Introduction
Total hip arthroplasty (THA) is an effective
surgical intervention for pain relief and function improvement in
elderly patients presenting with hip degeneration. However, a
variable amount of pain often accompanies this procedure
postoperatively (1,2). Inadequate control of postoperative pain
may influence early rehabilitation and result in delayed recovery
and prolonged hospitalization. Consequently, various studies have
been conducted to obtain an ideal analgesic strategy, which could
offer satisfactory analgesia with high safety.
Traditionally, post-THA analgesics include oral and
epidural analgesics, peripheral nerve blockers, and intra-articular
analgesia infusions, among which local analgesic injection is a
simple and effective method. At present, numerous options,
including opioids, non-steroidal anti- inflammatory agents and
local anesthetics are available for local injection (3–5). A
relatively novel approach for peripheral pain control is to use
magnesium sulphate. A recent study revealed magnesium had
antinociceptive effects in animal and human models of chronic pain
(6). It was shown to be effective as a
postoperative analgesic in orthopedic surgery (7,8). The
analgesic property of magnesium is associated with inhibition of
the N-methyl-D-aspartate (NMDA) receptor and modulation of calcium
channels (9). Furthermore, its
addition to local anesthetics prolongs anesthesia duration and
maximizes their effects (10,11). Clinical studies demonstrated that
intra- articular injection of magnesium effectively ameliorated
postoperative pain in arthroscopic knee surgery when compared with
a placebo (12,13). However, to the best of our knowledge,
its application to THA has not yet been reported.
Therefore the present study was performed to compare
and analyze the analgesic efficacy and safety of an intra-articular
magnesium sulfate injection with a placebo following THA. It was
hypothesized that patients receiving the intra-articular magnesium
sulfate injection would experience reduced postoperative pain when
compared with the control group.
Materials and methods
Participants
Between October 2012 and June 2014, a total of 60
patients underwent THA in the Second Affiliated Hospital of Wenzhou
Medical University (Wenzhou, China) and met the inclusion criteria,
thus were included in the current study. All procedures were
conducted in accordance with the ethical standards of the
responsible committee on human experimentation (institutional and
national) and with the Helsinki Declaration of 1964 and its later
amendments. Informed consent was obtained from all the patients
included in the study. The compliance was considered to be good, as
the majority of the outcome measurements were short-term
assessments.
Patients who underwent unilateral THA for hip
osteoarthritis or aseptic necrosis of the femoral head were
eligible for the current study. The diagnosis was predominantly
determined by local radiographs, and further confirmed with the
help of medical history, clinical examination, and additional
imaging examinations, such as computed tomography or magnetic
resonance imaging if necessary. The inclusion criteria were as
follows: Patients aged <80 years, weighing <80 kg, and with
an American Society of Anesthesiologists physical status class I or
II (14). The exclusion criteria were
as follows: i) History of bleeding diathesis or any previous
thromboembolic episodes; ii) recent use of therapeutic agents that
may distort the results; iii) exhibiting metabolic diseases, such
as hepatic or renal dysfunction, or serious cardiac disorders; iv)
exhibiting hypermagnesemia or associated diseases with the
potential to develop a hypermagnesemia; v) known allergies to one
of the medications included in the study.
Interventions
All patients were randomized into one of two groups
using computer-generated numbers. The magnesium group (n=30)
received 10 ml 10% magnesium sulphate and the control group (n=30)
received 10 ml normal saline solution. All patients received
celecoxib (COX-2 inhibitor; PPLLC, Dalian, China) postoperatively
with a dose of 200 mg twice a day. The patients received one dose
of cefazolin (2 g; Reyoung Pharmaceutical Co., Shandong, China)
intraoperatively, and the subsequent 4 doses of the same quantity
over 48 h postoperatively. A daily treatment of 4,000 IU low
molecular weight heparin was administrated subcutaneously for deep
venous thrombosis (DVT) prophylaxis.
Prior to surgery, all patients received an
intravenous bolus of 500 ml Ringer's lactate solution (Kelun
Pharmaceutical Co., Sichuan, China). General anesthesia was
required for all patients and all procedures were performed by the
same senior anesthetist. It was induced by total intravenous
anesthesia with propofol (2 mg/kg; Jiabo Pharmaceutical Co.,
Guangdong, China) and remifentanil (3 µg/kg; Nhwa Pharmaceutical
Co., Jiangsu, China), and maintained with nitrous oxide in oxygen
(60%) and isoflurane (1–2%; Lenuo Kefeng Pharmaceutical Co.,
Shandong, China). The depth of anesthesia was maintained by
adjusting the percentage of isoflurane. Vital signs, such as heart
rate (HR), blood pressure, respiratory rate and arterial oxygen
saturation were monitored continuously throughout surgery.
Hypotension (systolic blood pressure <80 mmHg or a 30% decrease
from baseline) was treated with Ringer's lactate solution, if
required followed by 5 mg intravenous epinephrine (Kingyork
Pharmaceutical Co., Tianjin, China). Bradycardia (HR <50 bpm)
was treated with 0.2 mg atropine (Kingyork Pharmaceutical Co.).
All surgical procedures were performed by the same
senior surgeon according to standard surgical routines.
Metal-on-metal total hip systems without bone cement (Smith &
Nephew, Memphis, TN, USA) were available for replacement. The
posterolateral approach was adopted, and a standard silicon drain
with an inner diameter of 16 mm was placed before closing the skin,
which was clamped for 2 h postoperatively and subsequently
released. Following closure of the capsule the test medication was
administrated. Patients in the magnesium group received 10 ml
magnesium sulphate solution (10% magnesium sulphate; 1g diluted in
10 ml normal saline) intra-articularly and those in the control
group received the same quantity of normal saline solution. All
cases were transferred to the post-anesthesia care unit and
observed for at least 2 h before being returned to the ward.
Morphine injection (10 mg) was administered
intravenously as an analgesic supplement once a day for 48 h after
surgery. If pain remained intolerable, an additional 5 mg per
administration was used and the total quantity was dependent on the
severity and general condition of the case. No other analgesics
were used during the study period. The drain at the surgical site
was removed 48 h postoperatively. The rehabilitation protocol was
identical for every patient. On postoperative day 1, the
anteroposterior and lateral radiographic views of the affected hip
were reexamined. Full weight bearing was permitted after removal of
the drains. The patients were permitted to be discharged when pain
was manageable with oral morphine and when they were able to walk
using crutches.
Outcome assessment
Preoperative variables, including age, gender,
diagnosis, body mass index (BMI) and surgical duration were
evaluated for each patient. The primary outcome was pain, as
assessed using the visual analogue score (VAS), which ranged from 0
mm (representing no pain) to 100 mm (representing the worst pain.
VAS was determined preoperatively and at hours 2, 4, 6, 12, 24 and
48, and on days 3, 7 and 14 at rest, and postoperatively at hours
24 and 48, and days 7 and 14 during activity. The secondary
outcomes included morphine consumption and Harris hip score (HHS)
(15). Morphine consumption was
calculated preoperatively and at hours 6, 12, 24 and 48, and HHS
was documented preoperatively, and at day 7 and 14 postoperatively.
Serum magnesium concentration was assessed in each patient. The
blood samples (10 ml) for calculating serum magnesium concentration
were obtained preoperatively and at hours 6 and 24 postoperatively.
Adverse events (AEs) were recorded within 14 postoperative
days.
Statistically analysis
All data were analyzed by the research team of our
department in conjunction with a medical statistician using the
latest version of SPSS 19.0 (IBM SPSS, Armonk, NY, USA). Continuous
data are presented as means ± standard deviation and analyzed using
paired or unpaired Student's t-test. Categorical data are presented
using proportions and analyzed using the χ2 test with
Fisher's exact test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Baseline demographic characteristics
and clinical data
All patients completed the study protocol. The
baseline demographic characteristics and clinical data are
presented in Table I. Two groups of
patients were well matched, with no significant differences in age,
gender, BMI, diagnosis, length of surgery, serum magnesium
concentration and total drainage fluid observed between the two
groups.
 | Table I.Demographic characteristics and
clinical data (values presented as means ± standard deviation). |
Table I.
Demographic characteristics and
clinical data (values presented as means ± standard deviation).
|
| Group |
|
|---|
|
|
|
|
|---|
| Parameter | Magnesium (n=30) | Control (n=30) | P-value |
|---|
| Age (years) | 66.2±9.8 |
65.1±10.9 | 0.683 |
| Gender |
|
| 0.299 |
| Male | 11 | 16 |
|
|
Female | 19 | 14 |
|
| Body mass index
(kg/m2) | 26.3±3.0 | 26.0±3.2 | 0.675 |
| Diagnosis (n) |
|
| 0.067 |
|
Osteoarthritis | 21 | 13 |
|
| Aseptic
necrosis | 9 | 17 |
|
| Length of surgery
(min) |
76.0±16.8 |
74.5±17.1 | 0.721 |
| Serum magnesium
concentration (mmol/l) |
|
|
|
|
Preoperative |
1.00±0.15 |
1.03±0.15 | 0.376 |
|
Postoperative, 6 h |
1.08±0.17 |
1.04±0.15 | 0.414 |
|
Postoperative, 24 h |
1.02±0.18 |
1.04±0.17 | 0.632 |
| Total
drainage fluid (ml) | 428.3±81.2 | 409.0±82.4 | 0.364 |
Outcome of VAS, consumption of
morphine and HHS
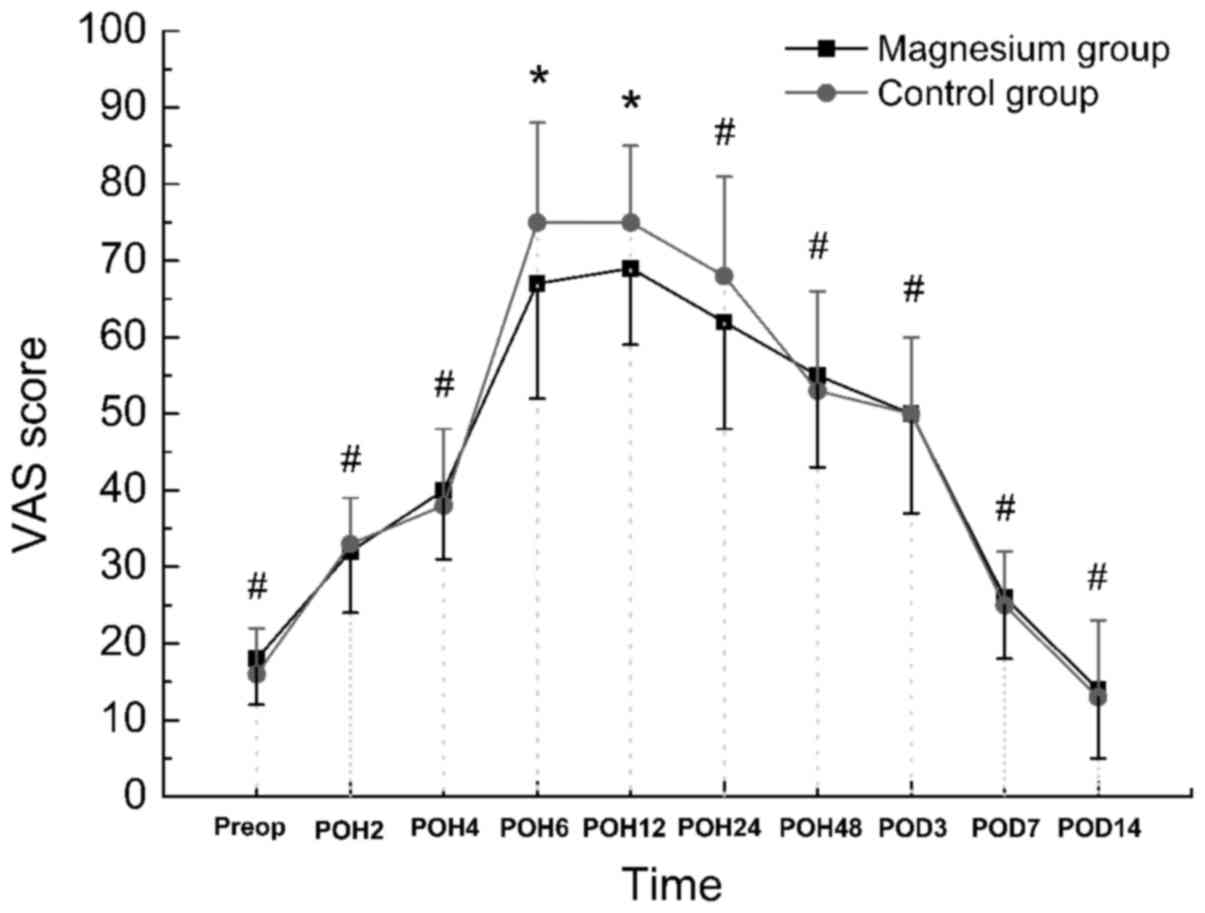
The outcome of VAS at rest was significantly lower
at postoperative hour 6 and 12 in the magnesium group as compared
with the control group (P<0.05), although the difference was
insignificant preoperatively and at postoperative hours 2, 4, 24
and 48, and days 3, 7 and 14 (P>0.05; Fig. 1). This indicator during activity was
also lower in the magnesium group at postoperative hour 24 compared
with the control group (P<0.05), although the difference was
insignificant preoperatively and at hour 48, and days 7 and 14
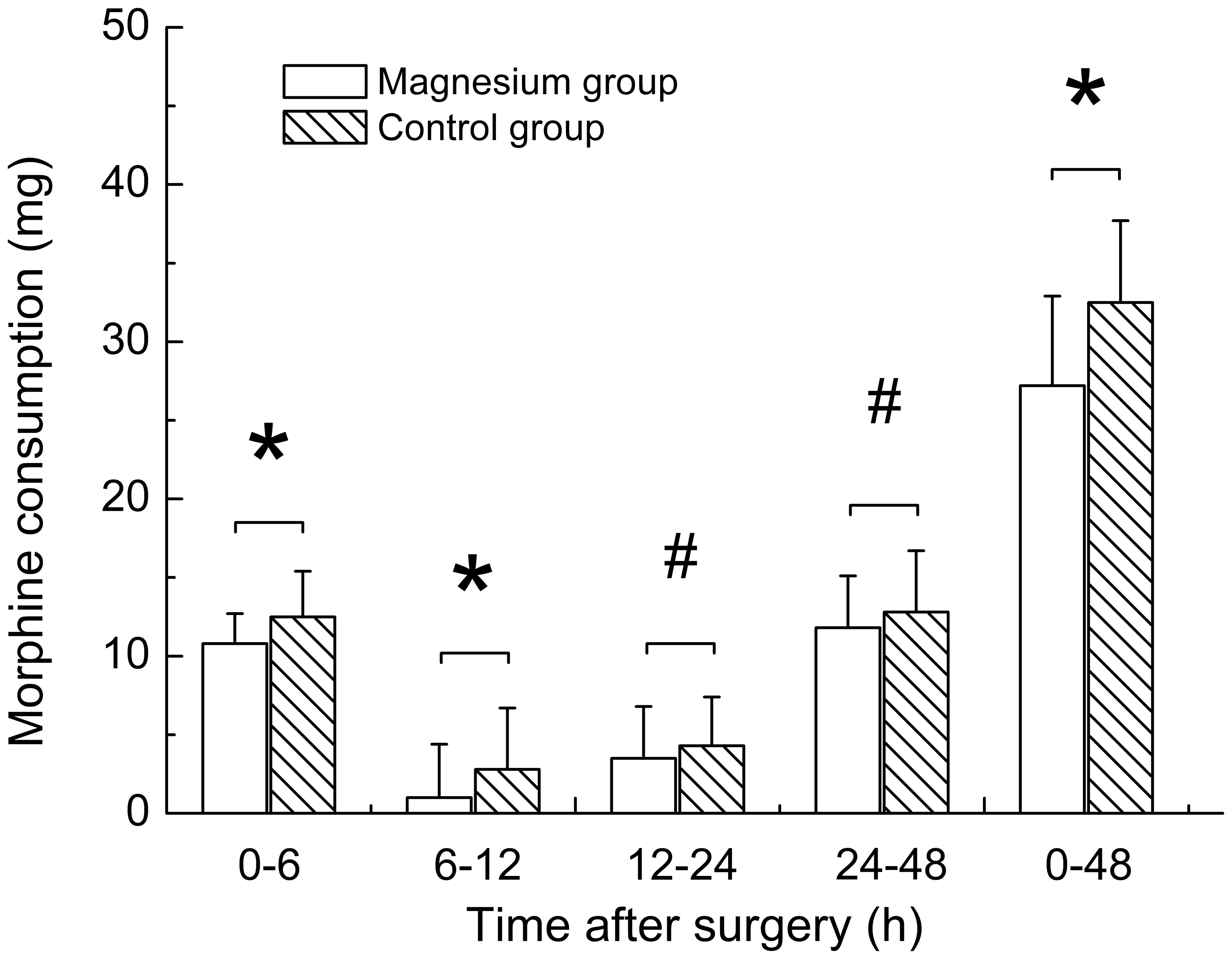
(P>0.05; Fig. 2). Following
surgery, the consumption of morphine at 0–6, 6–12 and 0–48 h (the
total quantity) in the magnesium group was significantly lower when
compared with the control group (P<0.05); however, no
significant differences were observed at 12–24 and 24–48 h between
the groups (P>0.05; Fig. 3). The
HHS was performed to evaluate postoperative recovery conditions. As
shown in Table II, although the
improvements from preoperative to postoperative scores were
statistically significant (P<0.05), no significant differences
were identified between the two groups (P>0.05). At the final
follow-up, 25 cases in the magnesium group and 26 in the control
group achieved excellent or good scores.
 | Table II.Outcome of Harris hip score (values
presented as means ± standard deviation). |
Table II.
Outcome of Harris hip score (values
presented as means ± standard deviation).
|
| Rating grade |
|
|---|
|
|
|
|
|---|
| Time | Group | Excellent
(90–100) | Good (80–89) | Fair (70–79) | Poor (<70) | P-value |
|---|
| Preoperative | Magnesium | 0 | 0 | 11 | 19 | 0.491 |
|
| Control | 0 | 1 | 13 | 16 |
|
| Postoperative day
7 | Magnesium | 4 | 15 | 11 | 0 | 0.279 |
|
| Control | 2 | 21 | 7 | 0 |
|
| Postoperative day
14 | Magnesium | 7 | 18 | 5 | 0 | 0.823 |
|
| Control | 9 | 17 | 4 | 0 |
|
AEs
A total of 17 patients (28%) reported at least one
AE and the proportions did not differ according to the treatment
group (Table III). Lower extremity
Doppler ultrasound scans were performed for every patient three
days post-surgery. Two cases in the magnesium group and one in the
control group were diagnosed with a DVT of the lower extremity.
These patients remained under observation for two weeks and no
serious AEs, such as pulmonary embolism or limb amputation
occurred. Those patients were advised to attend periodic outpatient
follow-up.
 | Table III.Adverse events. |
Table III.
Adverse events.
|
| Group
(n=30/group) |
|
|---|
|
|
|
|
|---|
| Adverse event | Magnesium, n
(%) | Control, n (%) | P-value |
|---|
| Deep venous
thrombosis | 2 (6.7) | 1 (3.3) | 1.000 |
| Vomiting | 1 (3.3) | 0 (0.0) | 1.000 |
| Nausea | 5 (16.7) | 3 (10.0) | 0.706 |
| Dizziness | 4 (13.3) | 6 (20.0) | 0.731 |
| Headache | 2 (6.7) | 2 (6.7) | 1.000 |
| Urine
retention | 0 (0.0) | 1 (3.3) | 1.000 |
Discussion
The present study indicates that intra-articular
injection of magnesium sulphate exerted analgesic effects following
THA, which decreased postoperative pain and morphine consumption in
the early stage of recovery either at rest or during activity.
In recent years, as a result of increasing numbers
of osteoarthritis cases and other degenerative disorders of the
joint, THA is being widely performed and has been demonstrated to
be a highly successful surgical intervention (16,17).
Although this procedure is effective, it is often associated with a
delayed rehabilitation and prolonged hospitalization, and is
accompanied by severe pain during the early postoperative period.
At present, various medications are available for postoperative
pain control in orthopedic surgeries, among which magnesium is
ideal, as it is safe and inexpensive. Although the underlying
mechanism of the antinociceptive effect remains unclear, its
interference with NMDA receptors and calcium channels appears to
affect postoperative pain. Previous studies revealed that NMDA
receptors exerted excitatory synaptic transmission effects and
possessed negative modulatory sites, and could be blocked by
magnesium in a voltage-dependent manner (18,19). In
animal models, NMDA receptor antagonists have been shown to have
analgesic properties in pain conditions, with similar effects also
observed in humans (20,21). Conversely, calcium has been shown to be
associated with the release of neurotransmitters and substances
implicated in nociceptive response (22), thus the blockage of calcium channels
may initiate the pain relief process (23).
Magnesium has been shown to exert its analgesic
effect by peripheral, intravenous or spinal infusion (24). With the advantages of simplicity and
minimal risk for complications, intra-articular administration of
magnesium has received increasing attention and interest. There is
evidence that NMDA-receptor exists in the peripheral terminal of
articular primary afferent fibers and cellular elements of the
joint (25). Previous studies revealed
that magnesium exerted its possible anti-nociceptive effect
predominantly via peripheral NMDA-receptor mechanisms when
administrated locally (26–28). Therefore, the method of intra-articular
injection was adopted in the present study.
The analgesic effect of magnesium by intra-articular
injection has been confirmed by various studies. Koltka et
al (29) conducted a randomized
clinical study on 120 patients undergoing arthroscopic
meniscectomy. In their study, the patients received magnesium
sulphate (500 mg diluted in 20 ml normal saline), levobupivacaine
(100 mg diluted in 20 ml normal saline), lornoxicam (8 mg diluted
in 20 ml normal saline) or 20 ml normal saline by intra-articular
injection prior to tourniquet deflation. Koltka et al
(29) found that magnesium sulphate
was a more effective analgesic than the placebo, although the most
effective was lornoxicam (29). In
another study performed by Bondok and Abd El-Hady (12), a total of 60 cases undergoing
arthroscopic knee surgery were randomly allocated to receive
intra-articular injection of either 10 ml magnesium sulphate (50
mg/ml) or 10 ml normal saline prior to tourniquet release. It was
observed that the magnesium group obtained a significant reduction
in pain scores and less total diclofenac consumption when compared
with the control group postoperatively. It was concluded that
intra-articular magnesium administration was effective for
postoperative analgesia (12). In the
present study, intra-articular administration of magnesium was
performed for pain management following THA, and the results
indicated that this strategy improved postoperative pain scores
when compared with the control group, which was consistent with the
above-mentioned reports.
No significant differences in preoperative data,
surgical duration and total drainage fluid were identified between
the two groups, nor were there differences in intraoperative
anesthesia, surgical approach and the types of implant; therefore,
associated factors that may affect postoperative VAS and
rehabilitation were excluded. The results of VAS at rest, at
postoperative hours 2 and 4 did not differ depending on the
treatment type, and the two were smaller than that at hour 6, which
may be due to residual anesthetic in the circulatory system. No
significant differences in VAS were found after postoperative day
2, as magnesium would be absorbed and metabolized by the body. The
result of HHS indicated that intra-articular administration of
magnesium may not facilitate functional recovery in the early
stages of recovery. However, the outcomes of VAS at postoperative
hours 6 and 12 at rest, and postoperative hour 24 during activity
were significantly lower in the magnesium group, and the
consumption of morphine at 0–6, 6–12 and 0–48 h were also
significantly reduced. Thus, it was concluded that magnesium
reduces postoperative pain when administered intra-articularly
following THA as compared with administration of normal saline.
In the present study, serum magnesium concentrations
were not influenced by intra-articular injection at a low dose (1
g), as the levels were comparable with those of the control group,
postoperatively and following surgery. All the serum magnesium
concentrations were within normal limits and no associated AE was
observed.
There were certain limitations of the present study.
A drainage system was applied and a small quantity of magnesium
sulphate was able to exude from it, thus it was hypothesized that a
lack of drainage could have contributed to greater analgesia
efficacy. In addition, the present study was monocentric;
therefore, the results may be biased, and a multicenter study may
strengthen the generalizability of the outcomes. Furthermore, the
present study was limited to the early postoperative analgesia and
complications, thus further studies of subsequent clinical outcomes
are required.
In conclusion, the present study indicates that the
administration of intra-articular magnesium sulphate provides
improved pain control and reduces the requirement for morphine in
the early postoperative period without increasing short-term
complications, when compared with a placebo.
References
|
1
|
McKenzie JC, Goyal N and Hozack WJ:
Multimodal pain management for total hip arthroplasty. Seminars in
Arthroplasty. 24:87–93. 2013. View Article : Google Scholar
|
|
2
|
Young AC and Buvanendran A: Pain
management for total hip arthroplasty. J Surg Orthop Adv. 23:13–21.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Badica CIG, Badica L, Grecu I, Bradis AA
and Grintescu IM: Opioids or NSAID in postoperative analgesia after
total hip arthroplasty? Comparison of adverse events: 14AP4-4. Eur
J Anaesthesiol. 24:177–178. 2007. View Article : Google Scholar
|
|
4
|
Fransen M and Neal B: Non-steroidal
anti-inflammatory drugs for preventing heterotopic bone formation
after hip arthroplasty. Cochrane Database Syst Rev.
2004:CD0011602004.
|
|
5
|
Kuchálik J, Granath B, Ljunggren A,
Magnuson A, Lundin A and Gupta A: Postoperative pain relief after
total hip arthroplasty: A randomized, double-blind comparison
between intrathecal morphine and local infiltration analgesia. Br J
Anaesth. 111:793–799. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Soave PM, Conti G, Costa R and Arcangeli
A: Magnesium and anaesthesia. Curr Drug Targets. 10:734–743. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abdulatif M, Amin SMM, Aboul-Ela A, Samuel
EWM and Abdel-Hakim SMA: Intra-articular versus intravenous
magnesium-sulfate as adjuvant to femoral nerve block in
arthroscopic knee surgery under general anesthesia: Randomized
controlled trial. Egypt J Anaesth. 31:239–246. 2015. View Article : Google Scholar
|
|
8
|
Saritas TB, Borazan H, Okesli S, Yel M and
Otelcioglu Ş: Is intra-articular magnesium effective for
postoperative analgesia in arthroscopic shoulder surgery? Pain Res
Manag. 20:35–38. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ekmekci P, Bengisun ZK, Akan B, Kazbek BK,
Ozkan KS and Suer AH: The effect of magnesium added to
levobupivacaine for femoral nerve block on postoperative analgesia
in patients undergoing ACL reconstruction. Knee Surg Sports
Traumatol Arthrosc. 21:1119–1124. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Farouk S and Aly A: A comparison of
intra-articular magnesium and/or morphine with bupivacaine for
postoperative analgesia after arthroscopic knee surgery. J Anesth.
23:508–512. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee AR, Yi HW, Chung IS, Ko JS, Ahn HJ,
Gwak MS, Choi DH and Choi SJ: Magnesium added to bupivacaine
prolongs the duration of analgesia after interscalene nerve block.
Can J Anaesth. 59:21–27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bondok RS and Abd El-Hady AM:
Intra-articular magnesium is effective for postoperative analgesia
in arthroscopic knee surgery. Br J Anaesth. 97:389–392. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Elsharnouby NM, Eid HE, Abou Elezz NF and
Moharram AN: Intraarticular injection of magnesium sulphate and/or
bupivacaine for postoperative analgesia after arthroscopic knee
surgery. Anesth Analg. 106:1548–1552. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rozner MA: The American Society of
Anesthesiologists physical status score and risk of perioperative
infection. JAMA. 275:1544. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mahomed NN, Arndt DC, McGrory BJ and
Harris WH: The Harris hip score: Comparison of patient self-report
with surgeon assessment. J Arthroplasty. 16:575–580. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim SJ, Huh J, Odrobina R and Kim JH:
Systemic review of published literature on Candida infection
following total hip arthroplasty. Mycopathologia. 179:173–185.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsertsvadze A, Grove A, Freeman K, Court
R, Johnson S, Connock M, Clarke A and Sutcliffe P: Total hip
replacement for the treatment of end stage arthritis of the hip: A
systematic review and meta-analysis. PLoS One. 9:e998042014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ascher P and Nowak L: Electrophysiological
studies of NMDA receptors. Trends Neurosci. 10:284–288. 1987.
View Article : Google Scholar
|
|
19
|
Coderre TJ, Katz J, Vaccarino AL and
Melzack R: Contribution of central neuroplasticity to pathological
pain: Review of clinical and experimental evidence. Pain.
52:259–285. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fisher K, Coderre TJ and Hagen NA:
Targeting the N-methyl-D-aspartate receptor for chronic pain
management. Preclinical animal studies, recent clinical experience
and future research directions. J Pain Symptom Manage. 20:358–373.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hewitt DJ: The use of NMDA-receptor
antagonists in the treatment of chronic pain. Clin J Pain.
16:(Suppl 2). S73–S79. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Memiş D, Turan A, Karamanlioğlu B, Süt N
and Pamukçu Z: The use of magnesium sulfate to prevent pain on
injection of propofol. Anesth Analg. 95:606–608. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Silverman R, Bendick PJ and Wasvary HJ: A
randomized, prospective, double-blind, placebo-controlled trial of
the effect of a calcium channel blocker ointment on pain after
hemorrhoidectomy. Dis Colon Rectum. 48:1913–1916. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arcioni R, Palmisani S, Tigano S,
Santorsola C, Sauli V, Romanò S, Mercieri M, Masciangelo R, De
Blasi RA and Pinto G: Combined intrathecal and epidural magnesium
sulfate supplementation of spinal anesthesia to reduce
post-operative analgesic requirements: A prospective, randomized,
double-blind, controlled trial in patients undergoing major
orthopedic surgery. Acta Anaesthesiol Scand. 51:482–489. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lawand NB, Willis WD and Westlund KN:
Excitatory amino acid receptor involvement in peripheral
nociceptive transmission in rats. Eur J Pharmacol. 324:169–177.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mercieri M, De Blasi RA, Palmisani S,
Forte S, Cardelli P, Romano R, Pinto G and Arcioni R: Changes in
cerebrospinal fluid magnesium levels in patients undergoing spinal
anaesthesia for hip arthroplasty: Does intravenous infusion of
magnesium sulphate make any difference? A prospective, randomized,
controlled study. Br J Anaesth. 109:208–215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Samir EM, Badawy SS and Hassan AR:
Intrathecal vs intravenous magnesium as an adjuvant to bupivacaine
spinal anesthesia for total hip arthroplasty. Egypt J Anaesth.
29:395–400. 2013. View Article : Google Scholar
|
|
28
|
Lin CY, Tsai PS, Hung YC and Huang CJ:
L-type calcium channels are involved in mediating the
anti-inflammatory effects of magnesium sulphate. Br J Anaesth.
104:44–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Koltka K, Koknel-Talu G, Asik M and
Ozyalcin S: Comparison of efficacy of intraarticular application of
magnesium, levobupivacaine and lornoxicam with placebo in
arthroscopic surgery. Knee Surg Sports Traumatol Arthrosc.
19:1884–1889. 2011. View Article : Google Scholar : PubMed/NCBI
|