Introduction
Nefopam is a novel type of analgesic drug that
exerts non-narcotic effects (1), and
thus differs from typical opioids and anti-inflammatory drugs
(2). Nevertheless, it effectively
elicits antinociception in the majority of noxious and thermal
models in rodents (3,4). The mechanisms of action of nefopam may
involve inhibition of monoamine reuptake in the central nervous
system and glutamatergic pathway, in which calcium channels serve a
role (5,6). Certain adverse reactions of nefopam,
including dizziness, headache, nausea, vomiting and sweating, have
been associated with its central mechanisms (7).
Non-steroid anti-inflammatory drugs (NSAIDs) are
clinically used in the management of chronic, inflammatory and
postoperative pains. They may inhibit cyclooxygenase (COX) enzyme
that catalyzes the conversion of arachidonic acid to generate
prostaglandins (PGs) (8–10). PGs are established to serve a role in
pain and inflammatory processes (11). Acetaminophen is among the most
commonly used analgesic and antipyretic drugs worldwide, either on
prescription or over the counter. The drug has generally replaced
aspirin and other salicylates in the treatment of mild to moderate
pains in conditions without inflammatory involvement, including
headache, toothache and dysmenorrhea (12,13).
However, the mechanisms of its action remain to be fully defined.
The antinociceptive effects of acetaminophen may occur in part
through inhibition of the central COX enzymes COX-2 and COX-3
(14).
Results from other studies on the underlying
mechanisms have also indicated the involvement of other receptors
and transmitters in the central nervous system (15,16). The
side effects of NSAIDs most commonly include gastrointestinal
problems, hepatotoxicity and nephrotoxicity (17).
In consideration of the proposed individual
mechanisms of nefopam and acetaminophen, the present study aimed to
investigate the analgesic efficacy of combined therapy with the two
drugs, and to determine whether it is possible to reduce the dose
of nefopam used. Three animal models were used: The first involved
intraperitoneal administration of acetic acid (AA) in mice, which
simulates acute inflammatory pain in the viscera and induces
abdominal writhing; the second and third models involved simulation
of acute thermal pains, evaluated through hot plate and tail flick
tests. Assessments of these models ultimately aimed to determine
the effects of combined drug therapy compared with nefopam
alone.
Materials and methods
Animals
The present animal experiments were conducted
according to the rules of animal experimentation and the Guide for
the Care and Use of Laboratory Animals of Shenyang Pharmaceutical
University (Shenyang, China), and the protocol was approved by the
Animal Ethics Committee of Shenyang Pharmaceutical University
(approval no: SYPU-IACUC-131220-138). The animals used were Kunming
mice aged 4–6 weeks old (n=249; male and/or female depending on the
assay as specified) and Sprague-Dawley male rats aged 6–8 weeks old
(n=77; sex ratio 1:1), obtained from the Animal Center of Shenyang
Pharmaceutical University. The animal weights ranged between 18–25
g for mice and 180–250 g for rats. The animals were group-housed (6
mice per cage) under standard environmental conditions (22±1°C,
humidity 60±5%, 12 h light/dark cycle) with free access to a
standard commercial diet and water ad libitum. After a 7 day
adaptation period, all experiments were performed during the light
phase.
Drugs
Nefopam hydrochloride and acetaminophen were
provided by Shenyang Funing Pharmaceuticals Co., Ltd. (Shenyang,
China). They were dissolved in 0.9% sterile saline (normal saline,
N.S.; Shenyang Zhiying Pharmaceutical Co., Ltd., Shenyang, China).
Nefopam and acetaminophen were intravenously (i.v.) administered at
a constant volume of 10 ml/kg body mass in the abdominal
constriction and hot plate tests, while the drugs were
intraperitoneally (i.p.) injected (10 ml/kg) in the rat tail flick
test to prevent tail pain from i.v. injection.
Determination of optimal drug
combination through orthogonal design
The orthogonal array method was used to optimize the
proportions of nefopam and acetaminophen (18–20). In
brief, the L9 (32) factorial design,
a factorial arrangement with two factors at three levels, was used
to indicate the optimal proportions of nefopam and acetaminophen in
combination (Table I). The detailed
experimental design is presented in Table II.
 | Table I.Orthogonal design for combination
treatment. |
Table I.
Orthogonal design for combination
treatment.
|
| Factor (mg/kg) |
|---|
|
|
|
|---|
| Level | Nefopam | Acetaminophen |
|---|
| 1 | 1.5 | 60 |
| 2 | 2.5 | 95 |
| 3 | 3.5 | 130 |
 | Table II.Experimental design based on L9
(32) orthogonal array and experimental results. |
Table II.
Experimental design based on L9
(32) orthogonal array and experimental results.
|
| Independent
variable (factors) |
|
|---|
|
|
|
|
|---|
| Run | Nefopam | Acetaminophen | Number of writhing
responses, mean ± SEM/20 min |
|---|
| 1 |
1 |
1 | 11.5±3.3 |
| 2 |
1 |
2 | 10.4±2.0 |
| 3 |
1 |
3 |
5.7±1.8 |
| 4 |
2 |
1 |
4.5±1.6 |
| 5 |
2 |
2 |
8.4±2.1 |
| 6 |
2 |
3 |
9.4±2.3 |
| 7 |
3 |
1 |
2.9±1.1 |
| 8 |
3 |
2 |
7.3±2.4 |
| 9 |
3 |
3 |
4.8±1.4 |
| K1 | 27.50 | 18.87 |
|
| K2 | 22.25 | 26.04 |
|
| K3 | 14.91 | 19.75 |
|
| R | 12.59 |
7.17 |
|
The analgesic activity of each combination was
evaluated through a preliminary abdominal constriction assay in
mice (21). Mice were randomized into
9 groups (n=10, sex ratio 1:1) and were respectively administered
the combinations of nefopam and acetaminophen (i.v.) according to
the design in Tables I and II. After 30 min, each mouse was injected
(i.p.) with 0.8% AA and was placed in an individual plastic cage
for observation. The number of writhing responses in a 20 min
observation period was counted by an investigator blinded to the
experimental groups, beginning 3 min after administration of the AA
solution. A writhing response was characterized as a wave of
contraction of the abdominal musculature followed by extension of
the hind limbs.
Abdominal constriction assay in
mice
Subsequently, persistent chemical pain was assessed
by abdominal constriction assay in mice. In the present test, male
and female mice (sex ratio 1:1) were used, randomized into 8
groups. The mice were respectively administered (i.v.) 0.9% N.S.
(n=10), nefopam (3.5 and 7.0 mg/kg; n=9 and 10), acetaminophen (60
and 120 mg/kg; n=9 and 10) or combinations of nefopam (N) and
acetaminophen (A) (1.75 N + 30 A, 3.5 N + 60 A, 7.0 N + 120 A
mg/kg; n=8, 10 and 8) 30 min prior to the abdominal constriction
test. Each mouse was injected (i.p.) with 0.8% AA at the start of
the test and was placed in an individual plastic cage for
observation. The number of writhing responses in a 20 min
observation period was counted as described above.
Hot plate test in mice
An RB-200 intelligent hot plate apparatus (Chengdu
Taimeng Science And Technology Co., Ltd., Chengdu, China) was used.
The temperature of the hot plate was set to 55±0.5°C and latency
was defined as the period from the time when the animal was placed
on the hot plate surface to the time when the animal licked its
back paw or jumped off to avoid thermal pain. Female mice were
used. The mice with latency of 5–30 sec by duplicated detection
were selected to be used in the formal experiment (21). The mice were randomized into 9 groups
and were respectively administered (i.v.) 0.9% N.S. (n=9), nefopam
(3.5 and 7.0 mg/kg; n=9 per group), acetaminophen (60, 120 and 240
mg/kg; n=10, 10 and 9) or combinations of N and A (1.75 N + 30 A,
3.5 N + 60 A, 7.0 N + 120 A mg/kg; n=9, 10 and 10). The latency of
hind paw licking was measured at 15, 30, 45 and 60 min after the
test compounds were administered. A maximal latency of 60 sec was
used to avoid damage to animal skin tissues for any mice exhibiting
thermal pain endurance.
Tail flick test in rats
A tail flick assay in rats was performed as
described in our previous study (21), for which an automated tail flick test
device (SW-200 Analgesia Meter; Chengdu Taimeng Science And
Technology Co., Ltd.) was used. Rat tails (distal 1/3rd) were
painted with black ink to enhance absorption of heat radiation. An
adjustable heat source was directly placed under the tail. The
assay is based on the animal flicking its tail away to avoid pain
induced by the source of heat. Tail flick latencies required for
the rat to remove its tail were determined in sec as an index of
nociceptive threshold (22). Pain
threshold elongation indicated the analgesic effect of the drug.
The rats with latency of 3–20 sec by duplicated detection were
selected to be used in the formal experiment. They were randomized
into 9 groups and were respectively administered (i.p.) 0.9% N.S.
(n=9), nefopam (2.5 and 5 mg/kg; n=9 per group), acetaminophen (42,
84 and 168 mg/kg; n=8 per group), or combinations of N and A (1.25
N + 21 A, 2.5 N + 42 A, 5.0 N +84 A mg/kg; n=9,9 and 8) prior to
their subjection to the tail flick test. The latency of tail
flicking was determined at 15, 30, 45, 60 and 75 min after drug
administration. The maximal latency was limited to 30 sec to
protect tail tissues from damage in instances of thermal pain
endurance.
Statistical analysis
Results from the groups were calculated as the mean
± standard error of mean. Statistical analysis was performed with
one-way analysis of variance and post hoc multiple comparison
between groups was performed with Fisher's protected least
significant difference test to compare the data, which were
analyzed with SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA).
Cumulative antinociceptive effect during the whole observation
period was determined according to the area under the curve (AUC)
of the temporal course to compare the complete antinociceptive
effects exerted by the tested drugs, alone or in combination. The
value of AUC was calculated with SigmaPlot 12.0 (Systat Software,
Inc., San Jose, CA, USA).
Results
Optimization of the proportion of
nefopam and acetami nophen
Three dose levels of individual doses of nefopam and
acetaminophen were represented by 1, 2 and 3, respectively
(Table I). The number of writhing
responses was determined to evaluate the analgesic effect of each
combination. Ki (i =1, 2, 3) represented the sum of
writhing responses for nefopam or acetaminophen at each level.
Considering that a reduced number of writhing responses indicated a
higher analgesic effect, the lower the value of Ki, the
greater the analgesic effect. The drug with higher R value was
considered to have a greater impact on the analgesic effect. As
depicted in Table II, nefopam was
the main factor influencing the analgesic effect of combination
therapy. The optimal formula for nefopam was the third level (3.5
mg/kg) and for acetaminophen the first level (60 mg/kg). Based on
the results under orthogonal design conditions, the optimum
proportions of the two drugs were indicated to be 3.5 mg/kg nefopam
plus 60 mg/kg acetaminophen (Table
II).
Antinociceptive effect on writhing
response in mice
The combinations of 1.75 N + 30 A, 3.5 N + 60 A and
7.0 N + 120 A mg/kg evoked antinociception dose-dependently in the
abdominal constriction assay (Fig.
1); writhing response number in each group was 21.4±2.26,
6.0±1.05 and 1.0±0.50, respectively. Acetaminophen used alone at a
dose of 60 mg/kg elicited significant antinociception, with
writhing number reduced to 25.1±1.49 during the 20 min period
(P<0.05), while 3.5 mg/kg nefopam reduced writhing number to
16.1±1.61 (P<0.01), compared with 0.9% N.S. control (36.7±2.58).
Co-injection of 3.5 mg/kg nefopam plus 60 mg/kg antinociception
(combination 3.5 N + 60 A mg/kg) significantly reduced the number
of writhing responses by 63% (to 6.0±1.05) compared with that of
3.5 mg/kg nefopam alone (P<0.01). High-dose nefopam (7.0 mg/kg)
exhibited significant analgesic efficacy with a writhing response
number of 3.0±0.58, as did the high-dose combination treatment (7.0
N+120 A mg/kg; 1.0±0.5), relative to N.S. control (P<0.01). The
high-dose combination decreased the number of writhing responses by
66% compared with high-dose nefopam alone, though there was no
significant difference between the groups. Additionally, there was
no significant difference in analgesic effect between the optimum
combination (3.5 N + 60 A mg/kg) and high-dose nefopam treatments
(Fig. 1).
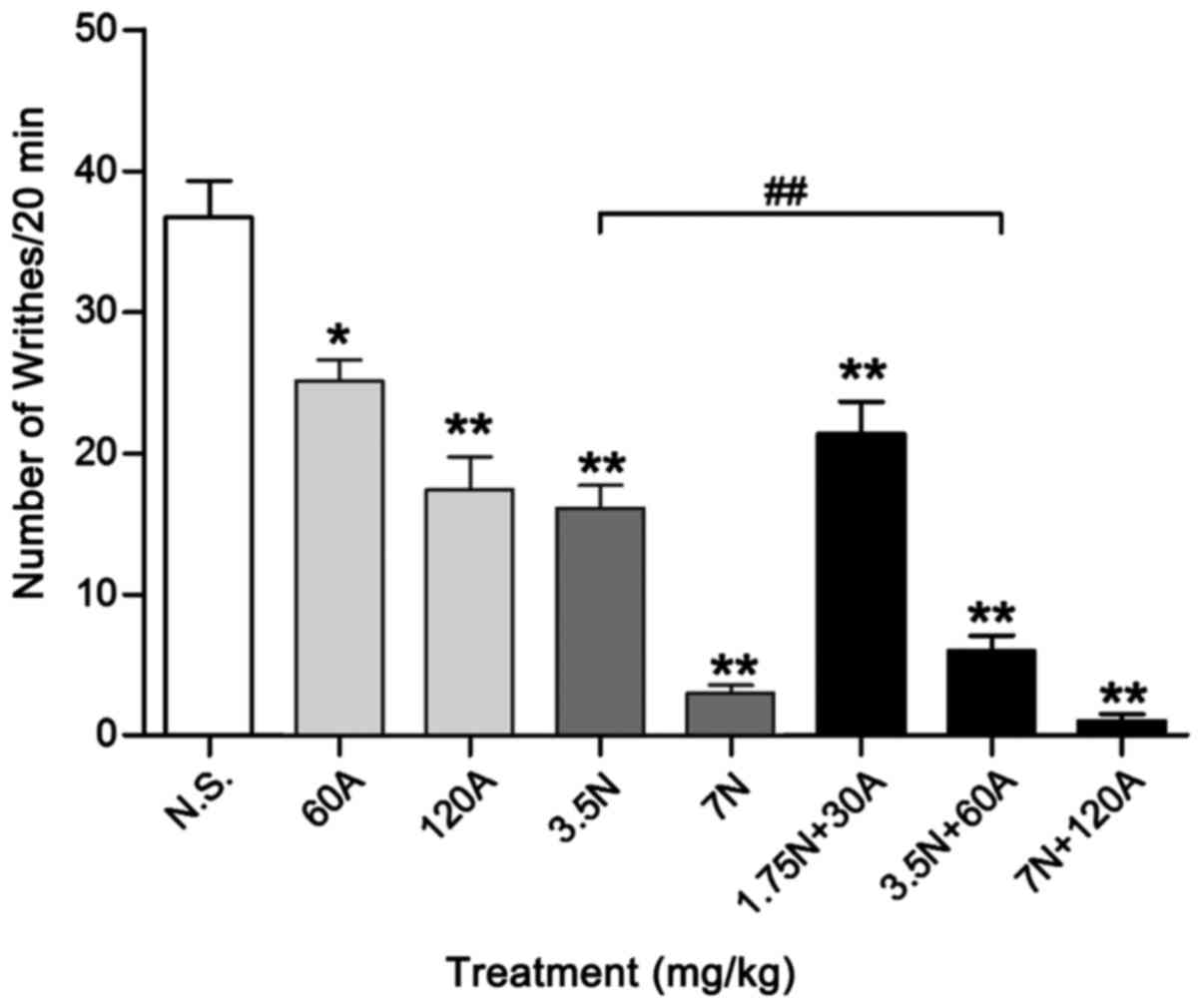 | Figure 1.Antinociceptive effects (number of
writhing responses) during the acetic acid-induced writhing test in
mice. The number of writhing responses were recorded in mice
following treatment with different doses of A alone (60 and 120
mg/kg, i.v.), N alone (3.5 and 7 mg/kg, i.v.) or N in combination
with A (1.75 N + 30 A, 3.5 N + 60 A and 7 N + 120 A mg/kg, i.v.).
Data are expressed as the mean ± standard error of the mean, n=8–10
per group. *P<0.05 and **P<0.01 vs. N.S. control;
##P<0.01 as indicated. A, acetaminophen; N, nefopam;
N.S., normal saline; i.v., intravenous. |
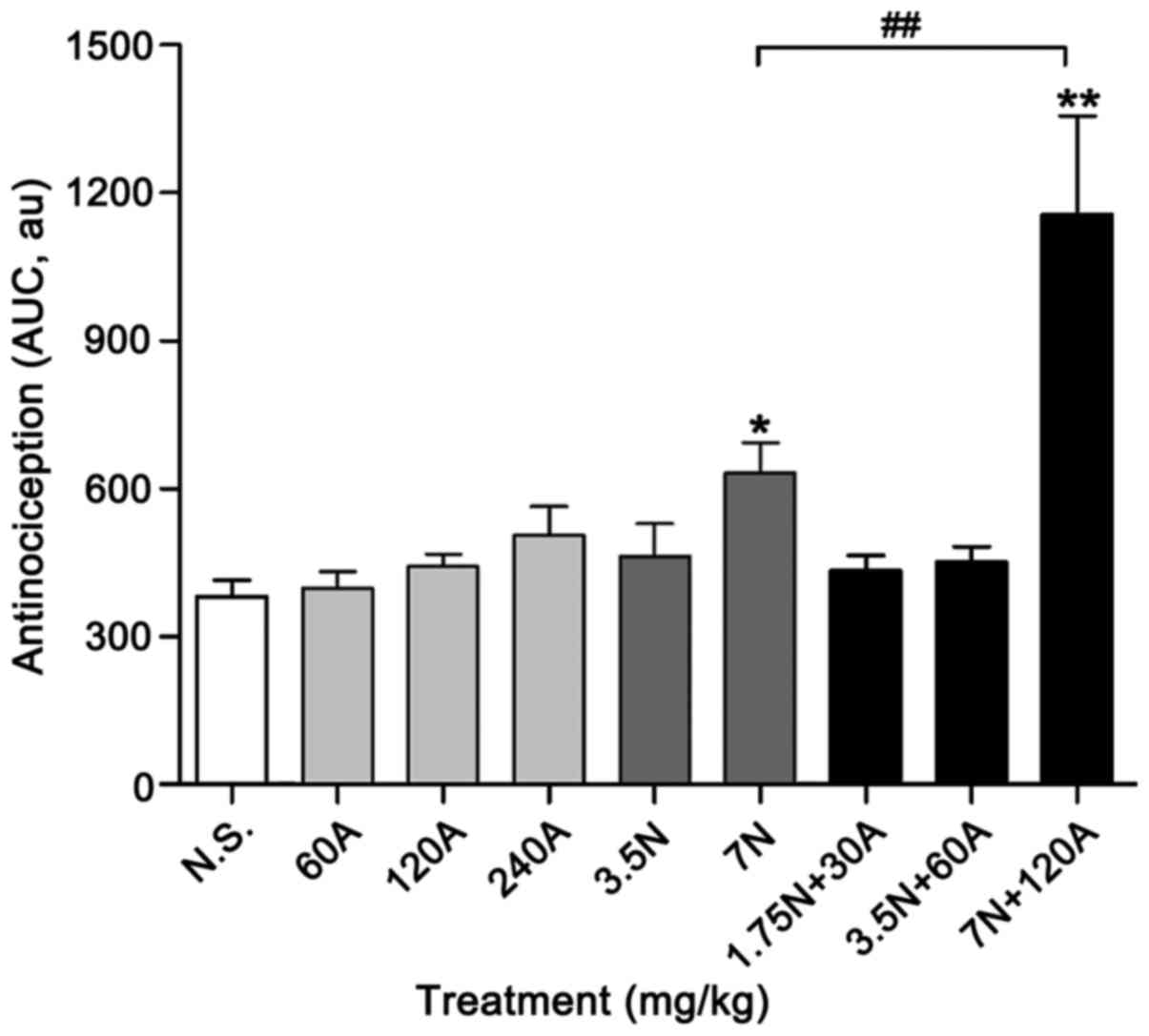
Antinociceptive effect on hot plate
latency in mice
Acetaminophen alone did not exhibit significant
antinociceptive activity in the hot plate assay even at a high dose
of 240 mg/kg (Fig. 2), and neither
did the low dose of nefopam (3.5 mg/kg; Fig. 3). The combination 3.5 N + 60 A mg/kg
exerted a significant antinociceptive effect at 15 min when
compared with N.S. control (P<0.05; Fig. 3). The analgesic effect of high-dose
nefopam (7.0 mg/kg) alone was significant at 15 min when compared
with that of the control (P<0.01), though was not sustained at
time points thereafter (Fig. 3). By
contrast, the high-dose combination (7 N + 120 A mg/kg) was
identified to produce a significant analgesic effect up to 30 min
after administration compared with the control (P<0.01; Fig. 3). Furthermore, the high-dose
combination exerted greater antinociceptive activity at 30 min
compared with high-dose nefopam alone (P<0.01; Fig. 3).
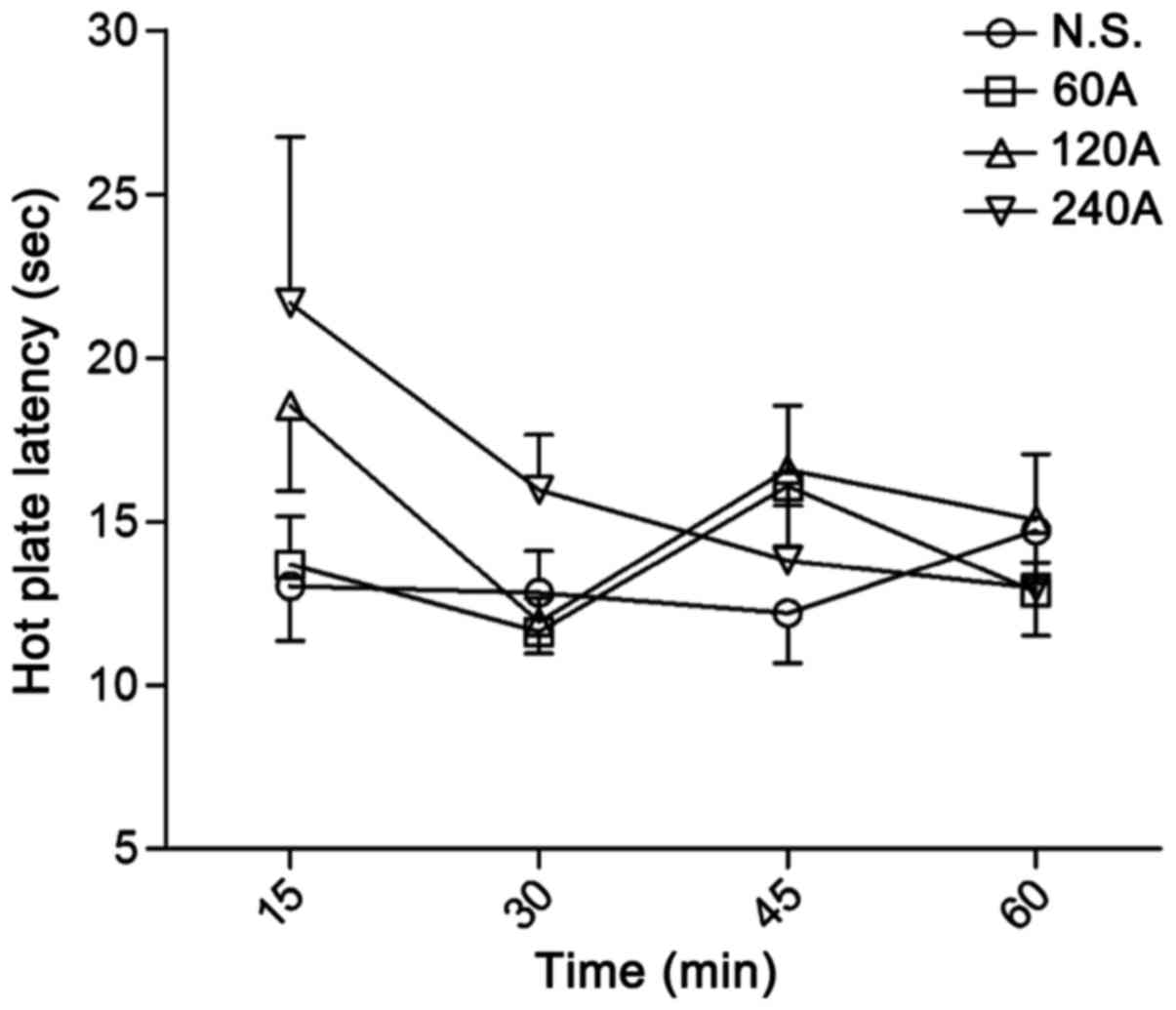 | Figure 2.Antinociceptive effect of A in the
hot plate test. Antinociceptive effect, based on prolonged paw
withdrawal latency in mice, was recorded following treatment with
different doses of A (60, 120 and 240 mg/kg, i.v.). Latency was
assessed at 15, 30, 45 and 60 min after drug administration. Data
are expressed as the mean ± standard error of the mean, n=9–10 per
group. No statistically differences were observed. A,
acetaminophen; N.S., normal saline; i.v., intravenous. |
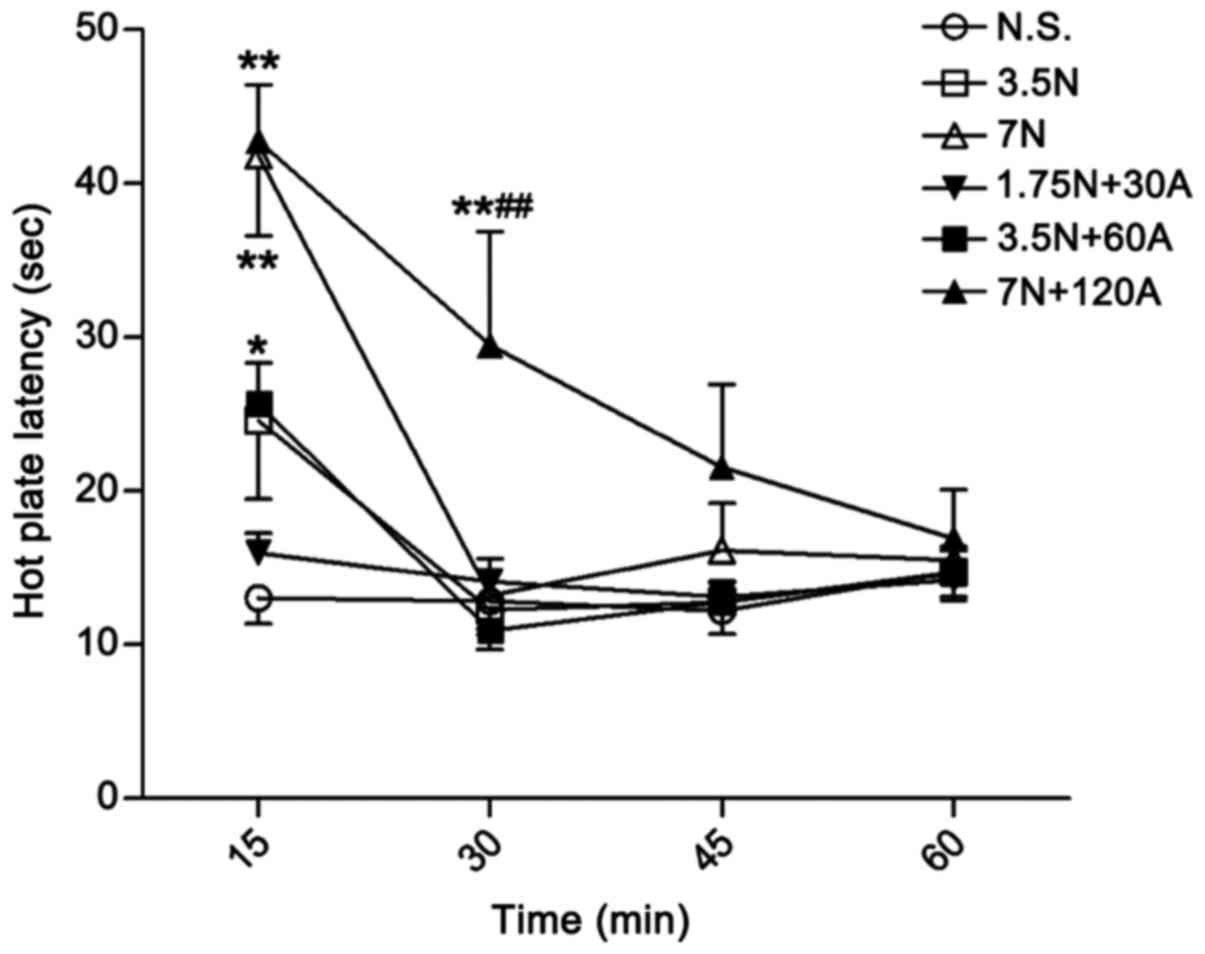 | Figure 3.Antinociceptive effect of N alone and
in combination in the mouse hot plate test. Paw withdrawal latency
in mice was recorded following treatment with different doses of N
alone (3.5 and 7 mg/kg, i.v.) and in combination with A (1.75 N +
30 A, 3.5 N + 60 A and 7 N + 120 A mg/kg, i.v.). Latency was
assessed at 15, 30, 45 and 60 min after drug administration. Data
are expressed as the mean ± standard error of the mean, n=9–10 per
group. *P<0.05 and **P<0.01 vs. N.S. control at the same time
point; ##P<0.01 vs. 7 N group at 30 min. A,
acetaminophen; N, nefopam; N.S., normal saline; i.v.,
intravenous. |
The cumulative effect of nefopam or acetaminophen
administered individually or in combination, expressed as the AUC
of the temporal course, also indicated the different
antinociceptive properties of the drugs used alone and in
combination (Fig. 4). According to
AUC values, acetaminophen at doses of 60, 120 and 240 mg/kg did not
exhibit significant antinociceptive activity in the hot plate
assay, though exerted dose-dependent effects; the area units (AU)
were 397.81±34.81, 442.54±82.59 and 505.85±58.09, respectively
(Fig. 4). The high-dose combination
(7 N + 120 A mg/kg) exhibited a greater cumulative antinociceptive
effect during the whole observation period (60 min), with an AUC
value of 1,156.95±199.30 AU, compared with that of high-dose
nefopam (7 mg/kg) alone (632.12±62.38 AU; P<0.01). Nevertheless,
both high-dose nefopam and combination exhibited significant
cumulative antinociception compared with the N.S. control
(381.6±33.9 AU; P<0.05 and P<0.01, respectively; Fig. 4).
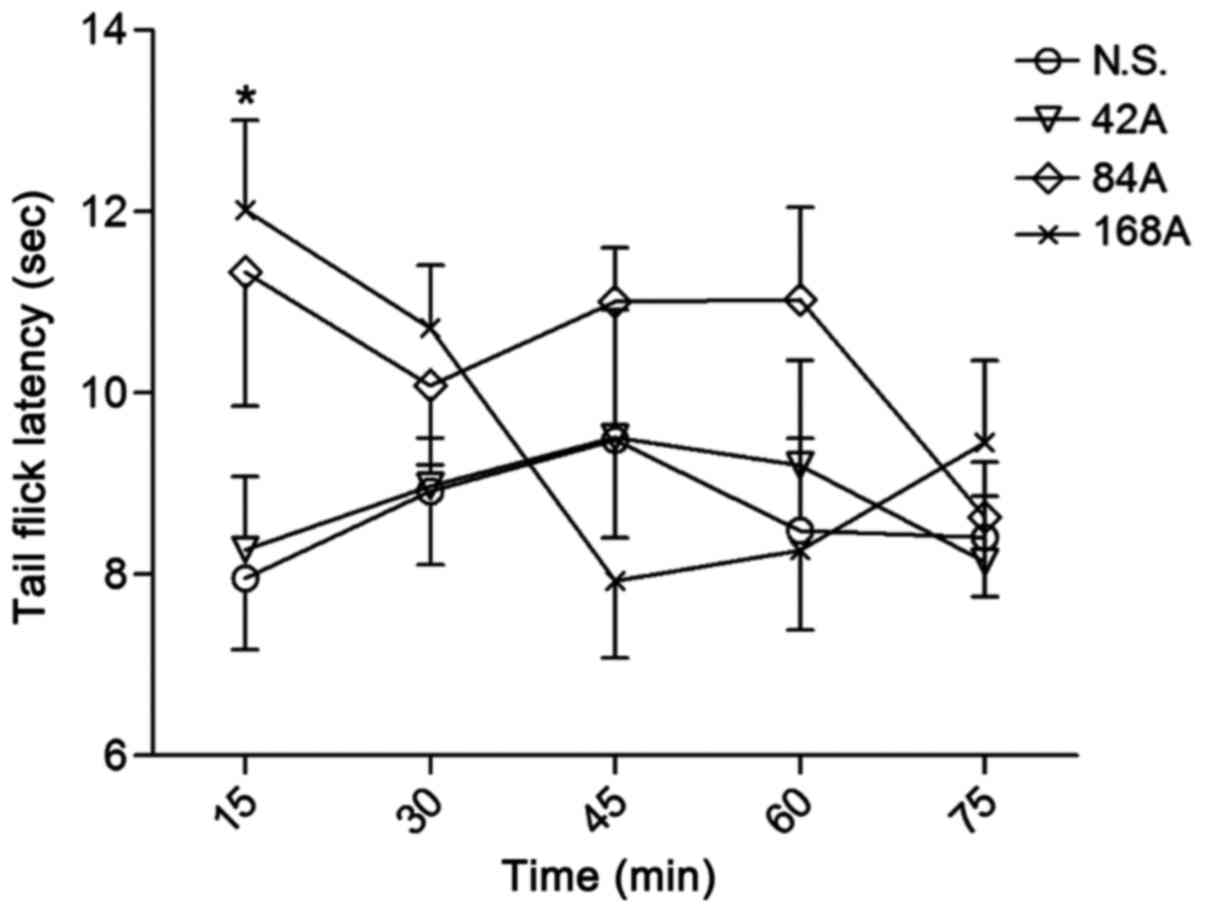
Antinociceptive effect on thermal tail
flick latency in rats
The highest dose of acetaminophen (168 mg/kg)
exhibited significant antinociceptive activity in the tail-flick
test at 15 min after drug administration (P<0.05), while the
lower doses (42 and 84 mg/kg) had no significant analgesic effect,
relative to N.S. control (Fig. 5).
Nefopam alone exerted a sustained analgesic effect at a dose of 5.0
mg/kg, which was significant at 30 (P<0.01), 45 (P<0.01), 60
(P<0.05) and 75 min (P<0.05; Fig.
6); while the lower dose of the drug (2.5 mg/kg) only exerted
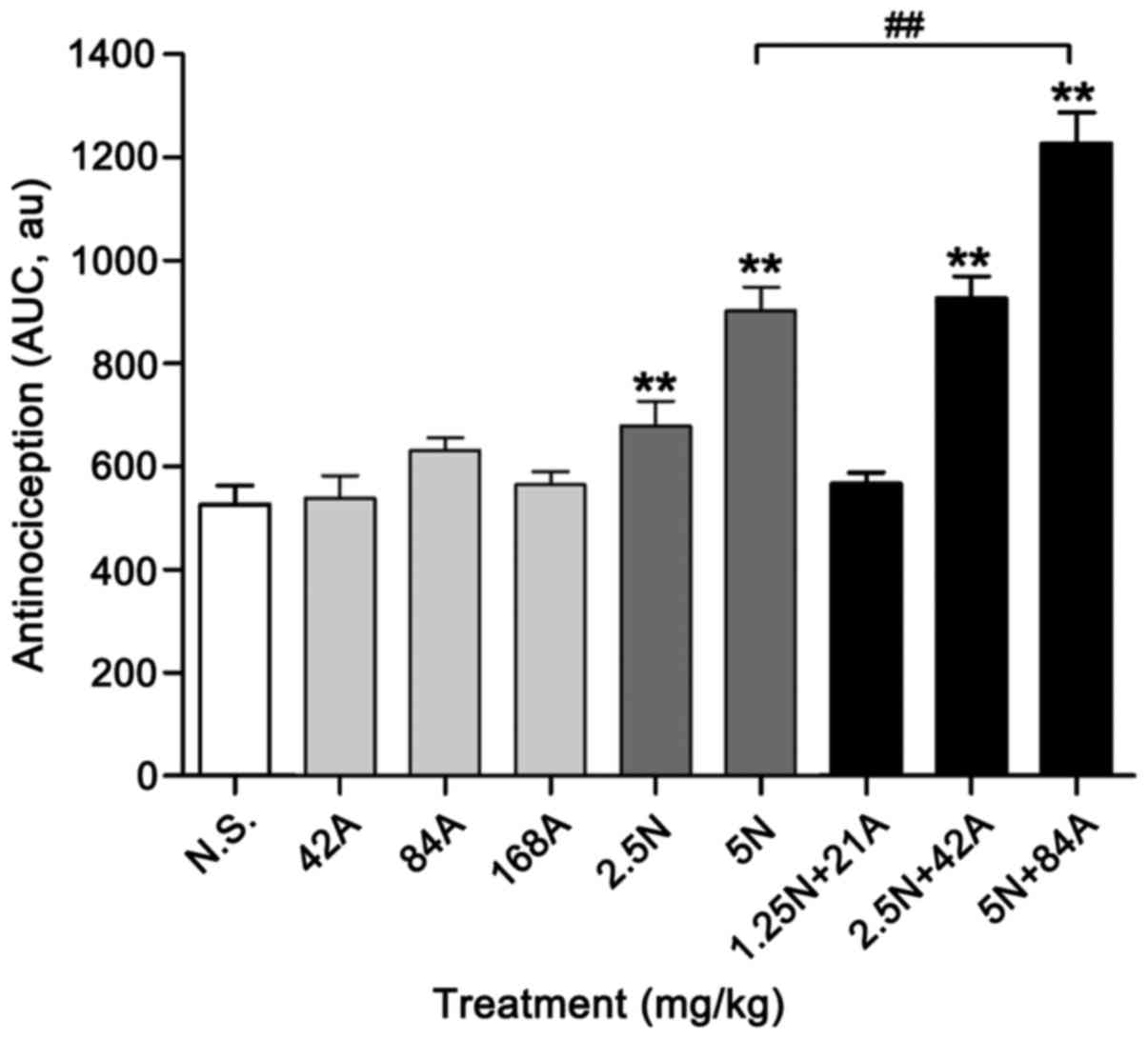
significant analgesic effect at 30 min (P<0.01; Fig. 6). By contrast, 42 mg/kg acetaminophen
combined with 2.5 mg/kg nefopam (combination 2.5 N + 42 A mg/kg)
exerted a significant analgesic effect on tail flick latency
compared with the control at all time points (P<0.05),
particularly between 15–45 min (P<0.01; Fig. 6). This antinociceptive effect was
maximized by co-injection of 5 mg/kg nefopam and 84 mg/kg
acetaminophen (combination 5 N + 84 A mg/kg) throughout the
observation period (P<0.01); additionally, maximum effect of the
5 N + 84 A mg/kg combination was observed at 30 min after drug
administration (Fig. 6). The
combinations of nefopam with acetaminophen produced greater
antinociceptive effect than that obtained by administration of the
corresponding nefopam concentration alone, and the analgesic effect
of the combination treatments occurred in a dose-dependent manner
(Fig. 6). The sustained effects over
75 min were subsequently expressed with AUC values, which verified
the assay results (Fig. 7). Notably,
both monotherapy and combined therapy exhibited antinociceptive
effects in a dose-dependent manner, and the higher dose combination
(5 N + 84 A mg/kg) exerted greater analgesic effect compared with
the higher dose of nefopam (5 mg/kg) alone (P<0.05; Fig. 7).
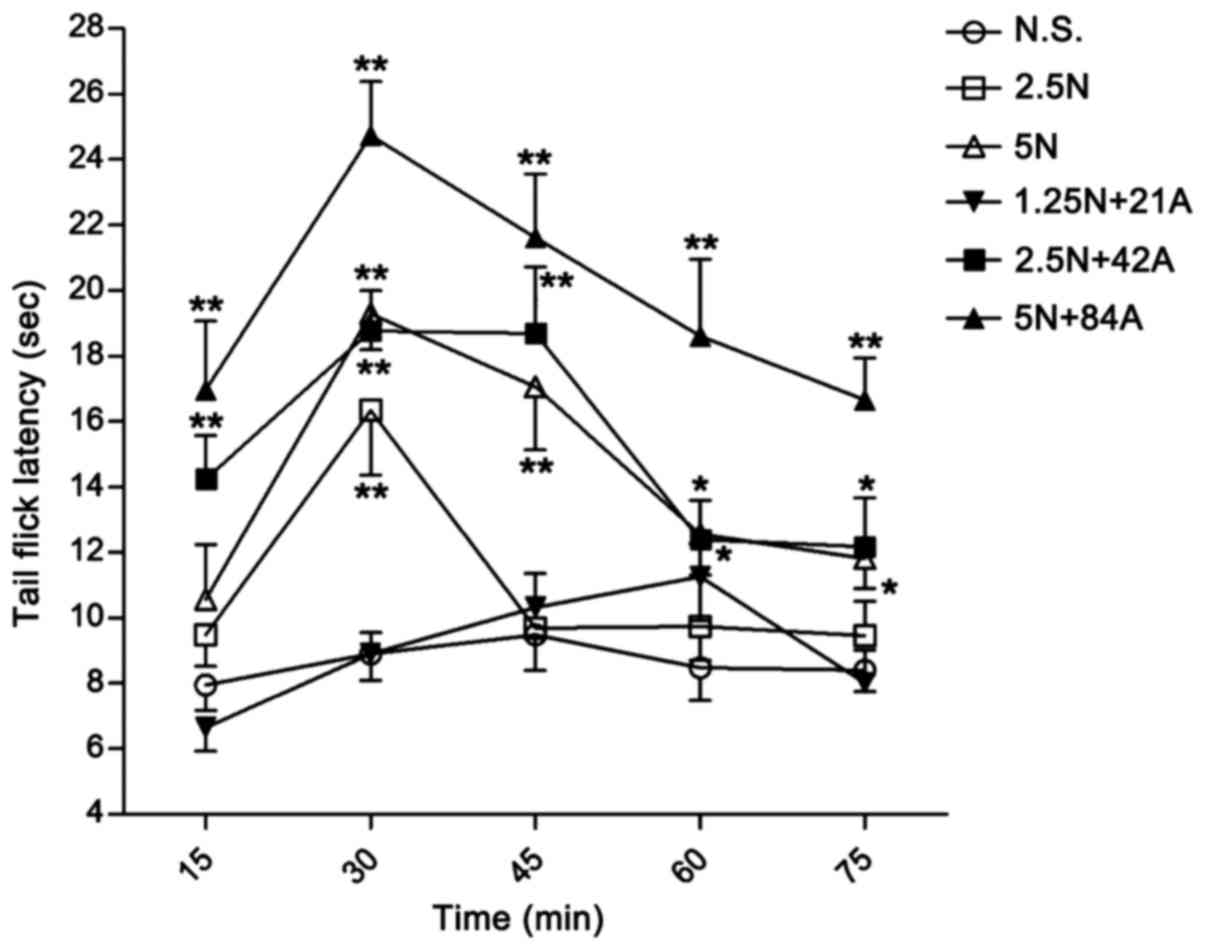 | Figure 6.Antinociceptive effect of N alone and
in combination in the tail flick test in rats. Tail flick latency
in rats was recorded following treatment with different doses of N
alone (2.5 and 5 mg/kg, i.p.) and in combination with A (1.25 N +
21 A, 2.5 N + 42 A, and 5 N + 84 A mg/kg, i.p.). Latency was
assessed at 15, 30, 45, 60 and 75 min after drug administration.
Data are expressed as the mean ± standard error of the mean, n=8–9
per group. *P<0.05 and **P<0.01 vs. N.S. control at the same
time point. A, acetaminophen; N, nefopam; N.S., normal saline;
i.p., intraperitoneal injection. |
Discussion
The main purpose for the development of combination
analgesics is to gain further efficacy and potency, and to thus
doses. It has been demonstrated that nefopam has synergistic
analgesic effects in humans and rodent animals when administered
concomitantly with nonsteroidal anti-inflammatory drugs (3,23–25). A previous clinical trial indicated
that a combination of nefopam and ketoprofen produced effective
analgesia with synergistic interaction in humans (23). Elstraete and Sitbon (24) reported that the combination of nefopam
and acetaminophen produced effective analgesia with a synergistic
interaction allowing a dose reduction of each drug. Furthermore, it
has been observed that coadministration of nefopam with ketoprofen
or paracetamol has synergistic effects in rats with
carrageenan-induced tactile allodynia, in the inflammatory phase of
the mouse formalin test, in thermal hyperalgesia in an incision
model of postoperative pain, and in the abdominal writhing response
in the AA test (3,25). The present study verified an additive
potentiation of acetaminophen and nefopam at the tested doses when
compared to their per se effect in rodents, and this positive
combination allowed dose reduction of each drug.
AA induces a writhing response when it is
intraperitoneally administered (26,27). It
has been reported that the agent may cause the indirect release of
noxious substances including bradykinins, serotonin, histamine and
PGs (28–30). The severity of abdominal constriction
induced by AA may be dependent on the production and release of
pro-inflammatory cytokines from resident peritoneal macrophages and
mast cells (31,32). Although the abdominal constriction
test is considered a sensitive method for investigating the
stimulation of local receptors in the abdomen, it is also
considered non-specific as the observed analgesic effects can not
be ascertained to involve a peripheral and/or central mechanism
(33). In the present study,
acetaminophen, nefopam and their combinations produced significant
antinociceptive effects in this visceral inflammatory model in
mice. The mechanism of action may be dependent on both peripheral
and/or central pathways.
Hot plate and tail flick tests were also performed
to confirm the additive effect of the two drugs in the present
study. The former is often used to investigate the analgesic
activity of a drug, which is probably achieved through its effect
on central the nervous system (34).
Using this test, the present study verified that combined
administration of nefopam and acetaminophen has potential as a
viable novel therapy for pain. Both methods of treatment, 7 mg/kg
nefopam alone and the combination 7 N + 120 A mg/kg, exhibited
analgesic effect, though their efficacy differed. Nefopam alone
produced a significant analgesic effect at 15 min, while the
combination was identified to have greater efficacy for a longer
period. Acetaminophen alone did not elicit significant
antinociceptive effect even at the highest dose of 240 mg/kg,
though markedly enhanced the antinociceptive effect of nefopam when
given concomitantly. Furthermore, the higher dose combination (5 N
+ 84 A kg/mg) in the rat tail flick test exhibited a peak
antinociceptive effect at 30 min, and significant antinociception
throughout the 75 min observation period compared with the control.
Notably, the effect of the higher dose combination in rats was
greater than that of nefopam alone at high dose (5 mg/kg; Fig. 7). Gong et al (35) identified that a combination of
tramadol and acetaminophen produced an additive antinociceptive
effect in the tail-flick test. In a previous study by Girard et
al (3), potent antinociceptive
properties of nefopam combined with ketoprofen were observed in a
mouse writhing abdominal test. The median effective dose (ED50) of
the two drug's coadministration was significantly lowered compared
with that of nefopam alone, and it was suggested that the nefopam
and ketoprofen interaction may be additive, with a possible
tendency toward synergy (3). Another
study also illustrated the enhanced antinociceptive properties of
combined administration of nefopam and acetaminophen in a writhing
test, in which a 36.3±5.5 mg/kg ED50 value was observed on
coadministration compared with 1.5±0.2 and 120.9±14.8 mg/kg for
nefopam and acetaminophen, respectively (25). Meanwhile, Miranda et al
(36) confirmed a synergistic
interaction between paracetamol and tramadol on their
coadministration via i.p. or intrathecal (i.t.) routes, with the
interaction index value of i.p. being similar to that of i.t. It
was suggested that the different mechanisms of action of
paracetamol and tramadol probably contributed to the analgesic
synergism between them (36). In the
present study, it was identified that the combination of nefopam
and acetaminophen additively reduced pain behavior and increased
antinociceptive activity, which is consistent with the results of
the previous studies. Additionally, the present results provide
novel understanding of the combined application of nefopam and
acetaminophen in other rodent pain models besides the mouse
writhing model.
In the present study, acetaminophen exhibited weak
analgesic action in the mouse hot plate model even at the highest
dose of 240 mg/kg and elicited weak antinociceptive effect in the
rat tail flick test at 168 mg/kg. Unlike most NSAIDs, acetaminophen
administration at therapeutic doses has little or no
anti-inflammatory or anti-platelet activity (29). Additionally, it does not exhibit the
typical side effect profile of NSAIDs, which includes
gastrointestinal tract problems and aspirin-induced asthma
(37,38). Although acetaminophen is among the
most popular and widely used analgesics, the mechanism of its
analgesic action remain uncertain, though the most suggested
mechanism involves inhibition of COX activity (39).
The COX-3 isoform of COX is inhibited by
acetaminophen at higher affinity than the two other isoforms (COX-1
and −2) (40), though the clinical
relevance of this is disputed (41).
Serotonin (also known as 5-hydroxytryptamine, 5-HT) exerts effects
via several subtypes of its cognate receptor, and its localization
in axon termini places these subtypes in prime location for the
modulation of pain, transmission and processing (42,43).
Previous studies have reported that acetaminophen may exert
antinociceptive effect through increasing the levels of serotonin
released from serotonergic neurons in the brainstem, known as
central mediation (42,43). Furthermore, results have suggested
that acetaminophen acts directly on the opioid receptor or
cannabinoid (CB) receptor, or indirectly by increasing levels of
the endogenous ligands including opioid or anandamide (44,45). It is
documented that the metabolism of acetaminophen in the brain and
dorsal root ganglia leads to the generation of
N-(4-hydroxyphenyl)-arachidonylamide (AM404), which prohibits the
cellular reuptake of CB (44). AM404
may also inhibit purified COX-1 and COX-2, which in turn reduces
the generation of prostaglandin in the brain (46). Although acetaminophen alone exhibited
little antinociceptive activity in the thermal stimulation model in
the present study, the effect of nefopam combined with
acetaminophen appeared additive in both the mouse hot plate test
and rat tail flick test. Furthermore, the analgesic duration was
lengthened when the two drugs were coadministered.
Nefopam, a non-opioid analgesic, is derived from
diphenhydramine, a histamine H1 receptor antagonist. A
primary hypothesis on the mechanism of analgesic action of nefopam
focuses on the inhibition of monoamine uptake in synapses, which
would lead to an increase in noradrenaline, dopamine and serotonin
(25). The analgesic action of
nefopam may involve the serotonergic or noradrenergic descending
pathways; a previous study demonstrated that depletion of spinal
noradrenaline attenuated the analgesic action of i.t. nefopam
during phase 1, but not during phase 2, of a formalin test
(47). Furthermore, it has been
confirmed in an in vivo animal pain model that monoamine
depletion inhibited nefopam antinociception, which indicated the
involvement of neuromediators in nefopam-induced analgesia
(6). More recently, a number of
studies have been conducted on the receptor subtypes that are
involved in nefopam antinociception. Girard et al (48) suggested that endogenous histamine may
indirectly modulate nefopam antinociception in the mouse writhing
and formalin tests to a low extent through histamine H3
receptors, due to a low affinity of nefopam with histamine
H3 receptors. Other results have illustrated that
nefopam antinociception may be blocked by serotonergic
5-HT1B receptor antagonist in the writhing test and
serotonergic 5-HT2C receptor antagonist in the formalin
test respectively, which indicates that the serotonergic system may
mediate nefopam antinociception directly and/or indirectly through
the serotonin 5-HT1B and 5-HT2C receptor
subtypes (49). Furthermore, the
analgesic activity of nefopam was adjusted by adrenergic
α1 and α2 receptors as well as dopaminergic
D2 receptors (49).
It is evident that the levels of serotonin may be
associated with the antinociceptive activity of acetaminophen and
nefopam through various mechanisms of action. In terms of their
combination in the present study, there was an apparent synergistic
analgesic effect. Regarding the potential target sites, it has been
indicated that the synergism of nefopam and acetaminophen may
occurs in connection with the descending serotonergic (5-HT)
pathways and spinal serotonin 5-HT receptors. It has been reported
that the potential effect of acetaminophen is indirectly/directly
related to 5-HT3 receptors at spinal sites (50,51) and
descending serotonergic pathways (52). Similarly, the action of nefopam have
been indicated to involve serotonergic pathways and 5-HT receptors
(49,53). Therefore, the analgesic activity of
the combination may be connected in part with serotonin. However,
further investigation into the molecular mechanism of the
antinociceptive potentiation between nefopam and acetaminophen
should now be performed, and the potential pharmacokinetic
interaction between the drugs should be studied in detail.
In conclusion, the data obtained from the present
study demonstrated that the combination of the non-opioid compound
nefopam with the atypical NSAIDs acetaminophen exerted more potent
antinociceptive effects than those of nefopam and acetaminophen
alone in mouse and rat models with acute and persistent pains.
Notably, the combined therapy of the two drugs appeared to increase
the antinociceptive effect. These results may provide pre-clinical
support for the application of combination therapy with nefopam and
acetaminophen in the management of acute pains.
Acknowledgements
Qian Li and Quankun Zhuang were the principal
investigators responsible for the experiment and performed the
majority of the experiments, and Qian Li wrote the original
manuscript. Yaru Gu and Cailing Dai were the experimental
assistants and aided in the revision of the manuscript. Xiaoxiao
Gao and Xiaomin Wang participated in the study. Huimin Wen and Xin
Li as assistant teachers aided to guide the experiment. Professor
Yuyang Zhang is the leader of the research group, and was
responsible for the study design and guidance, and for the accuracy
and authenticity of the manuscript.
This study was presented as a poster at the 6th
International Pharmaceutical Federation Pharmaceutical Sciences
World Congress May 21-May 24 2017 in Stockholm, Sweden.
References
|
1
|
Aveline C, Gautier JF, Vautier P, Cognet
F, Hetet HL, Attali JY, Leconte V, Leborgne P and Bonnet F:
Postoperative analgesia and early rehabilitation after total knee
replacement: A comparison of continuous low-dose intravenous
ketamine versus nefopam. Eur J Pain. 13:613–619. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Evans MS, Lysakowski C and Tramèr MR:
Nefopam for the prevention of postoperative pain: Quantitative
systematic review. Br J Anaesth. 101:610–617. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Girard P, Verniers D, Coppé MC, Pansart Y
and Gillardin JM: Nefopam and ketoprofen synergy in rodent models
of antinociception. Eur J Pharmacol. 584:263–271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Laboureyras E, Chateauraynaud J, Richebé P
and Simonnet G: Long-term pain vulnerability after surgery in rats:
Prevention by nefopam, an analgesic with antihyperalgesic
properties. Anesth Analg. 109:623–631. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cho SY, Park AR, Yoon MH, Lee HG, Kim WM
and Choi JI: Antinociceptive effect of intrathecal nefopam and
interaction with morphine in formalin-induced pain of rats. Korean
J Pain. 26:14–20. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim KH and Abdi S: Rediscovery of nefopam
for the treatment of neuropathic pain. Korean J Pain. 27:103–111.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Durrieu G, Olivier P, Bagheri H and
Montastruc JL: French network of pharmacovigilance centers:
Overview of adverse reactions to nefopam: An analysis of the French
pharmacovigilance database. Fundam Clin Pharmacol. 21:555–558.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Majchrzycki M, Kocur P and Kotwicki T:
Deep tissue massage and nonsteroidal anti-inflammatory drugs for
low back pain: A prospective randomized trial. Sci World J.
2014:2875972014. View Article : Google Scholar
|
|
9
|
Pongparadee C, Penserga E, Lee DJ, Chen
SL, Gill RS, Hamid A, Kumthornthip W, Liu Y, Meliala L, Misbach HJ,
et al: Current considerations for the management of musculoskeletal
pain in Asian countries: A special focus on cyclooxygenase-2
inhibitors and non-steroid anti-inflammation drugs. Int J Rheum
Dis. 15:341–347. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nikoda VV, Maiachkin RB and Bondarenko AV:
Clinical aspects of using patient-controlled analgesia with
nonsteroidal anti-inflammatory agents in postoperative period.
Anesteziol Reanimatol. 56–59. 2003.(In Russian). PubMed/NCBI
|
|
11
|
Vanegas H and Schaible HG: Prostaglandins
and cyclooxygenases [correction of cycloxygenases] in the spinal
cord. Prog Neurobiol. 64:327–363. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Isuru A, Rodrigo A, Wijesinghe C,
Ediriweera D, Premadasa S, Wijesekara C and Kuruppuarachchi L: A
randomized, double-blind, placebo-controlled trial on the role of
preemptive analgesia with acetaminophen [paracetamol] in reducing
headache following electroconvulsive therapy [ECT]. BMC Psychiatry.
17:2752017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Di Girolamo G, Sánchez AJ, De Los Santos
AR and González CD: Is acetaminophen, and its combination with
pamabrom, an effective therapeutic option in primary dysmenorrhoea?
Expert Opin Pharmacother. 5:561–570. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Botting RM: Inhibitors of cyclooxygenases:
Mechanisms, selectivity and uses. J Physiol Pharmacol. 57 Suppl
5:113–124. 2006.PubMed/NCBI
|
|
15
|
Ruggieri V, Vitale G, Pini LA and Sandrini
M: Differential involvement of opioidergic and serotonergic systems
in the antinociceptive activity of N-arachidonoyl-phenolamine
(AM404) in the rat: Comparison with paracetamol. Naunyn
Schmiedebergs Arch Pharmacol. 377:219–229. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sandrini M, Vitale G, Ruggieri V and Pini
LA: Effect of acute and repeated administration of paracetamol on
opioidergic and serotonergic systems in rats. Inflamm Res.
56:139–142. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roshi D, Toçi E, Burazeri G, Schröder-Bäck
P, Malaj L and Brand H: Users' knowledge about adverse effects of
non-steroidal anti-inflammatory drugs in Tirana, Albania. Mater
Sociomed. 29:138–142. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou C, Hu J, Ma H, Yagoub AE, Yu X, Owusu
J, Ma H and Qin X: Antioxidant peptides from corn gluten meal:
Orthogonal design evaluation. Food Chem. 187:270–278. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao J, Yuan Q, Cai W, Sun P, Ding L and
Jin F: Formulation, optimization, characterization, and
pharmacokinetics of progesterone intravenous lipid emulsion for
traumatic brain injury therapy. AAPS PharmSciTech. 18:1475–1487.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang YU, Bai W, Chen Y, Lin Y and Hu B:
Optimization of low-frequency low-intensity ultrasound-mediated
microvessel disruption on prostate cancer xenografts in nude mice
using an orthogonal experimental design. Oncol Lett. 10:2999–3007.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Du L, Pan H, Li L and Su X:
Enhanced analgesic effects of propacetamol and tramadol combination
in rats and mice. Biol Pharm Bull. 34:349–353. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheng J, Ma T, Liu W, Wang H, Jiang J, Wei
Y, Tian H, Zou N, Zhu Y, Shi H, et al: In in vivo evaluation of the
anti-inflammatory and analgesic activities of compound Muniziqi
granule in experimental animal models. BMC Complement Altern Med.
16:202016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Delage N, Maaliki H, Beloeil H, Benhamou D
and Mazoit JX: Median effective dose (ED50) of nefopam and
ketoprofen in postoperative patients: A study of interaction using
sequential analysis and isobolographic analysis. Anesthesiology.
102:1211–1216. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Van Elstraete AC and Sitbon P: Median
effective dose (ED50) of paracetamol and nefopam for postoperative
pain: Isobolographic analysis of their antinociceptive interaction.
Minerva Anestesiol. 79:232–239. 2013.PubMed/NCBI
|
|
25
|
Girard P, Niedergang B, Pansart Y, Coppé
MC and Verleye M: Systematic evaluation of the nefopam-paracetamol
combination in rodent models of antinociception. Clin Exp Pharmacol
Physiol. 38:170–178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
de la Puente B, Zamanillo D, Romero L,
Vela JM, Merlos M and Portillo-Salido E: Pharmacological
sensitivity of reflexive and nonreflexive outcomes as a correlate
of the sensory and affective responses to visceral pain in mice.
Sci Rep. 7:134282017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gu WW, Ao GZ, Zhu YM, Sun SC, Zhou Q, Fan
JH, Nobuhiko K, Ishidoh K, Zhang HL and Gao XM: Autophagy and
cathepsin L are involved in the antinociceptive effect of DMBC in a
mouse acetic acid-writhing model. Acta Pharmacol Sin. 34:1007–1012.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vliegenthart AD, Shaffer JM, Clarke JI,
Peeters LE, Caporali A, Bateman DN, Wood DM, Dargan PI, Craig DG,
Moore JK, et al: Comprehensive microRNA profiling in acetaminophen
toxicity identifies novel circulating biomarkers for human liver
and kidney injury. Sci Rep. 5:155012015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vellani V and Giacomoni C: Gabapentin
inhibits protein kinase C epsilon translocation in cultured sensory
neurons with additive effects when coapplied with paracetamol
(Acetaminophen). Sci World J. 2017:35959032017. View Article : Google Scholar
|
|
30
|
Lancaster EM, Hiatt JR and Zarrinpar A:
Acetaminophen hepatotoxicity: An updated review. Arch Toxicol.
89:193–199. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ribeiro RA, Vale ML, Thomazzi SM,
Paschoalato AB, Poole S, Ferreira SH and Cunha FQ: Involvement of
resident macrophages and mast cells in the writhing nociceptive
response induced by zymosan and acetic acid in mice. Eur J
Pharmacol. 387:111–118. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chan SL and Yeung JH: Polysaccharide
peptides from COV-1 strain of Coriolus versicolor induce
hyperalgesia via inflammatory mediator release in the mouse. Life
Sci. 78:2463–2470. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Maleki-Dizaji N, Fathiazad F and Garjani
A: Antinociceptive properties of extracts and two flavonoids
isolated from leaves of Danae racemosa. Arch Pharm Res.
30:1536–1542. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jokinen V, Lilius T, Laitila J, Niemi M,
Kambur O, Kalso E and Rauhala P: Do diuretics have antinociceptive
actions: Studies of spironolactone, eplerenone, furosemide and
chlorothiazide, individually and with oxycodone and morphine. Basic
Clin Pharmacol Toxicol. 120:38–45. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gong YH, Yu XR, Liu HL, Yang N, Zuo PP and
Huang YG: Antinociceptive effects of combination of tramadol and
acetaminophen on painful diabetic neuropathy in
streptozotocin-induced diabetic rats. Acta Anaesthesiol Taiwan.
49:16–20. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Miranda HF, Noriega V and Prieto JC:
Previous administration of naltrexone did not change synergism
between paracetamol and tramadol in mice. Pharmacol Biochem Behav.
102:72–76. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nagai J, Uesawa Y, Shimamura R and Kagaya
H: Characterization of the adverse effects induced by acetaminophen
and nonsteroidal anti-inflammatory drugs based on the analysis of
the Japanese adverse drug event report database. Clin J Pain.
33:667–675. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim YJ, Lim KH, Kim MY, Jo EJ, Lee SY, Lee
SE, Yang MS, Song WJ, Kang HR, Park HW, et al: Cross-reactivity to
acetaminophen and celecoxib according to the type of nonsteroidal
anti-inflammatory drug hypersensitivity. Allergy Asthma Immunol
Res. 6:156–162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hinz B, Cheremina O and Brune K:
Acetaminophen (paracetamol) is a selective cyclooxygenase-2
inhibitor in man. FASEB J. 22:383–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chandrasekharan NV, Dai H, Roos KL,
Evanson NK, Tomsik J, Elton TS and Simmons DL: COX-3, a
cyclooxygenase-1 variant inhibited by acetaminophen and other
analgesic/antipyretic drugs: Cloning, structure, and expression.
Proc Natl Acad Sci USA. 99:13926–13931. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Snipes JA, Kis B, Shelness GS, Hewett JA
and Busija DW: Cloning and characterization of cyclooxygenase-1b
(putative cyclooxygenase-3) in rat. J Pharmacol Exp Ther.
313:668–676. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bonnefont J, Daulhac L, Etienne M, Chapuy
E, Mallet C, Ouchchane L, Deval C, Courade JP, Ferrara M, Eschalier
A, et al: Acetaminophen recruits spinal p42/p44 MAPKs and GH/IGF-1
receptors to produce analgesia via the serotonergic system. Mol
Pharmacol. 71:407–415. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Courade JP, Chassaing C, Bardin L, Alloui
A and Eschalier A: 5-HT receptor subtypes involved in the spinal
antinociceptive effect of acetaminophen in rats. Eur J Pharmacol.
432:1–7. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mallet C, Daulhac L, Bonnefont J, Ledent
C, Etienne M, Chapuy E, Libert F and Eschalier A: Endocannabinoid
and serotonergic systems are needed for acetaminophen-induced
analgesia. Pain. 139:190–200. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bertolini A, Ferrari A, Ottani A, Guerzoni
S, Tacchi R and Leone S: Paracetamol: New vistas of an old drug.
CNS Drug Rev. 12:250–275. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Högestätt ED, Jönsson BA, Ermund A,
Andersson DA, Björk H, Alexander JP, Cravatt BF, Basbaum AI and
Zygmunt PM: Conversion of acetaminophen to the bioactive
N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent
arachidonic acid conjugation in the nervous system. J Biol Chem.
280:31405–31412. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jeong SH, Heo BH, Park SH, Kim WM, Lee HG,
Yoon MH and Choi JI: Spinal noradrenergic modulation and the role
of the alpha-2 receptor in the antinociceptive effect of
intrathecal nefopam in the formalin test. Korean J Pain. 27:23–29.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Girard P, Pansart Y, Coppé MC, Verniers D
and Gillardin JM: Role of the histamine system in nefopam-induced
antinociception in mice. Eur J Pharmacol. 503:63–69. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Girard P, Coppé MC, Verniers D, Pansart Y
and Gillardin JM: Role of catecholamines and serotonin receptor
subtypes in nefopam-induced antinociception. Pharmacol Res.
54:195–202. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tiippana E, Hamunen K, Kontinen V and
Kalso E: The effect of paracetamol and tropisetron on pain:
Experimental studies and a review of published data. Basic Clin
Pharmacol Toxicol. 112:124–131. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dogrul A, Seyrek M, Akgul EO, Cayci T,
Kahraman S and Bolay H: Systemic paracetamol-induced analgesic and
antihyperalgesic effects through activation of descending
serotonergic pathways involving spinal 5-HT7 receptors.
Eur J Pharmacol. 677:93–101. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Dam LJ, Hai L and Ha YM: Role of the
5-HT(7) receptor in the effects of intrathecal nefopam in
neuropathic pain in rats. Neurosci Lett. 566:50–54. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lee HG, Kim WM, Kim JM, Bae HB and Choi
JI: Intrathecal nefopam-induced antinociception through activation
of descending serotonergic projections involving spinal
5-HT7 but not 5-HT3 receptors. Neurosci Lett.
587:120–125. 2015. View Article : Google Scholar : PubMed/NCBI
|