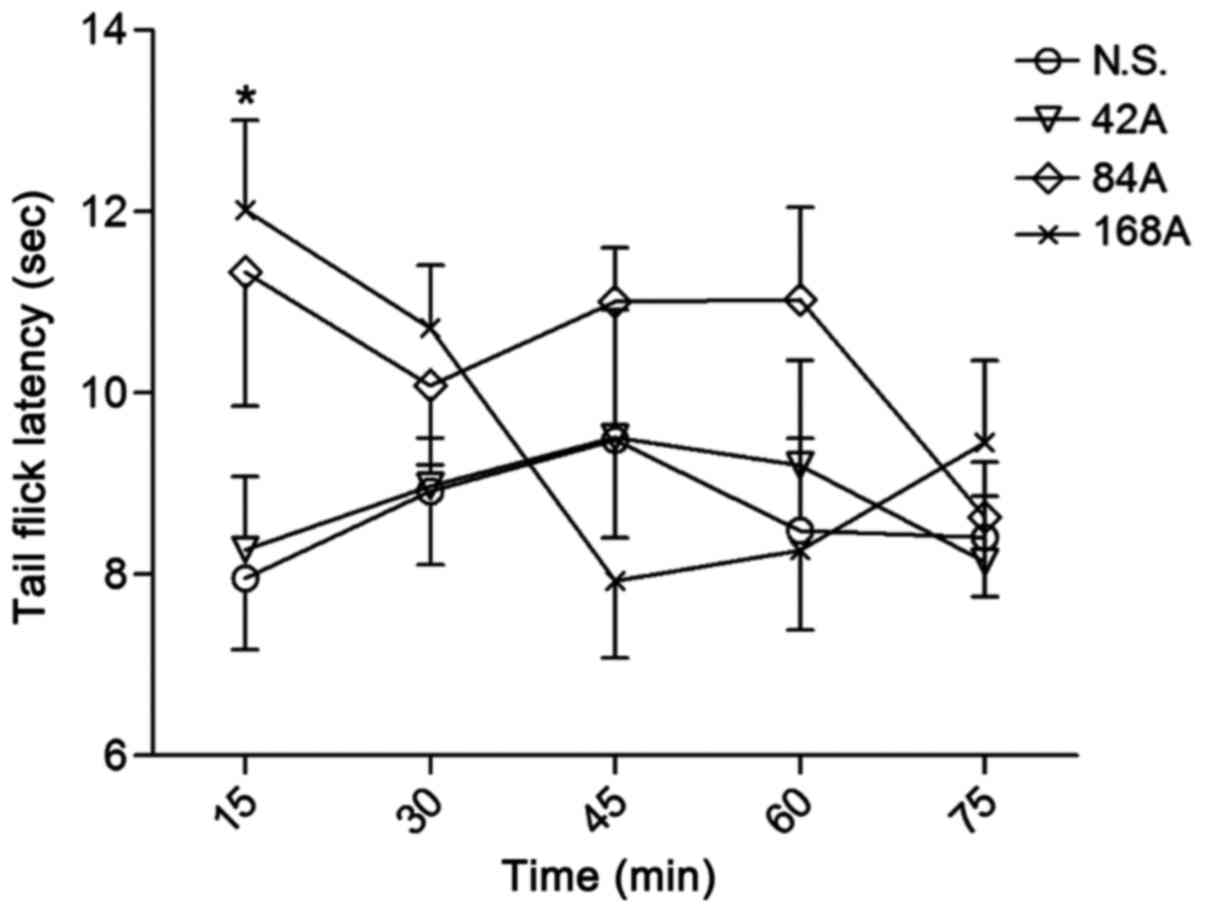
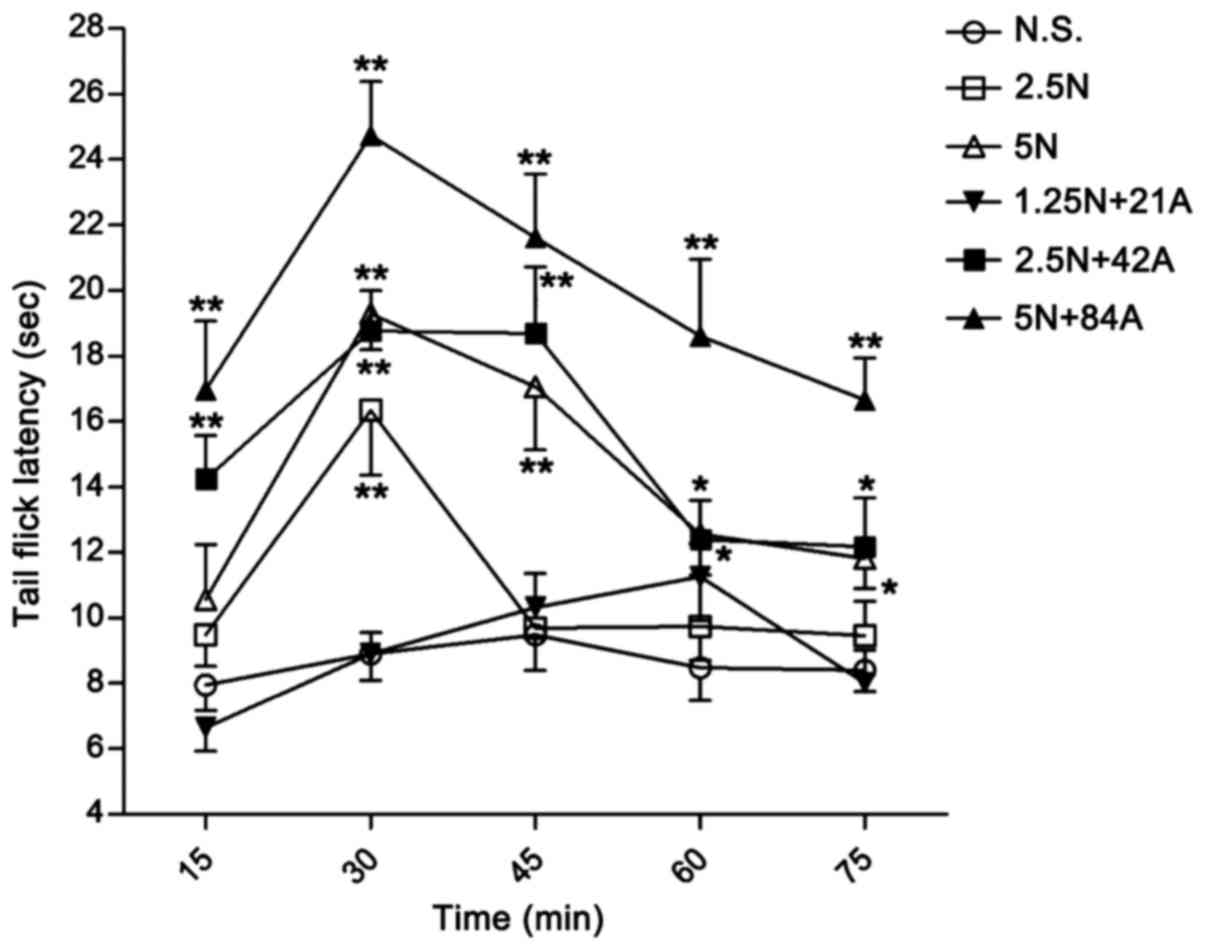
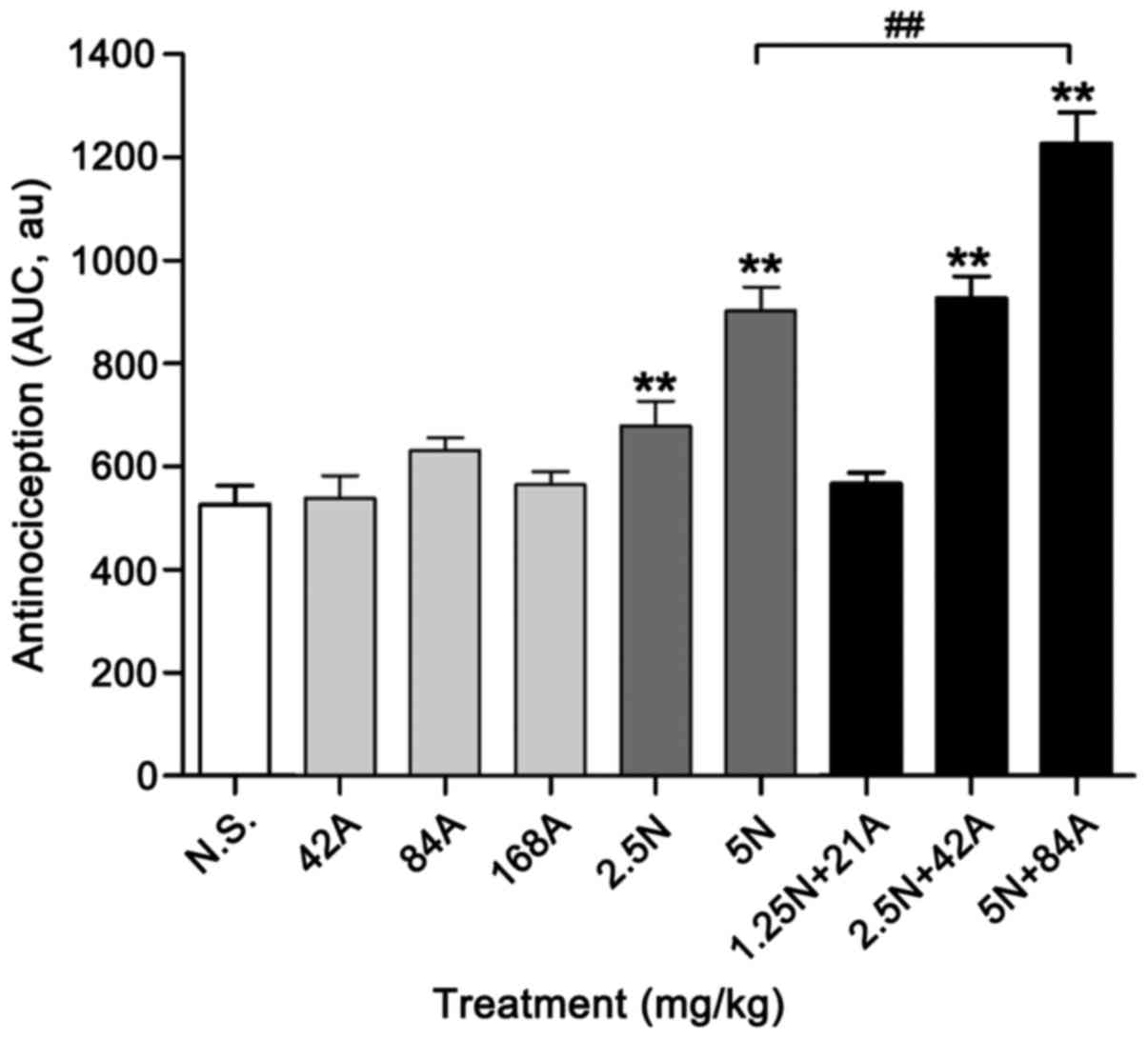
|
1
|
Aveline C, Gautier JF, Vautier P, Cognet
F, Hetet HL, Attali JY, Leconte V, Leborgne P and Bonnet F:
Postoperative analgesia and early rehabilitation after total knee
replacement: A comparison of continuous low-dose intravenous
ketamine versus nefopam. Eur J Pain. 13:613–619. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Evans MS, Lysakowski C and Tramèr MR:
Nefopam for the prevention of postoperative pain: Quantitative
systematic review. Br J Anaesth. 101:610–617. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Girard P, Verniers D, Coppé MC, Pansart Y
and Gillardin JM: Nefopam and ketoprofen synergy in rodent models
of antinociception. Eur J Pharmacol. 584:263–271. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Laboureyras E, Chateauraynaud J, Richebé P
and Simonnet G: Long-term pain vulnerability after surgery in rats:
Prevention by nefopam, an analgesic with antihyperalgesic
properties. Anesth Analg. 109:623–631. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cho SY, Park AR, Yoon MH, Lee HG, Kim WM
and Choi JI: Antinociceptive effect of intrathecal nefopam and
interaction with morphine in formalin-induced pain of rats. Korean
J Pain. 26:14–20. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim KH and Abdi S: Rediscovery of nefopam
for the treatment of neuropathic pain. Korean J Pain. 27:103–111.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Durrieu G, Olivier P, Bagheri H and
Montastruc JL: French network of pharmacovigilance centers:
Overview of adverse reactions to nefopam: An analysis of the French
pharmacovigilance database. Fundam Clin Pharmacol. 21:555–558.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Majchrzycki M, Kocur P and Kotwicki T:
Deep tissue massage and nonsteroidal anti-inflammatory drugs for
low back pain: A prospective randomized trial. Sci World J.
2014:2875972014. View Article : Google Scholar
|
|
9
|
Pongparadee C, Penserga E, Lee DJ, Chen
SL, Gill RS, Hamid A, Kumthornthip W, Liu Y, Meliala L, Misbach HJ,
et al: Current considerations for the management of musculoskeletal
pain in Asian countries: A special focus on cyclooxygenase-2
inhibitors and non-steroid anti-inflammation drugs. Int J Rheum
Dis. 15:341–347. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nikoda VV, Maiachkin RB and Bondarenko AV:
Clinical aspects of using patient-controlled analgesia with
nonsteroidal anti-inflammatory agents in postoperative period.
Anesteziol Reanimatol. 56–59. 2003.(In Russian). PubMed/NCBI
|
|
11
|
Vanegas H and Schaible HG: Prostaglandins
and cyclooxygenases [correction of cycloxygenases] in the spinal
cord. Prog Neurobiol. 64:327–363. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Isuru A, Rodrigo A, Wijesinghe C,
Ediriweera D, Premadasa S, Wijesekara C and Kuruppuarachchi L: A
randomized, double-blind, placebo-controlled trial on the role of
preemptive analgesia with acetaminophen [paracetamol] in reducing
headache following electroconvulsive therapy [ECT]. BMC Psychiatry.
17:2752017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Di Girolamo G, Sánchez AJ, De Los Santos
AR and González CD: Is acetaminophen, and its combination with
pamabrom, an effective therapeutic option in primary dysmenorrhoea?
Expert Opin Pharmacother. 5:561–570. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Botting RM: Inhibitors of cyclooxygenases:
Mechanisms, selectivity and uses. J Physiol Pharmacol. 57 Suppl
5:113–124. 2006.PubMed/NCBI
|
|
15
|
Ruggieri V, Vitale G, Pini LA and Sandrini
M: Differential involvement of opioidergic and serotonergic systems
in the antinociceptive activity of N-arachidonoyl-phenolamine
(AM404) in the rat: Comparison with paracetamol. Naunyn
Schmiedebergs Arch Pharmacol. 377:219–229. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sandrini M, Vitale G, Ruggieri V and Pini
LA: Effect of acute and repeated administration of paracetamol on
opioidergic and serotonergic systems in rats. Inflamm Res.
56:139–142. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roshi D, Toçi E, Burazeri G, Schröder-Bäck
P, Malaj L and Brand H: Users' knowledge about adverse effects of
non-steroidal anti-inflammatory drugs in Tirana, Albania. Mater
Sociomed. 29:138–142. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou C, Hu J, Ma H, Yagoub AE, Yu X, Owusu
J, Ma H and Qin X: Antioxidant peptides from corn gluten meal:
Orthogonal design evaluation. Food Chem. 187:270–278. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao J, Yuan Q, Cai W, Sun P, Ding L and
Jin F: Formulation, optimization, characterization, and
pharmacokinetics of progesterone intravenous lipid emulsion for
traumatic brain injury therapy. AAPS PharmSciTech. 18:1475–1487.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang YU, Bai W, Chen Y, Lin Y and Hu B:
Optimization of low-frequency low-intensity ultrasound-mediated
microvessel disruption on prostate cancer xenografts in nude mice
using an orthogonal experimental design. Oncol Lett. 10:2999–3007.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Du L, Pan H, Li L and Su X:
Enhanced analgesic effects of propacetamol and tramadol combination
in rats and mice. Biol Pharm Bull. 34:349–353. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheng J, Ma T, Liu W, Wang H, Jiang J, Wei
Y, Tian H, Zou N, Zhu Y, Shi H, et al: In in vivo evaluation of the
anti-inflammatory and analgesic activities of compound Muniziqi
granule in experimental animal models. BMC Complement Altern Med.
16:202016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Delage N, Maaliki H, Beloeil H, Benhamou D
and Mazoit JX: Median effective dose (ED50) of nefopam and
ketoprofen in postoperative patients: A study of interaction using
sequential analysis and isobolographic analysis. Anesthesiology.
102:1211–1216. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Van Elstraete AC and Sitbon P: Median
effective dose (ED50) of paracetamol and nefopam for postoperative
pain: Isobolographic analysis of their antinociceptive interaction.
Minerva Anestesiol. 79:232–239. 2013.PubMed/NCBI
|
|
25
|
Girard P, Niedergang B, Pansart Y, Coppé
MC and Verleye M: Systematic evaluation of the nefopam-paracetamol
combination in rodent models of antinociception. Clin Exp Pharmacol
Physiol. 38:170–178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
de la Puente B, Zamanillo D, Romero L,
Vela JM, Merlos M and Portillo-Salido E: Pharmacological
sensitivity of reflexive and nonreflexive outcomes as a correlate
of the sensory and affective responses to visceral pain in mice.
Sci Rep. 7:134282017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gu WW, Ao GZ, Zhu YM, Sun SC, Zhou Q, Fan
JH, Nobuhiko K, Ishidoh K, Zhang HL and Gao XM: Autophagy and
cathepsin L are involved in the antinociceptive effect of DMBC in a
mouse acetic acid-writhing model. Acta Pharmacol Sin. 34:1007–1012.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vliegenthart AD, Shaffer JM, Clarke JI,
Peeters LE, Caporali A, Bateman DN, Wood DM, Dargan PI, Craig DG,
Moore JK, et al: Comprehensive microRNA profiling in acetaminophen
toxicity identifies novel circulating biomarkers for human liver
and kidney injury. Sci Rep. 5:155012015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vellani V and Giacomoni C: Gabapentin
inhibits protein kinase C epsilon translocation in cultured sensory
neurons with additive effects when coapplied with paracetamol
(Acetaminophen). Sci World J. 2017:35959032017. View Article : Google Scholar
|
|
30
|
Lancaster EM, Hiatt JR and Zarrinpar A:
Acetaminophen hepatotoxicity: An updated review. Arch Toxicol.
89:193–199. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ribeiro RA, Vale ML, Thomazzi SM,
Paschoalato AB, Poole S, Ferreira SH and Cunha FQ: Involvement of
resident macrophages and mast cells in the writhing nociceptive
response induced by zymosan and acetic acid in mice. Eur J
Pharmacol. 387:111–118. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chan SL and Yeung JH: Polysaccharide
peptides from COV-1 strain of Coriolus versicolor induce
hyperalgesia via inflammatory mediator release in the mouse. Life
Sci. 78:2463–2470. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Maleki-Dizaji N, Fathiazad F and Garjani
A: Antinociceptive properties of extracts and two flavonoids
isolated from leaves of Danae racemosa. Arch Pharm Res.
30:1536–1542. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jokinen V, Lilius T, Laitila J, Niemi M,
Kambur O, Kalso E and Rauhala P: Do diuretics have antinociceptive
actions: Studies of spironolactone, eplerenone, furosemide and
chlorothiazide, individually and with oxycodone and morphine. Basic
Clin Pharmacol Toxicol. 120:38–45. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gong YH, Yu XR, Liu HL, Yang N, Zuo PP and
Huang YG: Antinociceptive effects of combination of tramadol and
acetaminophen on painful diabetic neuropathy in
streptozotocin-induced diabetic rats. Acta Anaesthesiol Taiwan.
49:16–20. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Miranda HF, Noriega V and Prieto JC:
Previous administration of naltrexone did not change synergism
between paracetamol and tramadol in mice. Pharmacol Biochem Behav.
102:72–76. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nagai J, Uesawa Y, Shimamura R and Kagaya
H: Characterization of the adverse effects induced by acetaminophen
and nonsteroidal anti-inflammatory drugs based on the analysis of
the Japanese adverse drug event report database. Clin J Pain.
33:667–675. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim YJ, Lim KH, Kim MY, Jo EJ, Lee SY, Lee
SE, Yang MS, Song WJ, Kang HR, Park HW, et al: Cross-reactivity to
acetaminophen and celecoxib according to the type of nonsteroidal
anti-inflammatory drug hypersensitivity. Allergy Asthma Immunol
Res. 6:156–162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hinz B, Cheremina O and Brune K:
Acetaminophen (paracetamol) is a selective cyclooxygenase-2
inhibitor in man. FASEB J. 22:383–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chandrasekharan NV, Dai H, Roos KL,
Evanson NK, Tomsik J, Elton TS and Simmons DL: COX-3, a
cyclooxygenase-1 variant inhibited by acetaminophen and other
analgesic/antipyretic drugs: Cloning, structure, and expression.
Proc Natl Acad Sci USA. 99:13926–13931. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Snipes JA, Kis B, Shelness GS, Hewett JA
and Busija DW: Cloning and characterization of cyclooxygenase-1b
(putative cyclooxygenase-3) in rat. J Pharmacol Exp Ther.
313:668–676. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bonnefont J, Daulhac L, Etienne M, Chapuy
E, Mallet C, Ouchchane L, Deval C, Courade JP, Ferrara M, Eschalier
A, et al: Acetaminophen recruits spinal p42/p44 MAPKs and GH/IGF-1
receptors to produce analgesia via the serotonergic system. Mol
Pharmacol. 71:407–415. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Courade JP, Chassaing C, Bardin L, Alloui
A and Eschalier A: 5-HT receptor subtypes involved in the spinal
antinociceptive effect of acetaminophen in rats. Eur J Pharmacol.
432:1–7. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mallet C, Daulhac L, Bonnefont J, Ledent
C, Etienne M, Chapuy E, Libert F and Eschalier A: Endocannabinoid
and serotonergic systems are needed for acetaminophen-induced
analgesia. Pain. 139:190–200. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bertolini A, Ferrari A, Ottani A, Guerzoni
S, Tacchi R and Leone S: Paracetamol: New vistas of an old drug.
CNS Drug Rev. 12:250–275. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Högestätt ED, Jönsson BA, Ermund A,
Andersson DA, Björk H, Alexander JP, Cravatt BF, Basbaum AI and
Zygmunt PM: Conversion of acetaminophen to the bioactive
N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent
arachidonic acid conjugation in the nervous system. J Biol Chem.
280:31405–31412. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jeong SH, Heo BH, Park SH, Kim WM, Lee HG,
Yoon MH and Choi JI: Spinal noradrenergic modulation and the role
of the alpha-2 receptor in the antinociceptive effect of
intrathecal nefopam in the formalin test. Korean J Pain. 27:23–29.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Girard P, Pansart Y, Coppé MC, Verniers D
and Gillardin JM: Role of the histamine system in nefopam-induced
antinociception in mice. Eur J Pharmacol. 503:63–69. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Girard P, Coppé MC, Verniers D, Pansart Y
and Gillardin JM: Role of catecholamines and serotonin receptor
subtypes in nefopam-induced antinociception. Pharmacol Res.
54:195–202. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tiippana E, Hamunen K, Kontinen V and
Kalso E: The effect of paracetamol and tropisetron on pain:
Experimental studies and a review of published data. Basic Clin
Pharmacol Toxicol. 112:124–131. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dogrul A, Seyrek M, Akgul EO, Cayci T,
Kahraman S and Bolay H: Systemic paracetamol-induced analgesic and
antihyperalgesic effects through activation of descending
serotonergic pathways involving spinal 5-HT7 receptors.
Eur J Pharmacol. 677:93–101. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Dam LJ, Hai L and Ha YM: Role of the
5-HT(7) receptor in the effects of intrathecal nefopam in
neuropathic pain in rats. Neurosci Lett. 566:50–54. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lee HG, Kim WM, Kim JM, Bae HB and Choi
JI: Intrathecal nefopam-induced antinociception through activation
of descending serotonergic projections involving spinal
5-HT7 but not 5-HT3 receptors. Neurosci Lett.
587:120–125. 2015. View Article : Google Scholar : PubMed/NCBI
|