Introduction
Skin maintains various homeostatic processes of the
body and acts as the first defense barrier against infection,
radiation and toxin exposure. However, skin is easily affected by
the natural aging process (i.e., intrinsic aging), as well as
environmental conditions (i.e., extrinsic aging), undergoing
aging-associated phenotypic and functional alterations in the
cellular and extracellular components (1,2). Skin aging
is a biological, inevitable process accompanied by a decreased
regenerative capacity, loss of dermal elasticity, impaired function
of the skin, and increased risk of cancer (3). Histologically, aging of the skin results
in altered epidermal architecture and morphology, reduced dermal
mast cells and fibroblasts, decreased collagen production,
epidermal thinning and diminished dermis vasculature (4,5). Mechanisms
for aging skin include exacerbated formation of free radicals
(reactive oxygen species; ROS), accumulation of mitochondrial DNA
mutations, progressive telomere shortening, ultraviolet (UV)
radiation, inflammation and hormonal changes, as well as other
factors that, taken together or alone, may accelerate skin aging
(6–8).
However, currently there are a variety of anti-aging agents
(7,8),
which are routinely used, although their therapeutic efficiency is
less than satisfactory. Therefore, novel therapeutic strategies for
the rejuvenation of aged skin are urgently required.
Mesenchymal stem cells (MSCs) are rare multipotent
stem cells of mesenchymal origin. Through identification with
specific surface markers, MSCs are isolated from the majority of
human tissue types, including bone marrow, adipose tissues,
umbilical cord blood and the dermis (9). MSCs derived from bone marrow (BM-MSCs)
possess remarkable self-renewing properties and exhibit a
multipotent differentiation profile under appropriate
differentiation conditions in vivo and in vitro
(10,11). MSCs facilitate tissue repair via cell
replacement from differentiated cells and/or remodeling the
microenvironment by releasing chemokines and growth factors. MSCs,
therefore, facilitate a variety of basic and clinical studies for
the treatment of a plethora of congenital and acquired diseases
(12). MSCs have been reported to
accelerate the healing of cutaneous wounds by systemic and/or local
administration (13–15). Animal experiments have confirmed the
anti-apoptotic and anti-oxidative capacities of MSCs (16), therefore, these cells may be considered
as an alternative therapeutic strategy for skin rejuvenation.
Conditioned serum-free medium from umbilical cord-derived MSCs may
protect against photo-aging induced by UV radiation in mouse skin
(17). Furthermore, adipose-derived
MSCs have been reported to improve UV radiation-induced wrinkles
and protect photo-aged dermal fibroblasts from oxidative stress
(18,19).
However, the underlying mechanisms by which BM-MSCs
exert their anti-aging effects have not been extensively
investigated. The aim of the current study was to evaluate the
anti-aging effects of BM-MSCs (using a skin aging model induced by
intradermal injection of D-galactose), as well as the underlying
mechanisms.
Materials and methods
Experimental animals
Thirty male Sprague-Dawley (SD) rats (age, 4–6
weeks), weighing 200±20 g, were purchased from the Experimental
Animal Center, the General Hospital of the Chinese People
Liberation Army (PLA; Beijing, China). They were maintained
separately in a constant environment (room temperature, 20–22°C;
room humidity, 40–60%, with free access to food and water) under a
12-h light/dark cycle. All procedures were performed with the
approval of the Committee on the Use of Live Animals in Teaching
and Research of the 309th Hospital of PLA.
Preparation and culture of rat
BM-MSCs
BM-MSCs with green fluorescent protein (GFP) from SD
rats were purchased from Cyagen Biosciences (Santa Clara, CA, USA).
The cells were centrifuged at 500 × g (20–22°C) for 5 min
and suspended in α-Minimum Essential Medium (Thermo Fisher
Scientific, Grand Island, NY, USA) supplemented with Gibco 10%
fetal bovine serum (Thermo Fisher Scientific) and then incubated at
37°C with 5% CO2. The cells were passaged at a ratio of
1:2 with 0.25% trypsin (Thermo Fisher Scientific), reseeded into
new flasks and cultured until reaching 80% confluence. The fourth
generation of BM-MSCs/GFP was collected, suspended in
phosphate-buffered saline (PBS) and used to inject the rats.
Establishment of the rat model of skin
aging
SD rats were randomly divided into three groups
(n=10/group) as follows: Normal control group; aging model group;
MSC-treated group (subcutaneous injection). The rats in the control
group received a daily subcutaneous injection of sterile saline (1
ml), and the other three groups were administered a daily
subcutaneous injection of 15% D-galactose (1,000 mg/kg in 1 ml
sterile saline) for 8 weeks (20).
In vivo transplantation of
BM-MSCs
Following successful preparation of the rat model of
skin aging, rats in the MSC-treated groups were given
3×106/ml BM-MSCs/GFP in 1 ml PBS by subcutaneous
multi-point injection at the midline of the dorsum for 4 weeks,
which was administered once per week. Rats in the control and model
groups were given the same quantity of PBS according to the same
routine at the same time points. All animals were allowed free
access to water and food, and were observed daily to assess the
general condition of the rats.
Fluorescence analysis for labeling and
trafficking of BM-MSCs in skin
The mice were sacrificed by cervical dislocation
after the experiments, and the skin tissue was immediately
collected and prepared for cryosectioning. Frozen sections
(thickness, 30 µm) were sliced from the skin tissues of the dorsum
and the GFP-positive cells were observed under a fluorescence
microscope (IX-51; Olympus Corporation, Tokyo, Japan).
Morphological and ultrastructure
analysis
Skin tissue samples from rats in each group were
obtained and fixed in 4% paraformaldehyde for 12 h, then embedded
in paraffin, and sliced into 5-µm sections. The sections were
mounted on glass slides, dewaxed, rehydrated with distilled water,
stained with hematoxylin and eosin, and observed under a light
microscope. Van Gieson stain was used for collagen fiber
staining.
For ultrastructural examination by transmission
electron microscopy (TEM; FEI Tecnai™, Hillsboro, OR, USA), the
skin tissue samples from rats were fixed in 2.5% pentanediol-2%
paraformaldehyde in 0.1 mol/l sodium phosphate for 12 h at 4°C.
After three washes with PBS, the samples were then secondarily
fixed in 1% osmium tetroxide (osmic acid) for 1.5 h. The samples
were thoroughly dehydrated in solutions of ethanol at increasing
concentrations of 50, 70, 80, 90 and 100% for 10 min each, followed
by immersion in 50% acetone for 1 h and in 33% acetone for 3 h. The
samples were transferred into fresh epoxy resin and polymerized at
60°C for 48 h. Finally, the fixed tissues were thin-sectioned to
60–80 nm using an ultramicrotome and subsequently subjected to TEM
for ultrastructural study.
Measurements of oxidant/antioxidant
parameters
Skin tissue samples were homogenized in normal
saline, and sonicated twice to produce homogenates, followed by
centrifugation at 1,000 × g (20–22°C) for 10 min and 4,000 × g
(4°C) for 15 min. The supernatant was collected and total proteins
were quantified using the bicinchonic acid (BCA) method. The
malondialdehyde (MDA) content, and superoxide dismutase (SOD) and
glutathione peroxidase (GSH-Px) activities were determined using a
total-SOD (T-SOD) assay kit (cat. no. A001-1), a MDA assay kit
(cat. no. A003-1) and a GSH-PX assay kit (cat. no. A0005) according
to the manufacturer's instructions (Jiancheng Biotech Co., Ltd.,
Nanjing, China). Briefly, the SOD activity was measured using the
xanthine oxidase method at a wavelength of 550 nm, the MDA content
was determined using the thiobarbituric acid colorimetric method at
a wavelength of 532 nm and the GSH-Px activity was detected using
the chemical colorimetric method at a wavelength of 412 nm. These
oxidant/antioxidant indicators (SOD, MDA and GSH-Px activity) were
measured using a 752 UV spectrophotometer (Hitachi, Ltd., Tokyo,
Japan).
Statistical analysis
Statistical analysis was performed using SPSS
software (version 12.0; SPSS, Inc, Chicago, IL, USA) and the
quantitative data are presented as the mean ± standard deviation.
Comparisons between two groups were performed by unpaired Student's
t-test and P<0.05 was considered to indicate a statistically
significant difference.
Results
General condition of the animals
The rats in the model group showed lower mood,
reduced appetite and water consumption, reduced activity, dull fur
and decreased skin elasticity in comparison with the rats in the
control group. However, the MSC-treated groups showed improvement
of these symptoms.
Comparison of oxidant/antioxidant
parameters among groups
Compared with the control group, rats in the model
group had a significantly increased MDA content (P<0.01), and
significantly decreased serum GSH-Px and SOD activities (Table I; P<0.05). Treatment with
3×106 BM-MSCs by subcutaneous injection led to a
significant increase in serum GSH-Px and SOD activities (P<0.05)
and decrease in MDA content when compared with the model group
(P<0.01), indicating that these treatments markedly ameliorated
aging-induced oxidative stress in skin.
 | Table I.Comparison of oxidant/antioxidant
parameters among groups (n=10/group). |
Table I.
Comparison of oxidant/antioxidant
parameters among groups (n=10/group).
|
| Group (means ±
standard deviation) |
|---|
|
|
|
|---|
| Parameter | Control | Model | Mesenchymal stem
cell-treated |
|---|
| Malondialdehyde
content (nmol/ml) |
5.7±0.6 | 13.2±0.9a |
8.6±0.5b |
| Superoxide dismutase
activity (nU/ml) | 148.1±10.1 | 95.0±7.5c |
132.8±8.3d |
|
Glutathione-Peroxidase activity
(U/ml) | 236.1±18.3 |
169.0±11.5c |
201.9±15.1d |
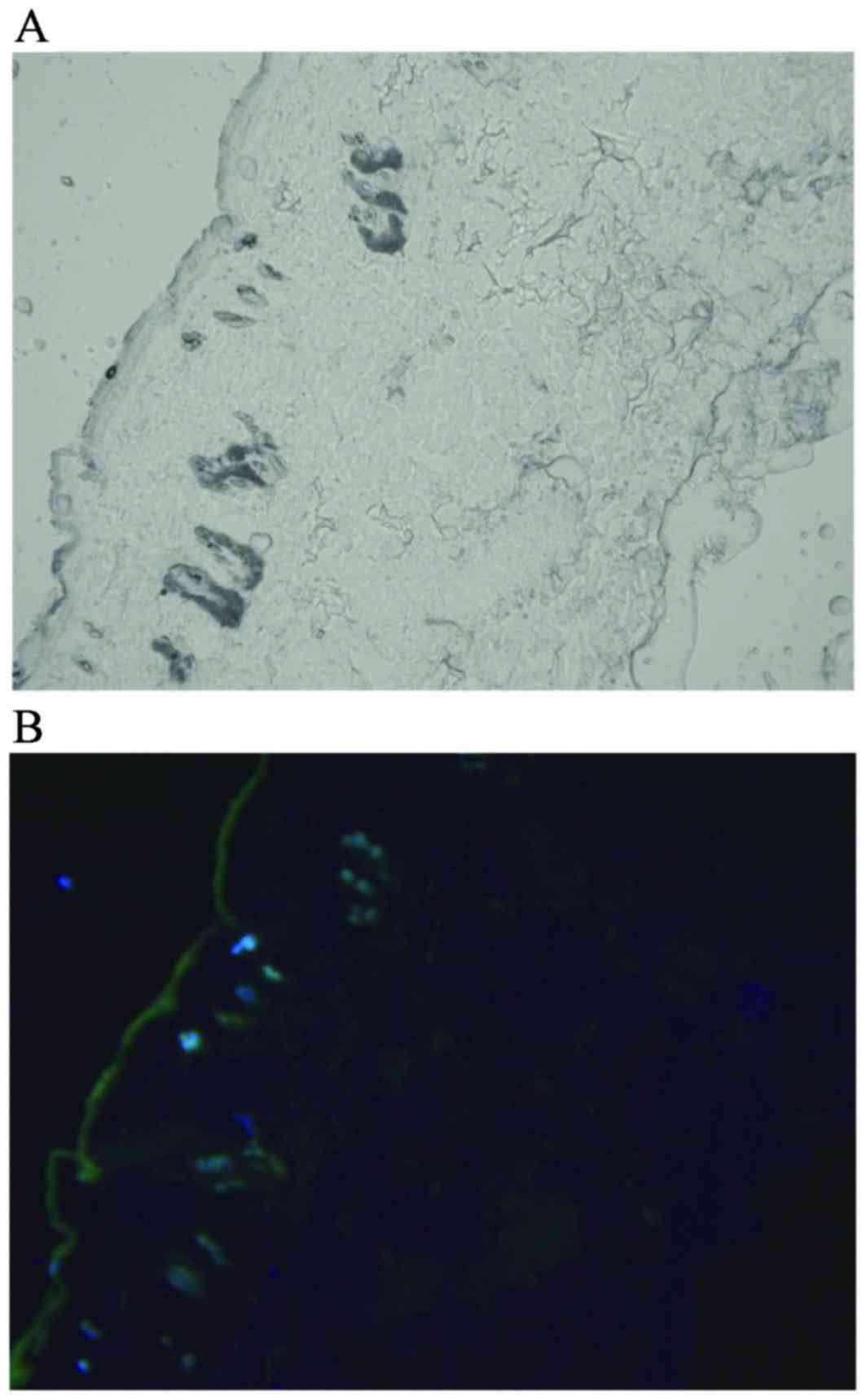
Trafficking of BM-MSCs in skin
Seven days after the final cell transplantation, the
GFP-positive cells were distributed in the dermis, which was
observed by fluorescence microscopy (Fig.
1).
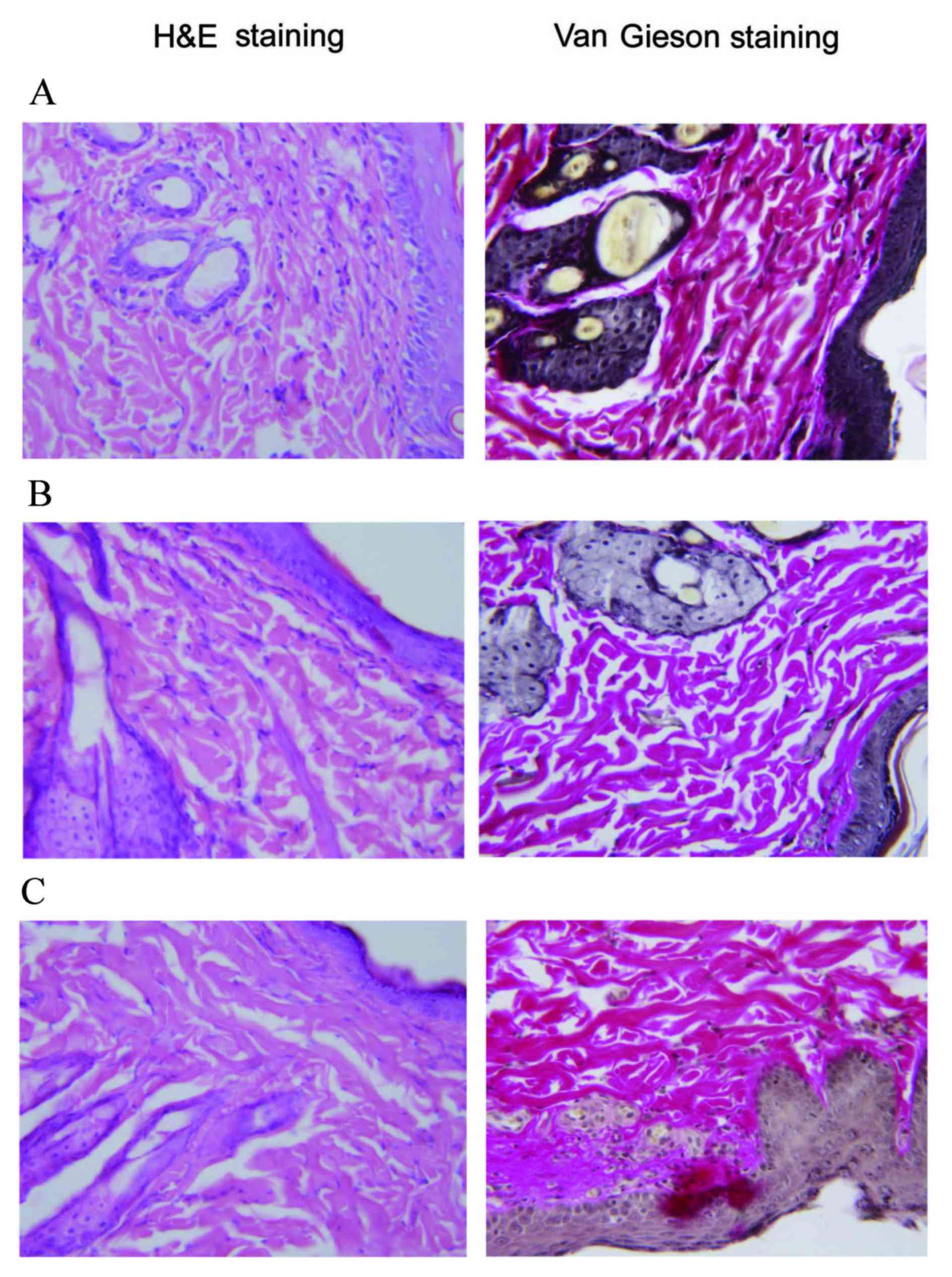
Histological and electron micrographic
findings
Histological sections of skin tissue samples were
stained with hematoxylin and eosin or Van Gieson stain, and
examined microscopically (Fig. 2).
Skin sections from the control group showed normal epidermal
morphology with regular arrangement of epidermal cell layers and
normal integral structures of the dermis. Collagen fibers were
neatly and densely arranged, and interwoven into a network. Rats in
the model group exhibited loosely arranged epidermal cell layers
and disorganized collagen fibers. Following BM-MSC treatment for 4
weeks, the histological abnormalities significantly improved, which
was evidenced by almost normal epidermal morphology and densely
arranged collagen fibers, which were similar to those observed in
the control group.
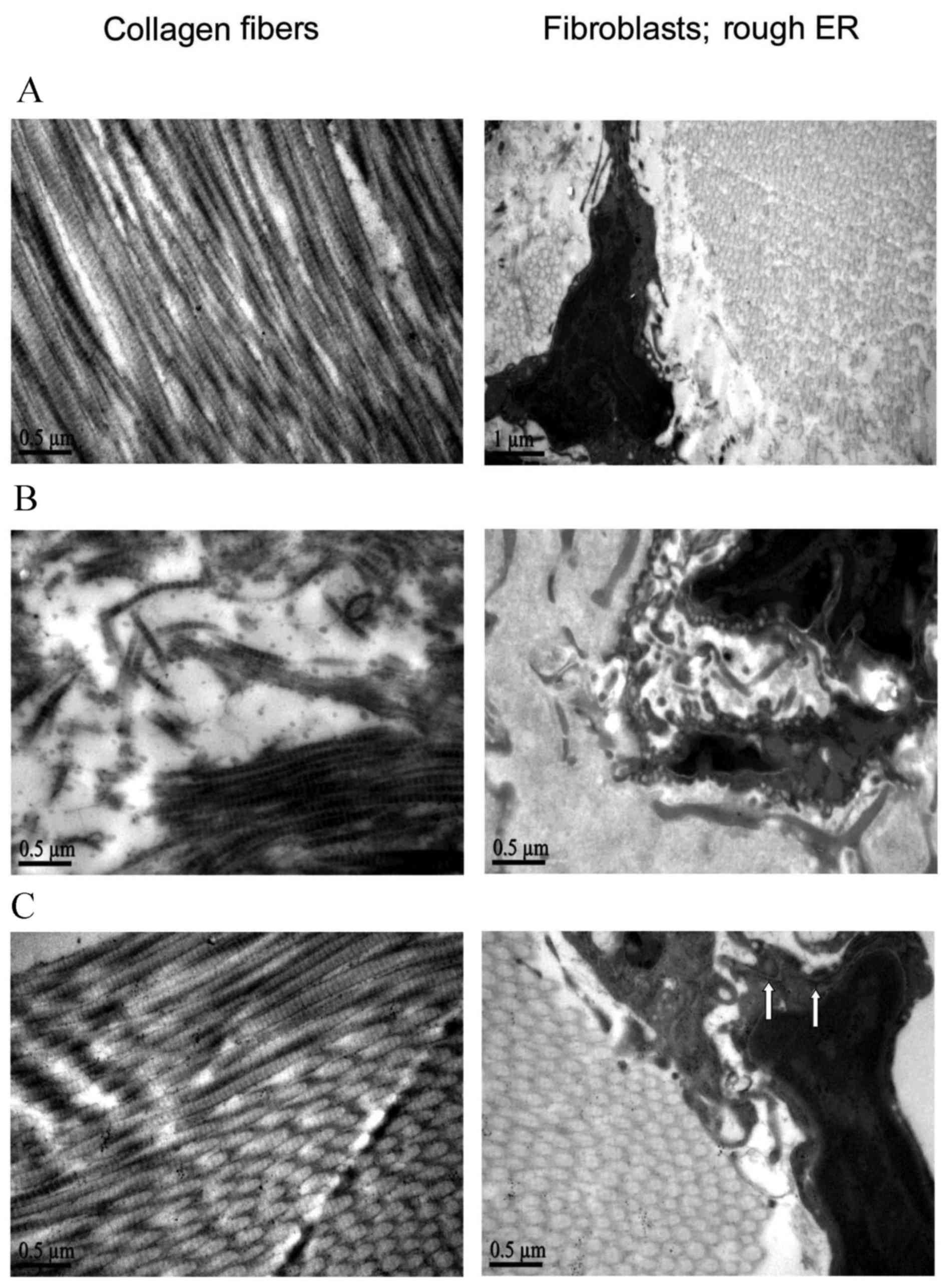
To assess the protective effects of BM-MSCs on
aging-induced skin lesions, ultrastructural analysis by TEM was
performed on the skin tissue samples (Fig.
3). Rats in the control group showed abundant, well-developed
rough endoplasmic reticulum and fibroblasts, surrounded by normal
collagen fibers of dense connective tissue, which were arranged
parallel to each other in neat rows within the dermal layer. In the
model group, the number of rough endoplasmic reticulum was
significantly reduced. The bundles of collagen fibers lost their
regular arrangement and presented as a loosely connected network.
In addition, broken or dissolved fibers were observed. Notably,
subsequent to 4-week BM-MSC treatment, the rats demonstrated marked
improvements in the ultrastructural abnormalities. Rough
endoplasmic reticulum with normal appearance were clearly visible
and the close arrangement of dermal collagen fibers was similar to
those in the control group.
Discussion
The present study provides evidence for the
anti-aging effects of BM-MSCs against D-galactose-induced skin
aging, as evidenced by substantial improvement in histological and
ultrastructural abnormalities, decreased expression of the
oxidant-associated marker, MDA and increases in the antioxidant
markers, SOD and GSH-Px. In addition, the BM-MSCs were retained 7
days after being injected into dermal tissues.
The dermal structure is primarily composed of
fibroblasts and extracellular matrix, which is rich in collagen and
elastic fibers. The collagen-rich extracellular matrix is
synthesized, organized and maintained by dermal fibroblasts. With
age, fibroblast cells gradually decrease in number, and exhibit
diminished capacity to synthesize collagen and elastic proteins,
resulting in laxity and decreased skin elasticity. Histological and
ultrastructural studies have revealed major alterations in the
dermal extracellular matrix in aged skin, with reduced fibroblasts
and decreased collagen production (5).
Chronic injection with D-galactose at a wide range of dosages has
been used to establish a skin aging model in rodents that resembles
intrinsic aging, characterized by typical changes of aging skin in
behavior, phenotype, histological appearance, as well as expression
of senescence markers (e.g., SOD and MDA) (20–22). In the
current study, rats in the model group were administered a daily
subcutaneous injection of D-galactose (1,000 mg/kg) for 8 weeks.
These rats showed poor mood, reduced appetite and water
consumption, reduced activity, dull fur and decreased skin
elasticity. In addition to typical symptoms of skin aging, this
model appeared to exhibit an increase in MDA content and decrease
in SOD and GSH-Px activity, and pathological characteristics of
aged skin (including loosely arranged epidermal cell layers and
disorganized collagen fibers) suggesting the successful
establishment of a skin aging model in SD rats in this study.
The beneficial effect of MSCs on disease has been
investigated in a variety of animal models and clinical trials. In
particular, BM-MSCs contribute to cutaneous wound healing by
promoting epidermal regeneration, as well as accelerating
endothelial cell formation (23,24). BM-MSCs
were originally described as plastic-adherent fibroblast-like cells
that are capable of differentiating into a variety of cell types,
including epidermal cells, endothelial cells and fibroblasts in the
presence of appropriate factors in vitro and in vivo
(15). Furthermore, MSCs enhance the
functions of keratinocytes and dermal fibroblasts in a paracrine
fashion (25). Thus, the potential of
BM-MSCs in the rejuvenation of aged skin were evaluated. In the
present study, BM-MSC treatment for 4 weeks restored
D-galactose-induced histological abnormalities to levels similar to
those of the controls. The present study hypothesized that through
releasing cytokines and growth factors, such as vascular
endothelial growth factor, platelet-derived growth factor,
transforming growth factor-β, BM-MSCs promote the proliferation of
fibroblasts, which stimulate production of collagen and elastic
fibers (25). As a consequence,
transplantation of BM-MSCs results in the activation of
fibroblasts, enhancement of extracellular matrix, and thereby
increases skin elasticity and reduces the appearance of wrinkles.
Indeed, experimental studies of cutaneous wounds treated with MSCs
revealed a relatively low level of direct engraftment of
transplanted cells into the epidermis, with fewer cells retained
over time (15). The low engraftment
rate of transplanted cells and short-term retention rates are
currently considered as barriers that potentially diminish the
benefits of cell therapy (26). Thus,
repeated transplants are proposed to enable the maximum therapeutic
effect of MSCs. In the current study, rats in the MSC-treated
groups were administered 3×106/ml BM-MSCs for 4 weeks,
once per week. Seven days after the final cell transplantation,
GFP-positive cells were observed to be distributed in the dermis
under a fluorescence microscope. Consistently, MSCs have been
reported to be detectable at 28 days after being injected into
dermal tissues despite a low retention of transplanted cells
(21). These data further indirectly
confirm the anti-aging effects of MSCs in skin.
Oxidative stress is currently considered as a
fundamental causative factor of aging, leading to intracellular
lipid peroxidation, abnormal protein oxidation reactions and the
accumulation of oxidative cellular damage (6). Aging appears to be a consequence of the
imbalance between overwhelming ROS production beyond the scavenging
ability, and substantial reduction in antioxidant defense during
the aging process (27). Thus,
attempts have been made to reduce excessive ROS in aged skin with
the aim of improving, or even rejuvenating, aged skin (28). ROS degrade polyunsaturated lipids to
form MDA, causing peroxidative tissue damage. SOD, an important
antioxidant enzyme, functions to catalyze the dismutation of
superoxide radicals to hydrogen peroxide and oxygen, defending the
skin against ROS-induced oxidative deterioration of DNA, proteins
and lipids by scavenging remaining ROS in cells (27). GSH-Px is a glutathione peroxidase that
functions as an anti-oxidant by catalyzing the reduction of
hydrogen peroxide to water and preventing lipid peroxidation
(29). Zhang et al (21) demonstrated that D-galactose induced
oxidative stress in mouse skin, as shown by the expression levels
of increased MDA and decreased SOD. Consistent with these findings,
the current study demonstrated that skin D-galactose-induced aging
produced large quantities of lipid peroxide MDA accompanied by a
decrease in the antioxidant enzymes, SOD and GSH-Px. Notably, MSCs
have been reported to exhibit antioxidative activity under various
conditions, by increasing the SOD and glutathione levels, and
modulating the activation of antioxidant-associated proteins
(30). Furthermore, MSCs exhibit
potent antioxidant effects that provide protection for dermal
fibroblasts and keratinocytes against oxidative stress, and
consequently accelerate skin wound healing (31–33). In the
present study, BM-MSCs were shown to cause a greater increase in
serum GSH-Px and SOD activities, and a significant decrease in MDA
content when compared with the model group. This indicates that
BM-MSCs promote an antioxidant response and ameliorate
aging-induced oxidative stress in the skin.
In conclusion, injection of BM-MSCs significantly
improve D-galactose-induced histological and ultrastructural
abnormalities of the skin, by promoting an antioxidant response and
by ameliorating aging-induced oxidative stress in aged skin. Thus,
BM-MSCs may be beneficial in the rejuvenation of aged skin.
However, further investigation is required to determine the exact
molecular mechanisms underlying the antioxidant effects of
MSCs.
Acknowledgements
The present study was supported by the National
Natural Science Foundation Project (grant nos. 81372051 and
81670009), the Medical and Senile Disease Foundation Project of
General Staff Headquarters (grant no. ZCWS14B06), the Beijing
Science and Technology ‘Capital Special’ Program (grant no.
Z151100004015199), and the Military Medical Science and Technology
Youth Training Program (grant no. 15QNP049).
References
|
1
|
Quan T and Fisher GJ: Role of
age-associated alterations of the dermal extracellular matrix
microenvironment in human skin aging: A mini-review. Gerontology.
61:427–434. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gunin AG, Kornilova NK, Vasilieva OV and
Petrov VV: Age-related changes in proliferation, the numbers of
mast cells, eosinophils, and cd45-positive cells in human dermis. J
Gerontol A Biol Sci Med Sci. 66:385–392. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McCullough JL and Kelly KM: Prevention and
treatment of skin aging. Ann N Y Acad Sci. 1067:323–331. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hwang KA, Yi BR and Choi KC: Molecular
mechanisms and in vivo mouse models of skin aging associated with
dermal matrix alterations. Lab Anim Res. 27:1–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Varani J, Dame MK, Rittie L, Fligiel SE,
Kang S, Fisher GJ and Voorhees JJ: Decreased collagen production in
chronologically aged skin: Roles of age-dependent alteration in
fibroblast function and defective mechanical stimulation. Am J
Pathol. 168:1861–1868. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cui H, Kong Y and Zhang H: Oxidative
stress, mitochondrial dysfunction, and aging. J Signal Transduct.
2012:6463542012.PubMed/NCBI
|
|
7
|
Panich U, Sittithumcharee G, Rathviboon N
and Jirawatnotai S: Ultraviolet radiation-induced skin aging: The
role of DNA damage and oxidative stress in epidermal stem cell
damage mediated skin aging. Stem Cells Int. 2016:73706422016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bhatia-Dey N, Kanherkar RR, Stair SE,
Makarev EO and Csoka AB: Cellular senescence as the causal nexus of
aging. Front Genet. 7:132016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lv FJ, Tuan RS, Cheung KM and Leung VY:
Concise review: The surface markers and identity of human
mesenchymal stem cells. Stem Cells. 32:1408–1419. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chamberlain G, Fox J, Ashton B and
Middleton J: Concise review: Mesenchymal stem cells: Their
phenotype, differentiation capacity, immunological features, and
potential for homing. Stem Cells. 25:2739–2749. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang J, Liao L and Tan J:
Mesenchymal-stem-cell-based experimental and clinical trials:
Current status and open questions. Expert Opin Biol Ther.
11:893–909. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Isakson M, de Blacam C, Whelan D, McArdle
A and Clover AJ: Mesenchymal Stem Cells and Cutaneous Wound
Healing: Current Evidence and Future Potential. Stem Cells Int.
2015:8310952015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hocking AM and Gibran NS: Mesenchymal stem
cells: Paracrine signaling and differentiation during cutaneous
wound repair. Exp Cell Res. 316:2213–2219. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen JS, Wong VW and Gurtner GC:
Therapeutic potential of bone marrow-derived mesenchymal stem cells
for cutaneous wound healing. Front Immunol. 3:1922012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu H, McTaggart SJ, Johnson DW and Gobe
GC: Original article anti-oxidant pathways are stimulated by
mesenchymal stromal cells in renal repair after ischemic injury.
Cytotherapy. 14:162–172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Q, Luo Z, He S, Peng X, Xiong S, Wang
Y, Zhong X, Zhou X, Eisenberg CA and Gao BZ: Conditioned serum-free
medium from umbilical cord mesenchymal stem cells has
anti-photoaging properties. Biotechnol Lett. 35:1707–1714. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim WS, Park BS, Park SH, Kim HK and Sung
JH: Antiwrinkle effect of adipose-derived stem cell: Activation of
dermal fibroblast by secretory factors. J Dermatol Sci. 53:96–102.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim WS, Park BS and Sung JH: Protective
role of adipose-derived stem cells and their soluble factors in
photoaging. Arch Dermatol Res. 301:329–336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhen YZ, Lin YJ, Li KJ, Zhang GL, Zhao YF,
Wang MM, Wei JB, Wei J and Hu G: Effects of rhein lysinate on
D-galactose-induced aging mice. Exp Ther Med. 11:303–308.
2016.PubMed/NCBI
|
|
21
|
Zhang S, Dong Z, Peng Z and Lu F:
Anti-aging effect of adipose-derived stem cells in a mouse model of
skin aging induced by D-galactose. PLoS One. 9:e975732014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wei H, Li L, Song Q, Ai H, Chu J and Li W:
Behavioural study of the D-galactose induced aging model in
C57BL/6J mice. Behav Brain Res. 157:245–251. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu Y, Zhao RC and Tredget EE: Concise
review: Bone marrow-derived stem/progenitor cells in cutaneous
repair and regeneration. Stem Cells. 28:905–915. 2010.PubMed/NCBI
|
|
24
|
Aoki S, Toda S, Ando T and Sugihara H:
Bone marrow stromal cells, preadipocytes, and dermal fibroblasts
promote epidermal regeneration in their distinctive fashions. Mol
Biol Cell. 15:4647–4657. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee SH, Jin SY, Song JS, Seo KK and Cho
KH: Paracrine effects of adipose-derived stem cells on
keratinocytes and dermal fibroblasts. Ann Dermatol. 24:136–143.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cassino TR, Drowley L, Okada M, Beckman
SA, Keller B, Tobita K, Leduc PR and Huard J: Mechanical loading of
stem cells for improvement of transplantation outcome in a model of
acute myocardial infarction: The role of loading history. Tissue
Eng Part A. 18:1101–1108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Treiber N, Maity P, Singh K, Ferchiu F,
Wlaschek M and Scharffetter-Kochanek K: The role of manganese
superoxide dismutase in skin aging. Dermatoendocrinol. 4:232–235.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen H, Long Y and Guo L: Antiaging Effect
of Inula britannica on Aging Mouse Model Induced by D-Galactose.
Evid Based Complement Alternat Med. 2016:60490832016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sandhu SK and Kaur G: Alterations in
oxidative stress scavenger system in aging rat brain and
lymphocytes. Biogerontology. 3:161–173. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim WS, Park BS and Sung JH: The
wound-healing and antioxidant effects of adipose-derived stem
cells. Expert Opin Biol Ther. 9:879–887. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim Y, Jo SH, Kim WH and Kweon OK:
Antioxidant and anti-inflammatory effects of intravenously injected
adipose derived mesenchymal stem cells in dogs with acute spinal
cord injury. Stem Cell Res Ther. 6:2292015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ayatollahi M, Hesami Z, Jamshidzadeh A and
Gramizadeh B: Antioxidant Effects of Bone Marrow Mesenchymal Stem
Cell against Carbon Tetrachloride-Induced Oxidative Damage in Rat
Livers. Int J Organ Transplant Med. 5:166–173. 2014.PubMed/NCBI
|
|
33
|
Zhuo W, Liao L, Xu T, Wu W, Yang S and Tan
J: Mesenchymal stem cells ameliorate ischemia-reperfusion-induced
renal dysfunction by improving the antioxidant/oxidant balance in
the ischemic kidney. Urol Int. 86:191–196. 2011. View Article : Google Scholar : PubMed/NCBI
|