Introduction
In recent decades, the prevalence of
gastroesophageal reflux disease (GERD) has increased in Japan
(1). This may be attributed to an
increase in gastric acid secretion, a decreased prevalence of
Helicobacter pylori infection, novel techniques that allow
more sensitive detection and diagnosis of GERD, and the amendment
of endoscopic finding criteria according to the modified Los
Angeles (LA) classification (2,3). The
occurrence of GERD symptoms, including heartburn and acid reflux,
at least once a week may considerably affect the quality of life
(QoL) of patients with GERD (4–7). As such,
the clinical practice guidelines for GERD established by the
Japanese Society of Gastroenterology emphasize the importance of
achieving complete resolution of symptoms (1). GERD is classified into two categories:
Non-erosive reflux disease (NERD) and erosive esophagitis (EE)
(8).
Proton pump inhibitors (PPIs) are recommended as the
first-line treatment for GERD as they are potent inhibitors of
gastric acid secretion (1,4). However, a previous study reported that
40–50% of patients with NERD and 6–15% of those with EE were
refractory to PPIs treatment (9). In
a recent multicenter prospective study from Japan, the endoscopic
healing rate of standard-dose PPIs was ~70% in patients with reflux
esophagitis (RE) of LA grade C and ~60% in patients with RE of LA
grade D (10). Due to these low
healing rates, many patients receiving the current standard
treatment are not satisfied with their gastrointestinal QoL
(10).
Vonoprazan is a novel potassium-competitive acid
blocker (P-CAB), part of a new class of gastric acid-suppressant
agents. Similar to PPIs, P-CABs inhibit gastric
H+,K+-adenosine triphosphatase (11). However, unlike PPIs, P-CABs inhibit
enzymes in a K+-competitive and reversible manner
(11,12). Vonoprazan is stable in gastric juice,
has a quick action and its effect lasts for a long time (13–15).
Vonoprazan undergoes substantial metabolic elimination
independently of CYP2C19 polymorphism (15). Therefore, the onset of the
acid-inhibitory effect of vonoprazan is more rapid than that of
esomeprazole in healthy Japanese adult male volunteers with the
CYP2C19 extensive metabolizer genotype (15). In a phase 3 clinical trial, the
endoscopic healing rate of RE following treatment with vonoprazan
20 mg for 8 weeks was 99% (16).
Recent studies have revealed that vonoprazan 20 mg is effective for
a majority of Japanese patients with RE refractory to PPIs
(17–19). However, to the best of our knowledge
no studies have evaluated the clinical value of maintenance therapy
with vonoprazan 10 mg in patients with RE.
The aim of the present study was to evaluate the
efficacy of vonoprazan 10 mg for maintenance therapy of healed RE
refractory to PPIs. The primary goal was to determine the
proportion of patients who had maintenance of healed RE refractory
to PPIs following 24 weeks of maintenance therapy with vonoprazan
10 mg. The secondary goal was to evaluate the proportion of
patients with symptomatic non-relapse at 24 weeks.
Materials and methods
Study design
This open-label, single-center, prospective study
was conducted at the Toyama City Hospital (Toyama, Japan) between
March 2015 and January 2017 following the approval of the
Institutional Ethics Committee (no. 2014-21). All patients provided
written informed consent prior to study enrollment. All study
procedures were performed in accordance with the 1964 Declaration
of Helsinki and its later amendments.
Patients and treatments
Patients aged ≥20 years, with endoscopically
diagnosed RE on the modified LA classification grade (3) between A and D were recruited for the
present study. RE refractory to PPIs was defined as patients who
had a Frequency Scale for the Symptoms of GERD (FSSG) (20) total score of ≥8 after ≥8 weeks of
treatment with standard doses of PPIs (rabeprazole 10 mg once
daily, omeprazole 20 mg once daily, esomeprazole 20 mg once daily
or lansoprazole 30 mg once daily). Patients with RE refractory to
PPIs who had no endoscopic evidence of erosive esophagitis
following oral administration of vonoprazan (Takeda Pharmaceutical
Company Ltd., Osaka, Japan) 20 mg once daily after breakfast for 4
weeks were eligible for inclusion in the present study Eligible
patients received maintenance therapy with vonoprazan 10 mg once
daily for 24 weeks. To avoid potential bias, concomitant treatment
with vonoprazan, H2-receptor antagonists, prokinetic agents,
mucosal protective factor enhancing agents and anticholinergic
agents was prohibited. Other pharmacological agents, namely herbal
medicine and antidepressive agents, thought to interact with the
study drug, were also prohibited.
Patients with serious organ dysfunctions or
malignant tumors, as well as those with concomitant
gastrointestinal diseases, including esophageal stricture,
achalasia, eosinophilic esophagitis, inflammatory bowel disease,
primary esophageal motility disorders, Zollinger-Ellison syndrome
and malabsorption syndrome, were excluded from the study.
Concomitant use of non-PPI drugs prior to providing informed
consent was permitted as long as the dosage remained stable
throughout the study period. Patient demographics and
characteristics are presented in Table
I.
 | Table I.Demographic and clinical
characteristics for patients with healed reflux esophagitis
refractory to PPIs. |
Table I.
Demographic and clinical
characteristics for patients with healed reflux esophagitis
refractory to PPIs.
| Parameter | Total (n=52) | FSSG <8
(n=25) | FSSG ≥8 (n=27) | P-value |
|---|
| Mean age
(range) | 66.0 (28–84) | 67.6 (45–84) | 64.6 (28–84) | 0.371 |
| Sex, M/F | 27/25 | 15/10 | 12/15 | 0.211 |
| Mean BMI | 22.7±5.9 | 24.5±3.6 | 22.9±2.8 | 0.067 |
| BMI ≥25 (%) | 18 (34.6) | 11 (44.0) | 7 (25.9) | 0.141 |
| Duration of
illness, months | 12.1±10.3 | 9.8±13.1 | 14.2±11.9 | 0.213 |
| Previous PPI
treatment, RPZ/OPZ/EPZ/LPZ | 21/9/15/7 | 13/5/4/3 | 8/4/11/4 | 0.201 |
| Los Angeles
classification grade prior to switching to vonoprazan 20 mg,
A/B/C/D | 25/14/9/4 | 10/8/5/2 | 15/6/4/2 | 0.724 |
| Helicobacter
pylori infection (%) | 12/29 (41.4) | 3/15 (20.0) | 9/14 (64.3) | 0.045 |
| Esophageal hiatus
hernia (%) | 17 (32.7) | 8 (32.0) | 9 (33.3) | 0.577 |
| Atrophy of gastric
mucosa (%) | 9
(17.3) | 4 (16.0) | 5 (18.5) | 0.551 |
| Functional
dyspepsia (%) | 10 (19.2) | 5 (20.0) | 5 (18.5) | 0.584 |
| Irritable bowel
disease (%) | 13 (25.0) | 4 (16.0) | 9 (33.3) | 0.131 |
| Diabetes mellitus
(%) | 2 (3.8) | 1 (4.0) | 1 (3.7) | 0.584 |
| Hyperlipidemia
(%) | 10 (19.2) | 4 (16.0) | 6 (22.2) | 0.416 |
| Hypertension
(%) | 13 (25.0) | 8 (32.0) | 5 (18.5) | 0.212 |
| Serum gastrin,
pg/ml | 1,059 | 1,189 | 930 | 0.239 |
Endoscopic assessments and
endpoint
The severity of esophagitis was determined by
endoscopic examination using the modified LA classification grade
between A and D, with M and N (3,21). This
adds additional grades N, which is defined as no apparent mucosal
change, and M, which is defined as minimal changes in the mucosa,
including erythema and/or whitish turbidity (3). Healing was defined as no detectable
erosive reflux (LA grade N or M). A diagnosis of hiatus hernia was
made when the retroflexed endoscope, under the condition of gastric
inflation, revealed gaping esophageal lumen allowing the squamous
epithelium to be viewed below (22).
Atrophic gastritis was diagnosed by endoscopy using the
Kimura-Takemoto endoscopic classification (C-1, C-2, C-3, O-1, O-2
and O-3) (23). Upper GI endoscopy,
which was performed transorally or transnasally in an unsedated
condition, was conducted at 24 weeks in order to evaluate the
presence or absence of esophageal mucosal break relapse. Morning
fasting gastrin levels in serum were measured at the completion of
maintenance therapy with vonoprazan 10 mg for 24 weeks, via
radioimmunoassay according to a Gastrin RIA kit®
produced by Fujirebio Diagnostics, Inc. (Tokyo, Japan). The primary
efficacy endpoint was the endoscopic remission rates (percentage of
patients with healing RE) at 24 weeks.
Symptomatic assessments and
endpoint
The incidence of GERD symptoms was determined using
the FSSG (20) and the
gastrointestinal QoL was assessed using the Japanese version of the
Gastrointestinal Symptom Rating Scale (GSRS) (24). The FSSG is a 5-point scale (never, 0;
occasionally, 1; sometimes, 2; often, 3; and always, 4) that rates
the frequency of 12 symptoms associated with the gastrointestinal
tract, which are divided into 2 subscales: Acid reflux-associated
symptoms, in which the sums of the respective scores of items 1, 4,
6, 7, 9, 10 and 12 are calculated; and dysmotility symptoms, in
which the sums of the respective scores of items 2, 3, 5, 8 and 11
are calculated. A total score of 8 points or higher is suggestive
of RE/GERD (20). The GSRS
questionnaire is a 7-point scoring system (no discomfort at all, 1;
minimal discomfort, 2; mild discomfort, 3; moderate discomfort, 4;
moderately severe discomfort, 5; severe discomfort, 6; and very
severe discomfort, 7) that rates the level of daily life discomfort
associate with 15 gastrointestinal tract symptoms, which are
divided into 5 subscales: Reflux (heartburn and acid
regurgitation), abdominal pain (abdominal pain, hunger pain and
nausea), indigestion (borborygmus, abdominal distension, eructation
and increased flatus), diarrhea (diarrhea, loose stools and urgent
need for defecation) and constipation (constipation, hard stools
and a feeling of incomplete evacuation) (24).
Patients were asked to complete the FSSG and GSRS
questionnaire at 0 (the baseline), 4, 8 and 24 weeks. Symptom
relief was defined as FSSG scores and GSRS scores that were similar
to or lower compared with the baseline. Symptomatic relapse was
assessed using acid reflux-related symptoms and defined as 3
consecutive days with moderate/severe heartburn and acid
regurgitation during the 24-week maintenance therapy. Patients with
symptomatic relapse were withdrawn from the study. Secondary
endpoints evaluated the proportion of patients with symptomatic
non-relapse at 24 weeks; patients reported the presence or absence
of epigastric pain, postprandial fullness and early satiation, and
rated the frequency, onset and severity of these symptoms. Those
with postprandial fullness or early satiation more than 1 day a
week and those with epigastric pain of more than mild severity at
least 1 day a week for over 6 months were defined as having
functional dyspepsia (FD) according to the Rome III criteria
(25). Additionally, patients with
abdominal pain or discomfort at least 3 days a month in the last 3
months were defined as having irritable bowel syndrome (IBS)
according to the Rome III criteria.
To evaluate the symptoms of RE without esophageal
erosions despite prior vonoprazan 20 mg treatment, patients were
divided into two groups; FSSG score ≥8 and FSSG score <8.
Associations between total FSSG score improvement (a decrease in
total score to ≤8) and the FSSG subscales and GSRS subscores were
assessed.
H. pylori infection was evaluated based on
anti-H. pylori IgG antibody titers, using a cutoff value of
10 U/ml according to the LZ Test® produced by Eiken
Chemical Co., Ltd. (Tokyo, Japan).
Statistical analysis
Analyses were performed for the intention-to-treat
(ITT) population. The distribution of LA classification grades
prior to treatment with vonoprazan 20 mg and following 24 weeks of
maintenance therapy with vonoprazan 10 mg were compared using the
Wilcoxon signed-rank test. The FSSG and GSRS scores were compared
prior to and following maintenance therapy with vonoprazan 10 mg.
Results are presented as the mean ± standard deviation or as number
(%) of patients. Intergroup comparisons were performed using the
χ2 test for categorical data and Student's t-test for
continuous data. The FSSG total score following 4 weeks of
treatment with vonoprazan 20 mg (prior to maintenance therapy) was
used as the response variable for the evaluation of patient
demographic and clinical characteristics. Additionally, the patient
demographic and clinical characteristics were also compared between
the FSSG score ≥8 and FSSG score <8 groups. Patient demographic
factors used as explanatory variables were age, gender, body mass
index (BMI), duration of illness, type and dose of previous PPI
treatment, presence or absence of Helicobacter pylori (H.
pylori) infection, presence or absence and details of
gastrointestinal complications (including hiatal hernia and atrophy
of gastric mucosa) and the presence or absence and details of
complications (including FD, IBS, diabetes mellitus, hyperlipidemia
and hypertension). Statistical analysis was performed using SPSS
16.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Study profile
The flow of patients through the study is presented
in Fig. 1. A total of 60 patients
with RE refractory to PPIs were enrolled in the present study. Of
these, 52 patients with healed RE refractory to PPIs received
maintenance therapy with vonoprazan 10 mg once daily for 24 weeks.
During maintenance therapy, the number of patients with symptomatic
relapse was 1 (1.9%) and 4 (7.7%) at 4 and 8 weeks, respectively. A
total of 4 patients were withdrawn because of loss to follow-up.
Finally, 43 patients (82.7%) successfully completed 24-week
maintenance therapy.
Patient demographic and clinical
characteristics
The eligible patients included in the present study
were 52% male (Table I). To evaluate
symptoms of RE without esophageal erosions despite prior vonoprazan
20 mg treatment, patients were divided into two groups according to
their FSSG score. In the total cohort of 52 patients, the mean age
and BMI were 66 years (28–84) and 22.7 kg/m2,
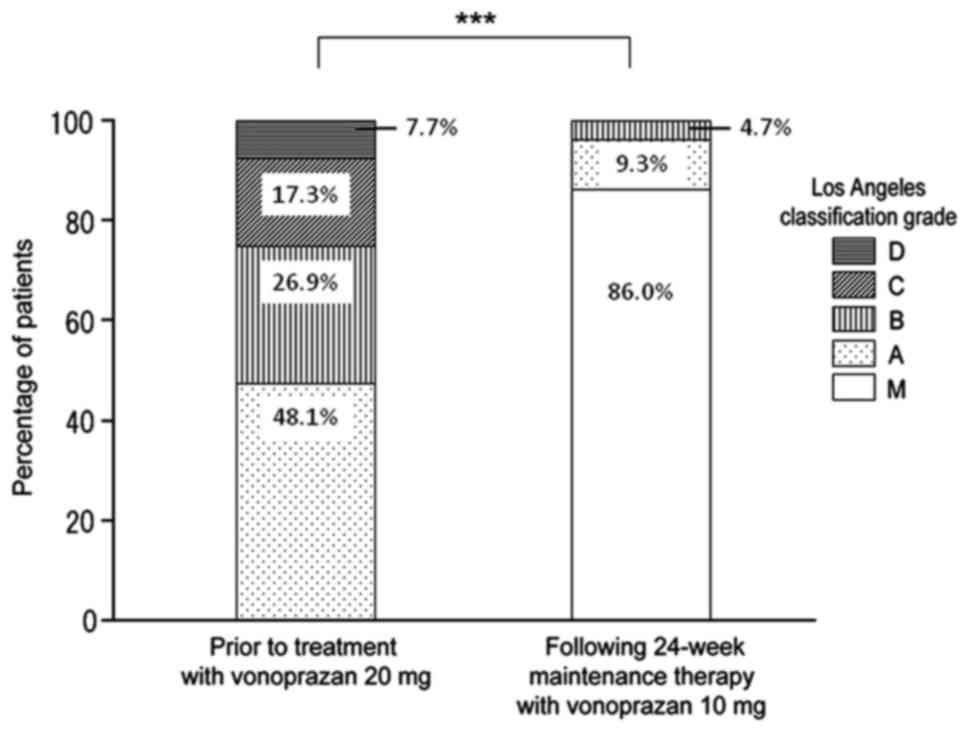
respectively. The LA classification prior to switching to
vonoprazan 20 mg was grade A in 48.1% of patients, grade B in
26.9%, grade C in 17.3% and grade D in 7.7%, respectively. Prior
PPI treatment was administered at standard dose in all patients.
The most frequently used PPI was rabeprazole 10 mg (40.4 % of
patients), following by esomeprazole 20 mg (28.8%), omeprazole 20
mg (17.3%) and lansoprazole 30 mg (13.5%). The mean duration of
illness was 12.1±15.0 months. Gastrointestinal complications were
observed in 57.7% of patients (hiatus hernia 32.7%, atrophic
gastritis 17.3%, FD 19.2% and IBS 25.0%, respectively). H.
pylori infection was significantly higher in the FSSG ≥8 group
compared with the <8 group (P=0.045; Table I). Otherwise, no significant
differences in patient demographic and clinical characteristics
were observed between the FSSG <8 and ≥8 groups (Table I).
Efficacy of maintenance therapy:
Endoscopy
Following 24 weeks of vonoprazan 10 mg maintenance
therapy, 37 patients were classified as grade M, and so the overall
non-relapse rate was 86.0% Of the 6 patients who experienced
esophageal mucosal break relapse, 4 were grade A and two were grade
B. Overall, LA grades significantly improved after 24 weeks of
maintenance therapy (P<0.001; Fig.
2).
Efficacy of maintenance therapy: GERD
symptoms and gastrointestinal QoL
The GERD symptoms and gastrointestinal QoL during
maintenance therapy are presented in Fig.
3. The symptomatic non-relapse rate for the acid
reflux-associated and dysmotility symptom scores of FSSG were 92.3
and 88.5% at 8 weeks and 86.5 and 80.8% at 24 weeks, respectively
(Fig. 3). Furthermore, the
symptomatic non-relapse rate for reflux, abdominal pain,
indigestion, diarrhea and constipation scores of GSRS were 88.5,
82.7, 76.9, 75.0 and 69.2% at 8 weeks and 86.5, 80.8, 75.0, 71.2
and 76.9% at 24 weeks, respectively (Fig.
3).
Association between FSSG total score
improvement and FSSG subscales
In the FSSG ≥8 group, changes in the acid
reflux-associated symptom scores were 9.26±3.2, 3.78±3.2 and
3.41±4.2 at 0, 8 and 24 weeks of maintenance therapy, respectively.
The scores were significantly lower at 8 and 24 weeks compared with
the baseline (P<0.001; Table II).
Changes in dysmotility symptom scores were 6.00±3.4, 3.22±2.9 and
2.89±3.9 at 0, 8 and 24 weeks of maintenance therapy, respectively.
The scores were significantly lower at both 8 and 24 weeks compared
with the baseline (P<0.01; Table
II).
 | Table II.Changes in FSSG for individual
symptoms. |
Table II.
Changes in FSSG for individual
symptoms.
|
| FSSG <8 group
(n=25) | FSSG ≥8 group
(n=27) |
|---|
|
|
|
|
|---|
| Variable | 0 weeks | 8 weeks | P-value | 24 weeks | P-value | 0 weeks | 8 weeks | P-value | 24 weeks | P-value |
|---|
| Acid
reflux-associated symptoms |
2.72±1.7 |
1.60±1.7 | 0.022 |
2.12±2.0 | 0.934 |
9.26±3.2 |
3.78±3.2 | <0.001 |
3.41±4.2 | <0.001 |
| Dysmotility
symptoms |
1.84±1.3 |
1.32±1.7 | 0.241 |
1.64±1.9 | 0.933 |
6.00±3.4 |
3.22±2.9 | 0.002 |
2.89±3.9 | <0.001 |
In the FSSG <8 group, changes in the acid
reflux-associated symptom scores were 2.72±1.7, 1.60±1.7 and
2.12±2.0 at 0, 8 and 24 weeks of maintenance therapy, respectively.
The scores were significantly lower at 8 weeks compared with the
baseline (P<0.05; Table II).
Changes in the dysmotility symptom scores were 1.84±1.3, 1.32±1.7
and 1.64±1.9 at 0, 8 and 24 weeks of maintenance therapy,
respectively. No significance differences in dysmotility symptom
scores of FSSG were observed during the 24-week treatment
period.
Association between FSSG total score
improvement and GSRS subscales
In the FSSG ≥8 group, the reflux scores of GSRS were
6.44±2.6, 3.81±1.7 and 3.37±1.9 at 0, 8 and 24 weeks of maintenance
therapy, respectively. The scores were significantly lower at 8 and
24 weeks after switching to vonoprazan 10 mg compared with the
baseline (P<0.001; Table III).
Abdominal pain (P<0.001) and indigestion (P<0.05) scores were
significantly lower at 8 and 24 weeks compared with the baseline
(Table III). The constipation score
was significantly lower at 24 weeks compared with the baseline
(P<0.05; Table III).
 | Table III.Changes in gastrointestinal symptom
rating scale for individual symptoms. |
Table III.
Changes in gastrointestinal symptom
rating scale for individual symptoms.
|
| FSSG <8 group
(n=25) | FSSG ≥8 group
(n=27) |
|---|
|
|
|
|
|---|
| Variable | 0 weeks | 8 weeks | P-value | 24 weeks | P-value | 0 weeks | 8 weeks | P-value | 24 weeks | P-value |
|---|
| Reflux |
3.96±1.7 |
2.84±1.0 | 0.009 |
2.96±1.2 | 0.135 |
6.44±2.6 |
3.81±1.7 | <0.001 |
3.37±1.9 | <0.001 |
| Abdominal pain |
3.92±1.3 |
3.92±1.5 | 0.991 |
3.84±1.4 | 0.909 |
6.81±2.9 |
4.30±1.7 | <0.001 |
4.30±2.3 | <0.001 |
| Indigestion |
6.83±2.7 |
5.64±2.0 | 0.074 |
6.20±2.3 | 0.429 |
10.4±4.2 |
8.15±2.8 | 0.024 |
8.33±4.3 | 0.044 |
| Diarrhea |
4.60±2.4 |
4.56±2.6 | 0.956 |
4.76±2.7 | 0.961 |
6.30±3.9 |
5.26±3.6 | 0.314 |
5.26±3.7 | 0.353 |
| Constipation |
5.84±3.0 |
5.56±3.0 | 0.744 |
5.44±3.1 | 0.539 |
6.63±3.2 |
5.56±1.8 | 0.132 |
5.22±2.0 | 0.034 |
In the FSSG <8 group, the reflux scores of GSRS
were 3.96±1.7, 2.84±1.0 and 2.96±1.2 at 0, 8 and 24 weeks of
maintenance therapy, respectively, the scores were significantly
lower at 8 weeks after switching to vonoprazan 10 mg compared with
the baseline (P<0.01; Table
III). Otherwise, no significant differences in GSRS subscale
scores were observed during the 24-week treatment period (Table III).
Safety
No serious adverse events were reported during the
study. Adverse events considered attributable to vonoprazan 10 mg
occurred in 1 of the 52 patients, who presented with mild abdominal
fullness. This symptom improved throughout the treatment period
with no intervention. The mean gastrin level was 1,059 pg/ml
(88–3,126; Table I).
Discussion
The results of the present study demonstrate for the
first time that vonoprazan 10 mg is a clinically 24-week
maintenance therapy for patients with healed RE in endoscopic and
symptomatic remission irrespective of whether RE refractory to PPIs
was initially healed by treatment with vonoprazan 20 mg. These
results indicate that vonoprazan 10 mg once daily prevented
esophageal mucosal break relapse in 37/43 patients (86.0%).
Maintaining control of symptoms and mucosal healing
is the most important goal of GERD therapy (26,27).
Long-term therapy with PPIs typically controls GERD more
effectively than other therapies including H2-receptor antagonists
and prokinetic agents (1). PPI
therapy relieves symptoms and controls esophagitis in most patients
with RE (28) and is recommended as
the first-line treatment for GERD (1). A number of clinical trials have examined
the efficacy of PPIs in GERD maintenance therapy, comparing
different PPI doses and different PPIs; large-scale studies
employing more >1,000 patients revealed that esomeprazole 20 mg
was superior to other PPIs (29–31).
During a period of 6 months, esomeprazole 20 mg once daily
effectively maintained endoscopic and symptomatic remission
(78.7–87.0%) in patients who had been successfully treated for RE
(29–33).
Although PPIs are recommended as the first-line
treatment for GERD (1), they also
possess a number of limitations, including incomplete gastric acid
suppression (especially at night), inter-patient variability in
efficacy due to CYP2C19 metabolism and the inconvenience of
requiring mealtime dosing to ensure adequate levels of the drug
during periods of H+,K+-ATPase activity. In
addition, PPIs are slow to achieve steady state inhibition of
gastric acid secretion, typically taking 3 to 5 days to achieve
maximum inhibition (34,35). The effect of vonoprazan 20 mg is
attributed to its potent (14–16) and
rapid (14,15) acid secretion suppressive effects.
Vonoprazan 20 mg was demonstrated to have a significantly greater
acid-inhibitory effect compared with esomeprazole 20 mg and
rabeprazole 10 mg in healthy Japanese subjects with the CYP2C19
extensive metabolizer genotype (15).
Recent studies revealed that vonoprazan 20 mg is an effective
treatment for a majority of Japanese patients with RE refractory to
PPI (17–19). Jenkins et al (14) reported that the mean efficacy rate of
10 mg vonoprazan in achieving a gastric acid pH of 4.0 or higher
was 63%. As the mean efficacy rate of achieving gastric pH 4.0 or
higher by standard-dose PPI is 50–65% (15,26,36,37),
the efficacy of vonoprazan 10 mg may be similar to or higher than
that of standard-dose PPI. However, the results of the present
study revealed that 24-week treatment with vonoprazan 10 mg once
daily successfully maintained endoscopic remission (86.0%) and
symptomatic remission (86.5%) in patients who had been successfully
treated by vonoprazan 20 mg for RE refractory to PPIs. This may be
why individual differences in the acid-suppressive effects of
vonoprazan were small, as vonoprazan is mainly metabolized by
CYP3A4 rather than CYP2C19 (38).
In the present study, 27 patients (51.9%) had a
total FSSG score of ≥8. In the FSSG ≥8 group, the mean acid
reflux-associated symptom scores of FSSG and mean reflux score of
GSRS improved significantly as early as 8 week after switching to
vonoprazan 10 mg. However, in the FSSG <8 group, the mean acid
reflux-associated symptom scores of FSSG and mean reflux score of
GSRS were significantly lower only at 8 weeks, with no significant
difference observed at the end of the 24-week maintenance period.
These results suggest that vonoprazan 10 mg may improve residual
GERD symptoms in patients receiving vonoprazan 20 mg.
In the FSSG ≥8 group, GSRS abdominal pain and
indigestion scores were lower at 8 and 24 weeks compared with at
the baseline. These results are in line with the results of a
previous study in which GSRS scores were evaluated after treatment
with the H2 blocker famotidine and the PPI omeprazole (39). It was reported that the combination
treatment significantly improved the acid reflux-associated
symptoms as well as the scores for abdominal pain and indigestion
(39). These findings suggest that
treatment with acid secretion suppressors may improve QoL
associated with gastrointestinal tract symptoms other than those
affecting the esophagus.
A study by Hori et al (40) indicated a high prevalence of FD and
IBS in patients with GERD, which is consistent with the findings of
the present study. Furthermore, Locke et al (41) conducted a study on gastrointestinal
symptoms using a medical questionnaire that confirmed the presence
of a complex overlap of symptoms involving the upper and lower
gastrointestinal tract, including acid reflux-associated symptoms,
dyspepsia symptoms, constipation and diarrhea. Similarly, the
majority of patients with RE in the present study reported diverse
gastrointestinal symptoms and complications.
Vonoprazan has been reported to have a favorable
safety and tolerability profile (13,14,16). The
median morning fasting gastrin level following the administration
of vonoprazan 20 mg for 4 weeks and vonoprazan 10 mg for 24 weeks
was 1,058 pg/ml, which is similar to that reported in a phase 3
clinical trial and previous studies (16,17,19).
Although PPI-induced hypergastrinemia does not appear to be
associated with carcinoid tumor development, follow-up monitoring
of gastrin levels in patients treated with vonoprazan may be
necessary.
The present study has several limitations. Firstly,
the study population was relatively small. Secondly, the
therapeutic efficacy of vonoprazan 10 mg in the present study is
presented as a result of the evaluation of changes in a single
group of patients in comparison with the baseline. Thirdly, the
results of the present study are subject to potential patient bias
given its open-label design. Fourthly, the presence of the CYP2C19
genotype was not investigated and 24-h gastric and esophageal pH
monitoring was not performed. In order to confirm the results
herein, multicenter investigations with a larger number of patients
with RE refractory to PPIs and control subjects is required.
In conclusion, the present study demonstrated that
vonoprazan 10 mg once daily is an effective 24-week maintenance
therapy for patients with healed RE refractory to PPIs.
Furthermore, vonoprazan 10 mg may improve residual GERD symptoms in
patients receiving vonoprazan 20 mg. These results suggest that
vonoprazan may be a better therapy for the maintenance treatment of
patients with healed RE than currently used first-line PPI
therapy.
References
|
1
|
Iwakiri K, Kinoshita Y, Habu Y, Oshima T,
Manabe N, Fujiwara Y, Nagahara A, Kawamura O, Iwakiri R, Ozawa S,
et al: Evidence-based clinical practice guidelines for
gastroesophageal reflux disease 2015. J Gastroenterol. 51:751–767.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fujiwara Y and Arakawa T: Epidemiology and
clinical characteristics of GERD in the Japanese population. J
Gastroenterol. 44:518–534. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miwa H, Yokoyama T, Hori K, Sakagami T,
Oshima T, Tomita T, Fujiwara Y, Saita H, Itou T, Ogawa H, et al:
Interobserver agreement in endoscopic evaluation of reflux
esophagitis using a modified Los Angeles classification
incorporating grades N and M: A validation study in a cohort of
Japanese endoscopists. Dis Esophagus. 21:355–363. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kahrilas PJ, Shaheen NJ and Vaezi MF;
American Gastroenterological Association Institute, ; Clinical
Practice and Quality Management Committee, : American
Gastroenterological Association Institute technical review on the
management of gastroesophageal reflux disease. Gastroenterology.
135:1392–1413, 1413.e1-1413.e5. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Joh T, Miwa H, Higuchi K, Shimatani T,
Manabe N, Adachi K, Wada T, Sasaki M, Fujiwara Y, Hongo M, et al
ARS Research Group, : Validity of endoscopic classification of
nonerosive reflux disease. J Gastroenterol. 42:444–449. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pace F, Negrini C, Wiklund I, Rossi C and
Savarino V; ITALIAN ONE INVESTIGATORS STUDY GROUP, : Quality of
life in acute and maintenance treatment of non-erosive and mild
erosive gastro-oesophageal reflux disease. Aliment Pharmacol Ther.
22:349–356. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wada T, Sasaki M, Kataoka H, Tanida S,
Itoh K, Ogasawara N, Oshima T, Togawa S, Kubota E, Yamada T, et al:
Efficacy of famotidine and omeprazole in healing symptoms of
non-erosive gastro-oesophageal reflux disease:
Randomized-controlled study of gastro-oesophageal reflux disease.
Aliment Pharmacol Ther. 21 Suppl 2:2–9. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fass R: Erosive esophagitis and nonerosive
reflux disease (NERD): Comparison of epidemiologic, physiologic,
and therapeutic characteristics. J Clin Gastroenterol. 41:131–137.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fass R, Shapiro M, Dekel R and Sewell J:
Systematic review: Proton-pump inhibitor failure in
gastro-oesophageal reflux disease - where next? Aliment Pharmacol
Ther. 22:79–94. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mizuno H, Matsuhashi N, Sakaguchi M, Inoue
S, Nakada K, Higuchi K, Haruma K and Joh T: Recent effectiveness of
proton pump inhibitors for severe reflux esophagitis: The first
multicenter prospective study in Japan. J Clin Biochem Nutr.
57:233–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Andersson K and Carlsson E:
Potassium-competitive acid blockade: A new therapeutic strategy in
acid-related diseases. Pharmacol Ther. 108:294–307. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hori Y, Imanishi A, Matsukawa J, Tsukimi
Y, Nishida H, Arikawa Y, Hirase K, Kajino M and Inatomi N:
1-[5-(2-Fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine
monofumarate (TAK-438), a novel and potent potassium-competitive
acid blocker for the treatment of acid-related diseases. J
Pharmacol Exp Ther. 335:231–238. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ashida K, Sakurai Y, Nishimura A, Kudou K,
Hiramatsu N, Umegaki E, Iwakiri K and Chiba T: Randomised clinical
trial: A dose-ranging study of vonoprazan, a novel
potassium-competitive acid blocker, vs. lansoprazole for the
treatment of erosive oesophagitis. Aliment Pharmacol Ther.
42:685–695. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jenkins H, Sakurai Y, Nishimura A, Okamoto
H, Hibberd M, Jenkins R, Yoneyama T, Ashida K, Ogama Y and
Warrington S: Randomised clinical trial: Safety, tolerability,
pharmacokinetics and pharmacodynamics of repeated doses of TAK-438
(vonoprazan), a novel potassium-competitive acid blocker, in
healthy male subjects. Aliment Pharmacol Ther. 41:636–648. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sakurai Y, Mori Y, Okamoto H, Nishimura A,
Komura E, Araki T and Shiramoto M: Acid-inhibitory effect of
vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10
mg in healthy adult male subjects - a randomised open-label
cross-over study. Aliment Pharmacol Ther. 42:719–730. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ashida K, Sakurai Y, Hori T, Kudou K,
Nishimura A, Hiramatsu N, Umegaki E and Iwakiri K: Randomised
clinical trial: Vonoprazan, a novel potassium-competitive acid
blocker, vs. lansoprazole for the healing of erosive oesophagitis.
Aliment Pharmacol Ther. 43:240–251. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hoshino S, Kawami N, Takenouchi N, Umezawa
M, Hanada Y, Hoshikawa Y, Kawagoe T, Sano H, Hoshihara Y, Nomura T,
et al: Efficacy of Vonoprazan for Proton Pump Inhibitor-Resistant
Reflux Esophagitis. Digestion. 95:156–161. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Okuyama M, Nakahara K, Iwakura N, Hasegawa
T, Oyama M, Inoue A, Ishizu H, Satoh H and Fujiwara Y: Factors
associated with potassium-competitive acid blocker non-response in
patients with proton pump inhibitor-refractory gastroesophageal
reflux disease. Digestion. 95:281–287. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Iwakiri K, Sakurai Y, Shiino M, Okamoto H,
Kudou K, Nishimura A, Hiramatsu N, Umegaki E and Ashida K: A
randomized, double-blind study to evaluate the acid-inhibitory
effect of vonoprazan (20 mg and 40 mg) in patients with proton-pump
inhibitor-resistant erosive esophagitis. Therap Adv Gastroenterol.
10:439–451. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kusano M, Shimoyama Y, Sugimoto S,
Kawamura O, Maeda M, Minashi K, Kuribayashi S, Higuchi T, Zai H,
Ino K, et al: Development and evaluation of FSSG: Frequency scale
for the symptoms of GERD. J Gastroenterol. 39:888–891. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lundell LR, Dent J, Bennett JR, Blum AL,
Armstrong D, Galmiche JP, Johnson F, Hongo M, Richter JE, Spechler
SJ, et al: Endoscopic assessment of oesophagitis: Clinical and
functional correlates and further validation of the Los Angeles
classification. Gut. 45:172–180. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kinoshita Y and Adachi K: Hiatal hernia
and gastroesophageal flap valve as diagnostic indicators in
patients with gastroesophageal reflux disease. J Gastroenterol.
41:720–721. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kimura K and Takemoto T: An endoscopic
recognition of the atrophic border and its significance in chronic
gastritis. Endoscopy. 1:87–97. 1969. View Article : Google Scholar
|
|
24
|
Hongo M, Fukuhara S and Green J:
Gastrointestinal-related QOL-QOL assessment using the Japanese
version of GSRS. Diagn Treat. 87:731–736. 1999.
|
|
25
|
Drossman DA: The functional
gastrointestinal disorders and the Rome III process.
Gastroenterology. 130:1377–1390. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dent J, Brun J, Fendrick A, Fendrick M,
Janssens J, Kahrilas P, Lauritsen K, Reynolds J, Shaw M, et al: An
evidence-based appraisal of reflux disease management - the Genval
Workshop Report. Gut. 44 Suppl 2:S1–S16. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
DeVault KR and Castell DO; American
College of Gastroenterology, : Updated guidelines for the diagnosis
and treatment of gastroesophageal reflux disease. Am J
Gastroenterol. 100:190–200. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Röhss K, Lind T and Wilder-Smith C:
Esomeprazole 40 mg provides more effective intragastric acid
control than lansoprazole 30 mg, omeprazole 20 mg, pantoprazole 40
mg and rabeprazole 20 mg in patients with gastro-oesophageal reflux
symptoms. Eur J Clin Pharmacol. 60:531–539. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lauritsen K, Devière J, Bigard MA,
Bayerdörffer E, Mózsik G, Murray F, Kristjánsdóttir S, Savarino V,
Vetvik K, De Freitas D, et al: Metropole study results:
Esomeprazole 20 mg and lansoprazole 15 mg in maintaining healed
reflux oesophagitis: Metropole study results. Aliment Pharmacol
Ther. 17:333–341. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Labenz J, Armstrong D, Lauritsen K,
Katelaris P, Schmidt S, Schütze K, Wallner G, Juergens H,
Preiksaitis H, Keeling N, et al: Esomeprazole 20 mg vs.
pantoprazole 20 mg for maintenance therapy of healed erosive
oesophagitis: Results from the EXPO study. Aliment Pharmacol Ther.
22:803–811. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Devault KR, Johanson JF, Johnson DA, Liu S
and Sostek MB: Maintenance of healed erosive esophagitis: A
randomized six-month comparison of esomeprazole twenty milligrams
with lansoprazole fifteen milligrams. Clin Gastroenterol Hepatol.
4:852–859. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vakil NB, Shaker R, Johnson DA, Kovacs T,
Baerg RD, Hwang C, D'Amico D and Hamelin B: The new proton pump
inhibitor esomeprazole is effective as a maintenance therapy in
GERD patients with healed erosive oesophagitis: A 6-month,
randomized, double-blind, placebo-controlled study of efficacy and
safety. Aliment Pharmacol Ther. 15:927–935. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Atug O, Giral A, Kalayci C, Dolar E,
Isitan F, Oguz D, Ovunc O, Ozgur O, Soykan I, Simsek I, et al
Turkish HEMANEX Study Group, : Esomeprazole in acute and
maintenance treatment of reflux oesophagitis: A multicentre
prospective study. Adv Ther. 25:552–566. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Piche T and Galmiche JP: Pharmacological
targets in gastro-oesophageal reflux disease. Basic Clin Pharmacol
Toxicol. 97:333–341. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sachs G, Shin JM and Howden CW: Review
article: The clinical pharmacology of proton pump inhibitors.
Aliment Pharmacol Ther. 23 Suppl 2:2–8. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shimatani T, Inoue M, Kuroiwa T, Xu J,
Mieno H, Nakamura M and Tazuma S: Acid-suppressive effects of
rabeprazole, omeprazole, and lansoprazole at reduced and standard
doses: A crossover comparative study in homozygous extensive
metabolizers of cytochrome P450 2C19. Clin Pharmacol Ther.
79:144–152. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Norris V, Baisley K, Dunn K, Warrington S
and Morocutti A: Combined analysis of three crossover clinical
pharmacology studies of effects of rabeprazole and esomeprazole on
24-h intragastric pH in healthy volunteers. Aliment Pharmacol Ther.
25:501–510. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yamasaki H, Kawaguchi N, Nonaka M,
Takahashi J, Morohashi A, Hirabayashi H, Moriwaki T and Asahi S: In
vitro metabolism of TAK-438, vonoprazan fumarate, a novel
potassium-competitive acid blocker. Xenobiotica. 47:1027–1034.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kusunoki H, Kusaka M, Kido S, Yamauchi R,
Fujimura Y, Watanabe Y, Kobori M, Miwa H, Tomita T, Kin Y, et al:
Comparison of the effects of omeprazole and famotidine in treatment
of upper abdominal symptoms in patients with reflux esophagitis. J
Gastroenterol. 44:261–270. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hori K, Matsumoto T and Miwa H: Analysis
of the gastrointestinal symptoms of uninvestigated dyspepsia and
irritable bowel syndrome. Gut Liver. 3:192–196. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Locke GR III, Zinsmeister AR, Fett SL,
Melton LJ III and Talley NJ: Overlap of gastrointestinal symptom
complexes in a US community. Neurogastroenterol Motil. 17:29–34.
2005.Soleceperum esequi ut reperum autem es sequat. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Quat Nimusa: Umquid maios rerrore mporum
evenis eaque sequam, nissitiae rem quam quuntusdae sequo te pro
corionsequid magnisint quis duntis ellicae prehent estrume ndanda
nonet, ut millace aquatur aspedi dolorro conessimust, a dis im
fuga. Itatemq uissusc illatem sernatu saerum qui omniam, sinum
fugitium alit facescient.
|