, Milan, Italy) at room temperature. The stained
sections were observed with a Zeiss Axiophot microscope (Carl Zeiss
AG, Oberkochen, Germany) in bright field. The assessment of
specimens by optical microscopy following H&E staining focused
on features of the collagen fiber network, cellular infiltration
and the re-epithelialization process.
Staining for collagen fibers was performed with a
modified Picrosirius Red (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) procedure (13,14). In brief, following rehydration, the
sections were incubated for 1 h at room temperature in 0.1% Sirius
Red in a saturated picric acid solution, and then washed twice in
1% acetic acid for 15 sec. This was followed by staining with
hematoxylin for 5 min at room temperature, rinsing in water for 5
min, dehydration and mounting of the slides with DPX
(Sigma-Aldrich; Merck KGaA). The sections were then examined under
polarized light with the Zeiss Axiophot microscope equipped with a
polarizing filter.
Elastic fibers were stained with orcein staining
reagents (Bio-Optica SpA) according to the manufacturer's protocol.
Following rehydration, the sections were treated with 5 drops of
potassium permanganate solution (reagent A) and 5 drops of acid
activation buffer (reagent B) for 4 min. After washing with
distilled water, the sections were treated with 10 drops of oxalic
acid solution (reagent C) for 1 min, and then washed with distilled
water. Subsequently, 20 drops of alcoholic reagent (reagent D) were
placed onto the bottom of an incubation box, the sections were
introduced into the box, and each section was treated with 10 drops
of orcein solution (reagent E) for 20 min. Following washing with
distilled water, the sections were treated with 10 drops of
differentiation solution (reagent F) for 2 min. Finally, the
sections were stained with hematoxylin for 5 min at room
temperature, washed in water for 5 min, dehydrated, mounted and
observed with the Zeiss Axiophot optical microscope.
Staining for proliferating cell nuclear antigen
(PCNA) was used to quantify the cell turnover rates of the
different culture groups. The sections were treated with 0.3%
hydrogen peroxide for 30 min at room temperature to block
endogenous peroxidase activity, and then were immersed in a citrate
buffer and treated for antigenic retrieval with steam for 30 min,
which was followed by blocking of non-specific sites with
Background Sniper reagent (Biocare Medical, LLC, Pacheco, CA, USA)
for 10–15 min at room temperature.
The sections were then incubated with primary mouse
anti-PCNA antibody overnight at 4°C (1:4,500; cat. no. 152; Biocare
Medical, LLC), then with MACH 1 mouse probe for 15 min and
Horseradish Peroxidase (HRP)-Polymer for 30 min (MACH1 Universal
HRP-Polymer Detection system; Biocare Medical, LLC), according to
the manufacturer's instructions. Following incubation with betazoid
diaminobenzidine (Biocare Medical, LLC) for 5 min at room
temperature, the sections were dehydrated, mounted with DPX
mounting medium and examined. Between each the immunostaining
steps, the sections were washed with 0.15 M Tris-buffered saline,
pH 7.4 (0.05 M Tris buffer containing 0.1 M sodium chloride). The
observations were performed with the Zeiss Axiophot optic
microscope equipped with a Nikon Digital Sight DS-5M camera (Nikon
Corporation, Tokyo, Japan). Positive nuclei were counted in all
fields of view for each section by the same two independent
operators at the different time points.
Data analysis
Data processing and analysis was performed for the
PCNA counts. The mean count of the two independent operators'
values was calculated. The mean value of the two measurements on
the same sample was estimated for each experimental condition and
time point. The ratio between the mean values of the samples
cultured under the different experimental conditions (Comano
water/control) was also calculated at each time point.
Results
H&E staining
Collagen fibers
At T1 the control samples exhibited
absence of the papillary dermis and a compact and regular
arrangement of collagen fibers in the reticular dermis. A newly
formed loose unstructured connective tissue was also observed. At
T2 the fibers had increased in size and compactness, and
exhibited an orientation perpendicular to the skin surface. At
T3 the fibers appeared smaller in size compared with
T2, while their orientation had become parallel to the
skin surface (Fig. 1).
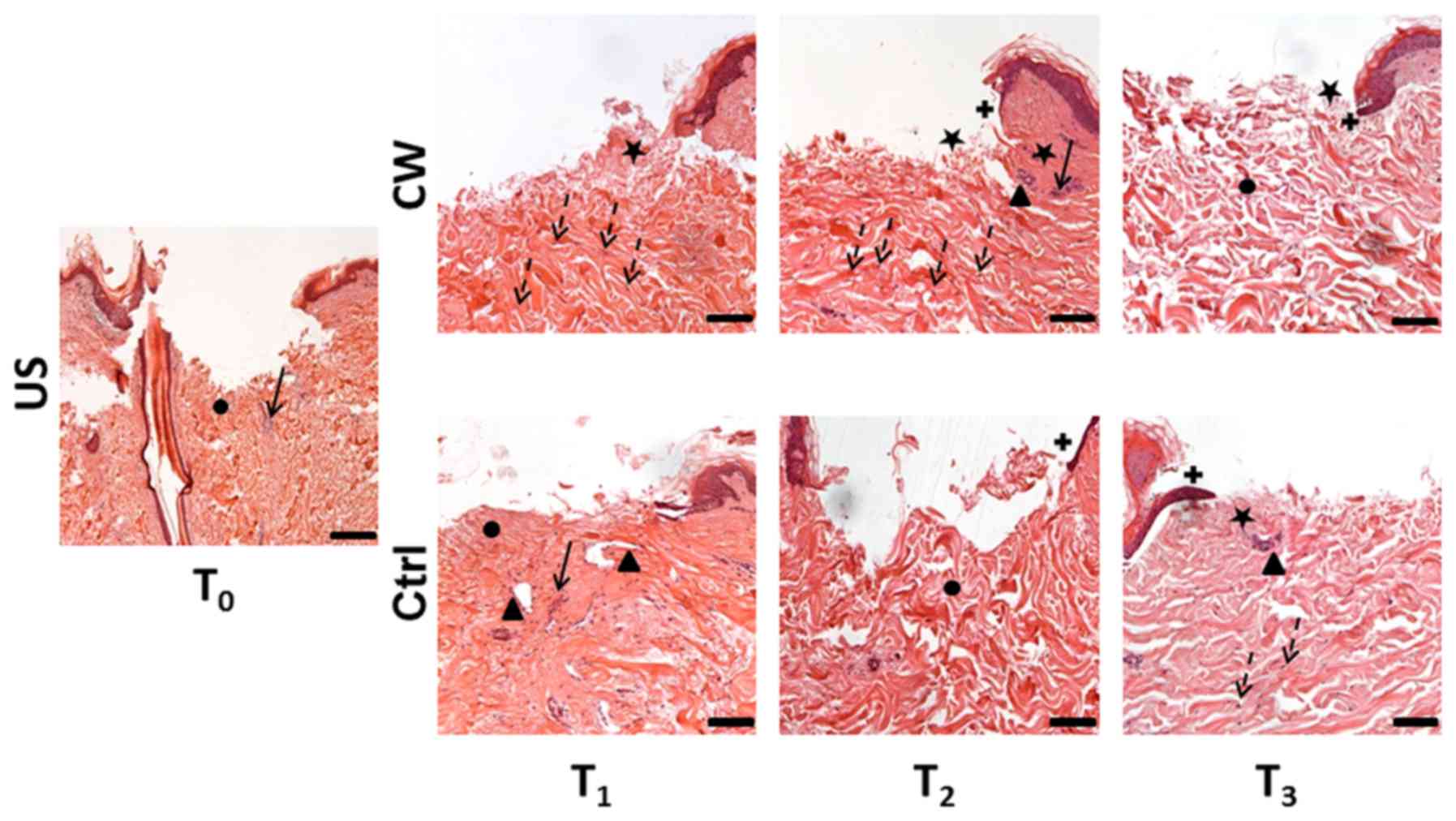 | Figure 1.Hematoxylin and eosin staining.
Representative images of the US sample at T0; Ctrl
samples cultured in DMEM with saline solution applied to the
central wound; and CW-treated samples cultured in DMEM powder
reconstituted with filtered CW and with filtered CW applied to the
central wound at T1, T2 and T3.
Histological features are indicated as follows: Black circle, loose
dermis; black triangle, new tissue; black arrow, inflammatory
perivascular cellular infiltration; black star, papillary dermis;
dotted arrow, fibroblasts; black cross, re-epithelialization.
Magnification, ×5 (US); scale bar, 200 µm; and ×10 (CW and Ctrl);
scale bar, 100 µm. US, untreated skin; Ctrl, control; DMEM,
Dulbecco's modified Eagle's medium; CW, Comano spring water;
T0–3, 0–72 h. |
At T1 the treated samples exhibited a
regenerated regular papillary dermis and a structured reticular
dermis comprised of collagen fibers with a slight perpendicular
orientation to the skin surface. At T2 some active
regeneration in the papillary dermis was observed, and the collagen
fibers in the reticular dermis exhibited regular structure with a
more parallel orientation to the skin surface. At T3 the
papillary dermis exhibited an active regeneration similar to that
at T2; the collagen fibers in the reticular dermis
exhibited regular structure, though their orientation had become
more irregular, which was concomitant with an increase in
interfiber spaces (Fig. 1).
Cellular infiltration
Inflammatory cell infiltration was detected around
the newly formed vascular structures. In the control samples at
T1, increased inflammatory infiltration was observed
compared with the US samples. This infiltration progressively
decreased in the T2 and T3 samples. In the
treated samples, inflammatory infiltration was absent at
T1 and T3, while at T2, slight
infiltration similar to that in the US was observed.
In the control samples at T1, some
fibroblasts were observed in the dermis, which were markedly
reduced by T3. Conversely, in the treated samples, few
fibroblasts were observed in the dermis at T1, while
increased fibroblasts were observed by T3 (Fig. 1).
Re-epithelialization
The control samples lacked re-epithelialization at
T1, though exhibited early basal cell
re-epithelialization at T2 and signs of multi-layered
re-epithelialization at T3 (Fig. 1).
In the treated samples, a similar sequence of
re-epithelialization was observed, with absence of
re-epithelialization at T1, early single-layered
re-epithelialization at T2, and multi-layered
re-epithelialization at T3 (Fig. 1).
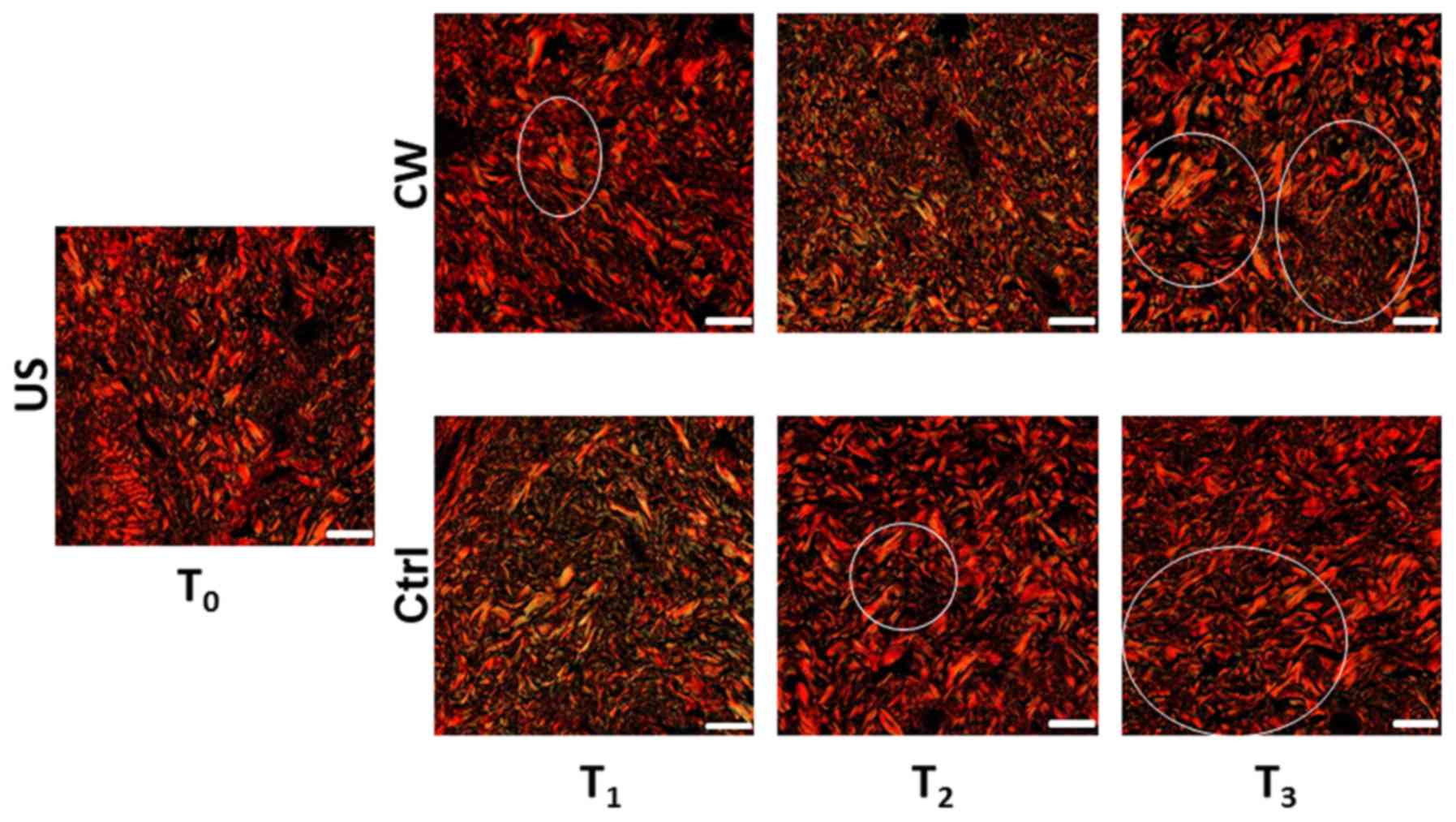
Picrosirius Red staining
At T1, the control samples exhibited
diffuse green-yellow birefringence suggestive of early collagen
fiber regeneration. At T2, a red birefringence was
observed, indicating a reduction in the collagen regeneration
process. This remained stable at T3 (Fig. 2).
At T1, the treated samples exhibited some
green birefringence. At T2, this developed into a
diffuse green-yellow birefringence. At T3, regions of
green-yellow birefringence were observed (Fig. 2).
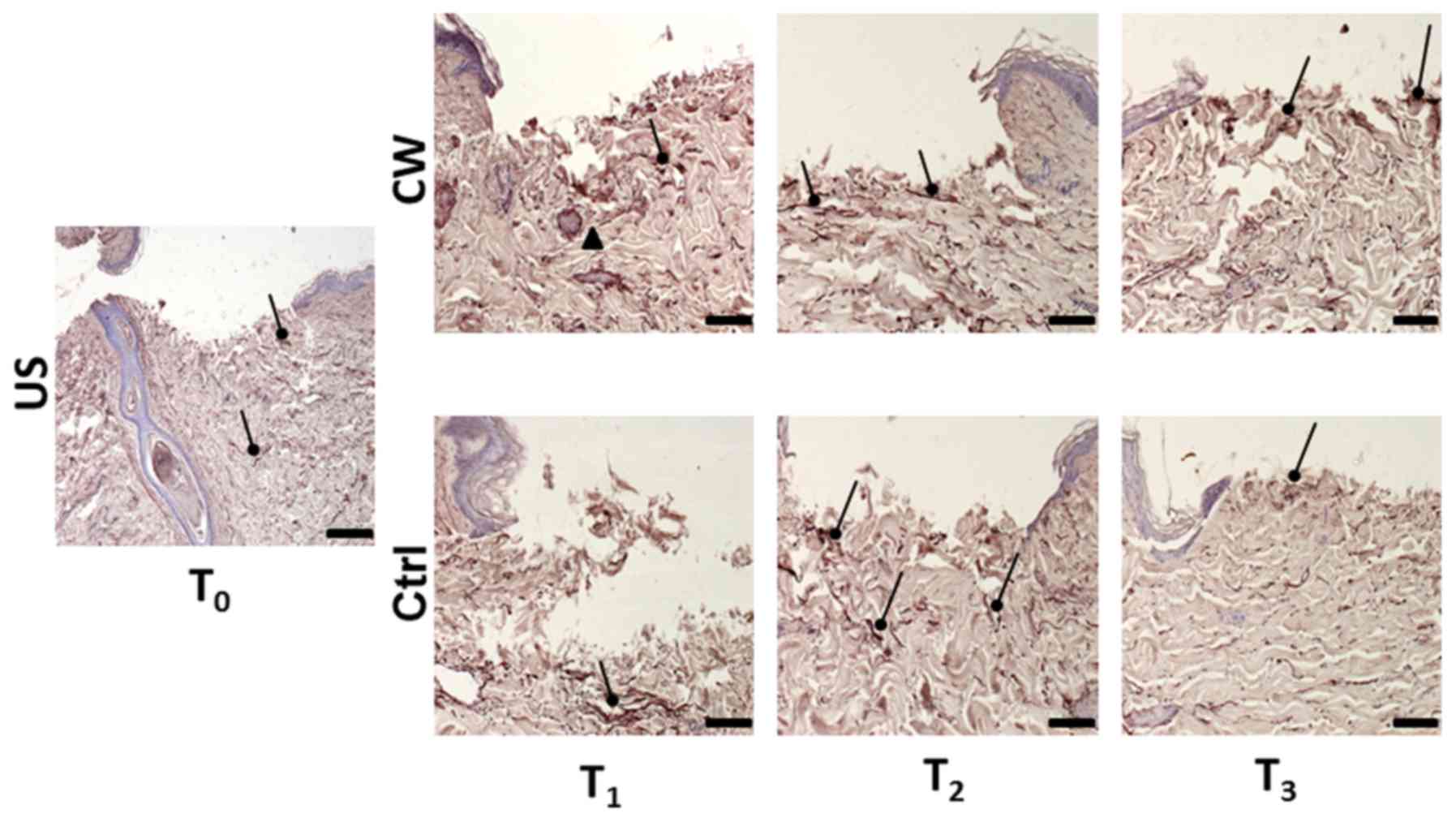
Orcein staining
At T1, the control samples exhibited
diffuse staining and a slight parallel orientation of elastic
fibers to the skin surface. At T2, a reduction in
staining was observed, and the orientation of the elastic fibers
had become more perpendicular to the skin surface. Further reduced
staining was observed at T3 and fiber distribution was
similar to that in the US, with the fibers reverted to a more
parallel orientation (Fig. 3).
At T1, the treated samples exhibited
diffuse staining and an irregular orientation of the elastic fibers
to the skin surface. At T2, a marked parallel
orientation of the elastic fibers to the skin surface was observed,
and the elastic fibers were concentrated between the papillary and
reticular dermis. This concentration of fibers remained stable at
T3, though both perpendicular and parallel orientations
were observed. Fiber distribution throughout the dermal layers also
became similar to that of the US (Fig.
3).
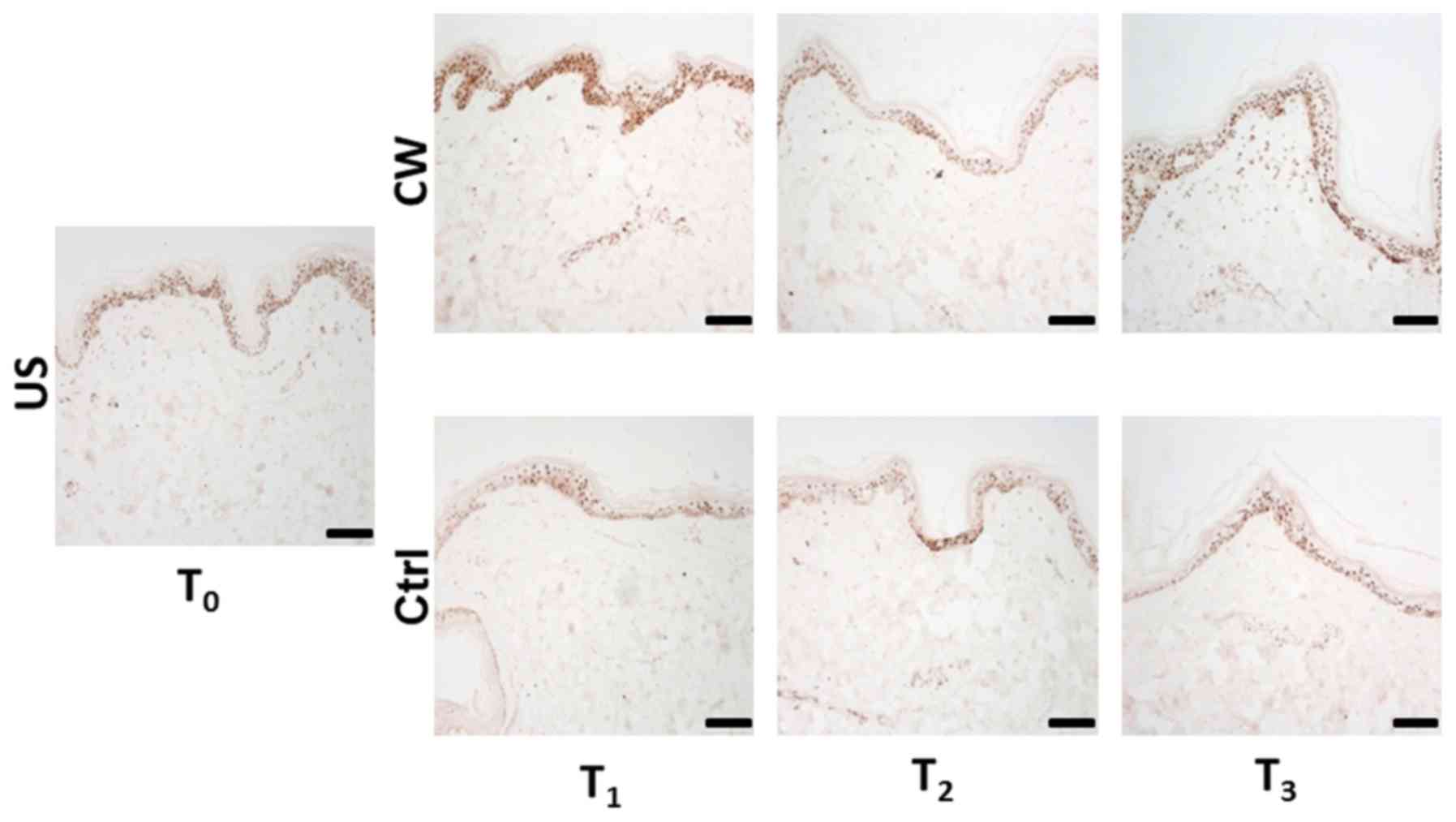
PCNA staining
At T1, the control samples exhibited a reduction in
the number of PCNA-positive nuclei compared with the US stained at
T0. At T2, compared with T1, a
slight increase in the PCNA-positive nuclei count was observed;
however, the number of positive nuclei remained lower than in the
US. At T3, a stable positive nuclei count compared with
T2 was exhibited.
At T1, the treated samples exhibited a
notable increase in the count of PCNA-positive nuclei compared with
the US at T0. At T2, the PCNA-positive nuclei
count was markedly decreased compared with that at T1
and slightly reduced compared with the US. At T3, the
positive nuclei count was increased compared with that at
T2 and somewhat higher than that in the US.
Additionally, the treated samples exhibited increased counts of
PCNA-positive nuclei compared with the control samples, notably at
T1 and T3 (Fig.
4).
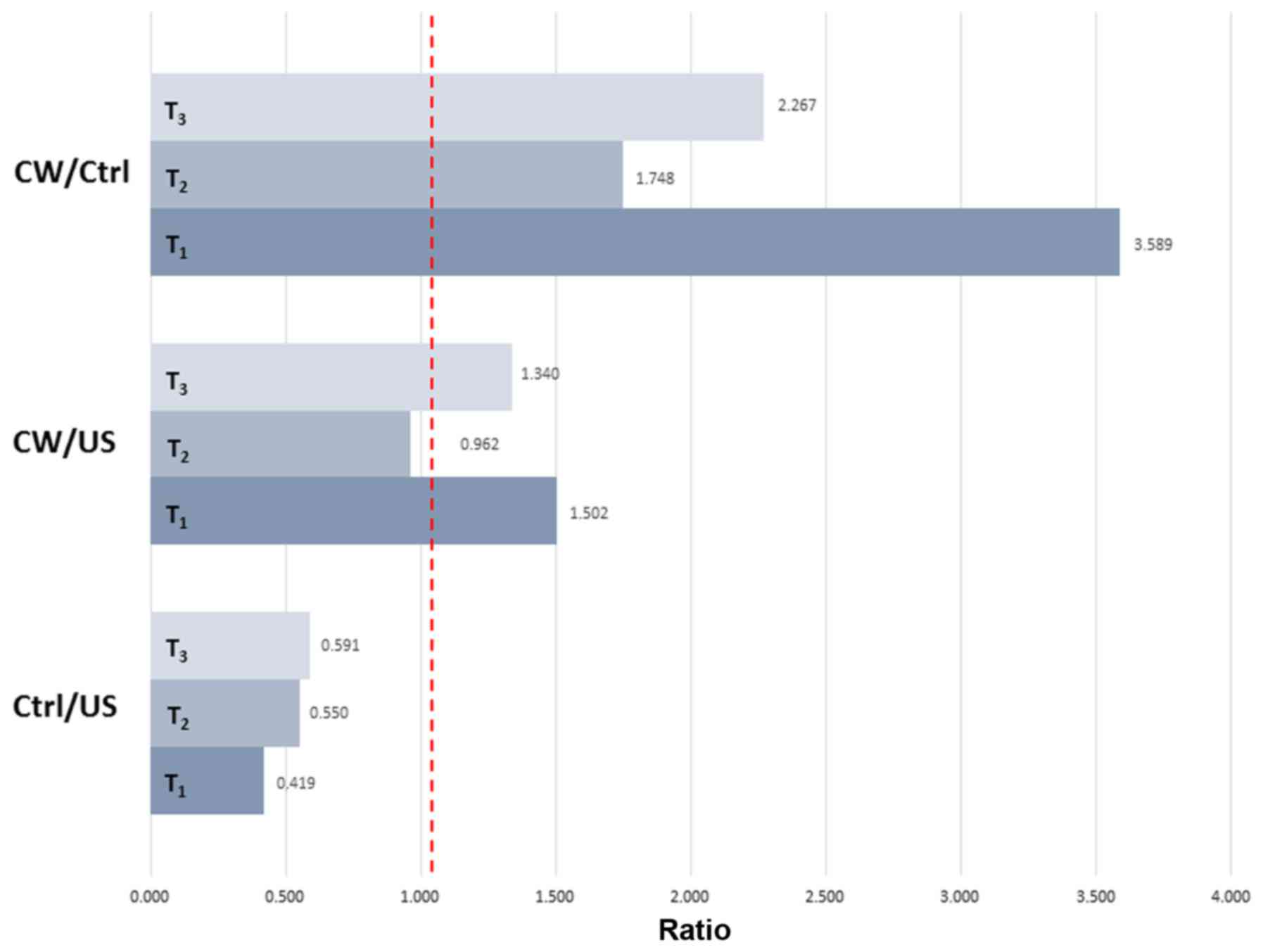
The ratio of PCNA-positive nuclei between the
treated and control samples decreased from T1 to
T2 and increased from T2 to T3. A
similar trend was observed for the ratio between the treated and US
samples, although at lower values. The ratio between the controls
and US underwent progressive marginal increases from T1
to T3 (Fig. 5).
Discussion
In the present study an ex vivo human skin
model was used to evaluate the physiology of human skin during the
wound repair process.
A number of strategies that have used skin samples
of differing thickness, different types of culture media and
different platforms for support have previously been used to
maintain full-skin culture ex vivo (10). The model used in the current study was
based on full-thickness skin punch biopsies containing a central
skin loss injury that were cultured in DMEM, of which the
reliability and effectiveness for reproducing the human skin
physiology in previous research trials have been indicated
(11,12).
A previous study by our group on a rabbit wound
healing model in vivo demonstrated a significant increase in
overall keratinocyte proliferation and a corresponding reduction in
the local inflammatory response following application of Comano
spring water (4). These results were
further supported by our previous study of human skin fibroblast
cultures in vitro (5).
Previous data suggests that the bioactive effects of some spring
waters may be correlated not only to their specific mineral
composition, but also to the complex activity of the resident
non-pathogenic bacterial flora, of which the composition and
biological features are largely unknown at present (6,7).
The spring waters certified as bacteriologically
pure are considered to be best termed as pathogen germ-free
(15). In the Comano spring water, a
total of 12 different bacterial strains have recently been
identified (Aeromonas encheleia, Aeromonas
hydrophila, Bacillus simplex, Brevundimonas
vesicularis, Chromobacterium violaceum, Citrobacter
youngae, Cupriavidus campinensis, Empedobacter
brevis, Pantoea agglomerans, Pseudomonas putida,
Pseudomonas stutzeri and Streptococcus mitis)
(8). All of these bacterial strains,
despite showing a rare potential virulence, demonstrate overall
favorable metabolic activity that includes the ability to promote
water self-purification (16), to
degrade soil contaminants such as herbicides, pesticides and
organic solvents (17–20), and to produce antifungi, antibacterial
and antioxidant substances (21–24). The
presence of other unknown bacterial species is also being
demonstrated by ongoing genomic sequential analysis (9). Therefore, the overall non-pathogenic
bacterial populations of the Comano spring water, comprehensively
termed microbiota, may be responsible for its regenerative
properties. These properties may be related to the production of so
far unknown substances that promote regeneration, probably in
synergy with macro and micro mineral elements of the spring water
(25).
In the present study, filtered Comano spring water
was used to obtain germ-free water that retained the supposed
bioactive bacterial metabolites (26). Investigations into the Comano spring
water microbiota by our group are now focusing on the extraction of
bacterial lysates from the bacterial strains considered most
influential, and a forthcoming study plans to assess their
regenerative properties on the experimental model used in the
current study.
In the present experimental model, control skin
biopsies were cultured with DMEM and saline solution was applied to
the central skin loss region. The biological events that occurred
over the 72 h observation period paralleled histological features
previously observed for an ex vivo human skin model, whereby
the absence of re-epithelialization and a rich inflammatory cell
infiltrate with progressive late identification of fibroblasts were
documented (27).
The current study identified favorable biological
events in the human skin samples treated with filtered Comano
spring water. The most notable effects were evident in the dermis.
A markable anti-inflammatory effect by reducing overall dermal cell
infiltration when compared with the controls was appreciated. The
reduction in cellular infiltrate in the dermis was concomitant with
fibroblast recruitment, suggesting a favorable modulation of the
local cell proliferative phase.
The PCNA immunostaining demonstrated a notable
stimulation of cell proliferation in the samples treated with
Comano spring water compared with the controls; the samples treated
with the spring water not only failed to exhibit the expected
reduction in cellular vitality following tissue explantation, but
also exhibited an increase in cell proliferation compared with the
baseline skin values. Most notably, cell proliferation appeared
highest in the treated samples at T1 and T3
when compared with the US at T0. Considering a ratio
equal to 1 is the condition for no difference between the
experimental conditions, and that only the treated samples
exhibited a ratio >1, this indicates a regenerative capacity of
Comano spring water.
A regenerative process was also indicated by an
increase in papillary dermis in the treated samples compared with
the controls.
Although the observation time of 72 h was relatively
short, interesting effects following the application of filtered
spring water were observed in the dermal collagen fiber network.
Signs of collagen fiber degeneration were appreciated at a later
time (T3) than in the controls, with an increase in
matrix deposition, and an increase in the spaces amongst the
fibers. The selective green staining with Picrosirius Red,
indicative of active collagen fiber regeneration through the
production of small-sized collagen fibers (28), suggested an early regenerative
activity in the treated samples, that, despite fading by
T3, remained higher than in the control samples. A
similar trend was observed for the elastic fiber network, which
exhibited signs of regeneration in the treated samples throughout
the observation period.
In conclusion, the previously implicated
regenerative properties of the Comano spring water were confirmed
in the current ex vivo human skin wounding model. Reduction
of inflammatory cell infiltration with selective fibroblast
recruitment was identified following application of the Comano
spring water. These favorable cellular effects matched an increase
in neo-collagen synthesis and a stimulatory effect on elastic fiber
regeneration. All of these effects are potentially associated with
the functions of the active metabolites produced by the spring
water's native microbiota. Thus, preparations of the spring water,
as a natural remedy, may have clinical efficacy in promoting tissue
regeneration and wound healing.
Acknowledgements
The present study was partially funded by ALMaUST
Onlus, Milano, Italy (grant no. 1514), Istituto GB Mattei, Terme di
Comano, Stenico, Italy (grant no. 24147) and Fondazione Anna Villa
e Felice Rusconi Onlus, Varese, Italy (grant no. 24988). Professor
Angela Faga and Dr Giovanni Nicoletti designed the experiments,
analyzed the data and wrote the paper; Dr Marco Saler and Dr
Federica Riva performed the experiments; Dr Alberto Malovini
analyzed the data; Dr Tommaso Pellegatta, Dr Marco Mario Tresoldi
and Dr Viola Bonfanti contributed to the data collection and
analysis and paper writing. The authors wish to thank Dr Laura
Villani at the Maugeri Clinical Scientific Institutes for her help
in reviewing and interpreting the histological preparations.
References
|
1
|
Belizário JE and Napolitano M: Human
microbiomes and their roles in dysbiosis, common diseases, and
novel therapeutic approaches. Front Microbiol. 6:10502015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kanno E, Kawakami K, Ritsu M, Ishii K,
Tanno H, Toriyabe S, Imai Y, Maruyama R and Tachi M: Wound healing
in skin promoted by inoculation with Pseudomonas aeruginosa
PAO1: The critical role of tumor necrosis factor-α secreted from
infiltrating neutrophils. Wound Repair Regen. 19:608–621. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kostarnoy AV, Gancheva PG, Logunov DY,
Verkhovskaya LV, Bobrov MA, Scheblyakov DV, Tukhvatulin AI,
Filippova NE, Naroditsky BS and Gintsburg AL: Topical bacterial
lipopolysaccharide application affects inflammatory response and
promotes wound healing. J Interferon Cytokine Res. 33:514–522.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Faga A, Nicoletti G, Gregotti C, Finotti
V, Nitto A and Gioglio L: Effects of thermal water on skin
regeneration. Int J Mol Med. 29:732–740. 2012.PubMed/NCBI
|
|
5
|
Nicoletti G, Saler M, Pellegatta T,
Malovini A, Faga A, Scalise A and Riva F: Effects of a spring water
on human skin fibroblasts in vitro cultures: Preliminary results.
Acta Vulnol. 14:196–201. 2016.
|
|
6
|
Aries MF, Fabre P, Duplan H, Hernandez
Pigeon H, Galliano MF, Castex-Rizzi N, Bessou-Touya S and Nguyen T:
I-modulia, an Aquaphilus dolomiae extract, stimulates innate
immune response through Toll-like receptor activation. J Am Acad
Dermatol. 70 Suppl 1:AB632014. View Article : Google Scholar
|
|
7
|
Mahe YF, Perez MJ, Tacheau C, Fanchon C,
Martin R, Rousset F and Seite S: A new Vitreoscilla
filiformis extract grown on spa water-enriched medium activates
endogenous cutaneous antioxidant and antimicrobial defenses through
a potential Toll-like receptor 2/protein kinase C, zeta
transduction pathway. Clin Cosmet Investig Dermatol. 6:191–196.
2013.PubMed/NCBI
|
|
8
|
Nicoletti G, Corbella M, Jaber O, Marone
P, Scevola D and Faga A: Non-pathogenic microflora of a spring
water with regenerative properties. Biomed Rep. 3:758–762. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jousson O: Culture-independent versus
culture-dependent approaches for microbial community analysis of a
thermal spring with therapeutic propertiesEMBL Symposium
Heidelberg. New Approaches and Concepts in Microbiology; Germany:
June 27–30–2017
|
|
10
|
Nakamura M, Rikimaru T, Yano T, Moore KG,
Pula PJ, Schofield BH and Dannenberg AM Jr: Full-thickness human
skin explants for testing the toxicity of topically applied
chemicals. J Invest Dermatol. 95:325–332. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mori M, Rossi S, Ferrari F, Bonferoni MC,
Sandri G, Riva F, Tenci M, Del Fante C, Nicoletti G and Caramella
C: Sponge-like dressings based on the association of chitosan and
sericin for the treatment of chronic skin ulcers. II. Loading of
the hemoderivative platelet lysate. J Pharm Sci. 105:1188–1195.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fontana F, Mori M, Riva F, Mäkilä E, Liu
D, Salonen J, Nicoletti G, Hirvonen J, Caramella C and Santos HA:
Platelet lysate-modified porous silicon microparticles for enhanced
cell proliferation in wound healing applications. ACS Appl Mater
Interfaces. 8:988–996. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Junqueira LCU, Bignolas G and Brentani RR:
Picrosirius staining plus polarization microscopy, a specific
method for collagen detection in tissue sections. Histochem J.
11:447–455. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Montes GS and Junqueira LC: The use of the
Picrosirius-polarization method for the study of the biopathology
of collagen. Mem Inst Oswaldo Cruz. 86 Suppl 3:1–11. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Éditeur officiel du Québec: Regulation
respecting bottled water. Food Products Act. Chapter P-29, s. 40.
http://www.legisquebec.gouv.qc.ca/en/pdf/cr/S-2.1,%20R.%2013.pdfApril
24–2017
|
|
16
|
Kompanets EV, Isaeva NM and Balakhnin IA:
Bacteria of the genus Aeromonas and their role in
aquaculture. Mikrobiol Zh. 54:89–99. 1992.(In Russian). PubMed/NCBI
|
|
17
|
Erguven GO and Yildirim N: Efficiency of
some soil bacteria for chemical oxygen demand reduction of
synthetic chlorsulfuron solutions under agiated culture conditions.
Cell Mol Biol (Noisy-le-grand). 62:92–96. 2016.PubMed/NCBI
|
|
18
|
Han L, Zhao D and Li C: Isolation and
2,4-D-degrading characteristics of Cupriavidus campinensis
BJ71. Braz J Microbiol. 46:433–441. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheriaa J, Mosrati R, Ladhari N and
Bakhrouf A: Acclimated biomass that degrades sulfonated naphthalene
formaldehyde condensate. Pak J Biol Sci. 11:1588–1593. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li C, Yang J, Wang X, Wang E, Li B, He R
and Yuan H: Removal of nitrogen by heterotrophic
nitrification-aerobic denitrification of a phosphate accumulating
bacterium Pseudomonas stutzeri YG-24. Bioresour Technol.
182:18–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
El Amraoui B, El Amraoui M, Cohen N and
Fassouane A: Antifungal and antibacterial activity of marine
microorganisms. Ann Pharm Fr. 72:107–111. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Durán M, Faljoni-Alario A and Durán N:
Chromobacterium violaceum and its important metabolites -
review. Folia Microbiol (Praha). 55:535–547. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hoshino T: Violacein and related
tryptophan metabolites produced by Chromobacterium
violaceum: Biosynthetic mechanism and pathway for construction
of violacein core. Appl Microbiol Biotechnol. 91:1463–1475. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rezzonico F, Smits TH, Montesinos E, Frey
JE and Duffy B: Genotypic comparison of Pantoea agglomerans
plant and clinical strains. BMC Microbiol. 9:2042009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pellegatta T, Saler M, Bonfanti V,
Nicoletti G and Faga A: Novel perspectives on the role of the human
microbiota in regenerative medicine and surgery. Biomed Rep.
5:519–524. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liang Z and Keeley A: Filtration recovery
of extracellular DNA from environmental water samples. Environ Sci
Technol. 47:9324–9331. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Safferling K, Sütterlin T, Westphal K,
Ernst C, Breuhahn K, James M, Jäger D, Halama N and Grabe N: Wound
healing revised: A novel reepithelialization mechanism revealed by
in vitro and in silico models. J Cell Biol. 203:691–709. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu W, Jong Hong S, Jia S, Zhao Y, Galiano
RD and Mustoe TA: Application of a partial-thickness human ex vivo
skin culture model in cutaneous wound healing study. Lab Invest.
92:584–599. 2012. View Article : Google Scholar : PubMed/NCBI
|