Introduction
Stem cells in biological research provide a
significant source of information and, thus, contribute to the
development of novel therapeutic strategies. In particular, the
ability to cultivate stem cells and human hematopoietic progenitor
cells in vitro is the fundamental basis for investigating
hematopoiesis. An improved understanding of the mechanisms that
regulate cell proliferation and differentiation during the stages
of hematopoiesis would further elucidate the molecular
characteristics of diseases (which are characterized by excessive
expansion or a functional defect of certain immature blood
components), and facilitate the identification of substances that
are able to specifically protect healthy cells from the action of
cytotoxic drugs.
Hematopoietic stem and progenitor cells (HSPCs) may
be isolated from peripheral blood (PB), bone marrow (BM) or
umbilical cord blood (CB) (1).
Although significant improvements to cell
cryopreservation procedures have been achieved (2), the improvement of current
cryopreservation protocols that lead to a high mortality rate after
thawing for the crystal formations that arise during freezing is a
primary goal. Specifically, fast cooling forms intracellular ice
crystals, which results in cell destruction and slow cooling forms
ice crystals in the extracellular space, with consequent cellular
dehydration. Selection of a cryoprotectant, as well as a suitable
freezing rate serves to protect cells from these adverse effects
(3,4).
Cryoprotectants are divided into two classes:
Penetrating and nonpenetrating (5).
The penetrating cryoprotectants include glycerol and
1,2-propanediol and dimethyl sulfoxide (Me2SO); the
latter is commonly used for HSPC cryopreservation (6). The non penetrating cryoprotectants
comprise polyvinyl pyrrolidone, trehalose, fructose, sucrose and
glucose.
Trehalose is a non-toxic disaccharide of glucose
that preserves the structural integrity of the cells during
freezing and thawing (7).
Specifically, trehalose is found in numerous organisms, such as
nematodes and yeasts, which are capable of surviving during
freezing and drying (8,9). Previous studies have used this
disaccharide for cryopreservation of human cells, such as platelets
(10), red blood cells (11), sperm (12), oocytes (13), pancreatic islets (14) and fetal skin (15). Furthermore, the use of an alternative
protocol with trehalose for clinical applications to cryopreserve
stem cells obtained from CB and bone marrow (BM) has previously
been reported in the literature (16,17) and in a
study by Scheinkonig et al (18) from mobilized PB stem cells (PBSCs).
Conversely, in the current study, trehalose was administered, only
for research purposes, to cryopreserve pure HSPCs, and not
mobilized HSPCs, that were isolated from the PB of healthy
individuals.
Materials and methods
Cytokines, antibodies and
chemicals
Ficoll-Hypaque (density, 1.077 g/ml) was purchased
from Pharmacia Biotech, Uppsala, Sweden. A CD34 MultiSort kit
human, midiMACS separator, MACS multistand, LS columns, and
anti-CD34 fluorescein isothiocyanate (FITC), human (clone: AC136;
cat. no. 130-081-001), anti-CD61 PE, human (clone: Y2/51; cat. no.
130-081-501), mouse IgG2a-FITC isotype control (cat. no.
130-091-837) and mouse IgG1-PE isotype control (cat. no.
130-092-212) antibodies (all dilutions, 1:10) were purchased from
Miltenyi Biotec, Inc. (Auburn, CA, USA); BIT 9500 Serum Substitute
(Stem cell Technologies, Inc., Vancouver, BC, Canada); Gibco
Iscove's Modified Dulbecco's Medium (IMDM) was obtained from Thermo
Fisher Scientific, Inc. (Waltham, MA, USA) and recombinant human
TPO was from PeproTech, Inc. (Rocky Hill, NJ, USA).
Me2SO, D-(+)-Trehalose dihydrate, catalase from bovine
liver, lipoprotein, low density lipoproteins (LDLs) from human
plasma, trypan blue and May-Grünwald-Giemsa were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Nalgene® Mr.
Frosty® Cryo 1°C Freezing Containers were purchased from
Thermo Fisher Scientific, Inc.
Purification and characterization of
CD34+ cells
Hematopoietic stem and progenitor cells (HSPCs) were
obtained from the PB of 80 healthy donors supplied by IOM SPA
(Viagrande, Italy) under an approved Institutional Review Board
protocol (project ID code: 454_1; 6 February 2008, IOM
Institutional Review Board, Viagrande, Italy) and subsequent to
obtaining informed consent.
Briefly, mononuclear cells (MNCs) were isolated from
PB (20–30 ml) by Ficoll-Hypaque. After recovery of the cell
suspension, 150–300×106 MNCs were magnetically labeled
with CD34 Micro Beads and loaded onto a MACS® Column,
which was placed in the magnetic field of the midi MACS separator.
The CD34+ cells are retained within the column and
unlabeled cells run through. After the column was removed from the
magnetic field, the magnetically retained CD34+ cells
were eluted as the positively selected cell fraction. The purity
was evaluated by flow cytometry of the FITC-labeled anti-CD34 FITC
antibody (dilution, 1:10) according to the manufacturer's
instructions. Cells were analyzed by FACSCalibur (BD Biosciences)
using BD CellQuest™ Pro (version 6.0; BD Biosciences) software for
fluorescence intensity analysis. The CD34+ morphology
was evaluated by May-Grünwald-Giemsa staining. Cells were
cytocentrifuged onto glass slides, stained with May-Grünwald-Giemsa
and observed by light microscopy; the percentage of
CD34+ cells ranged from 89 to 98%.
Freezing media preparation for
cryopreservation
Freezing media preparation for cryopreservation
of human CD34+ cells with different trehalose
concentrations or the classical method
Freezing media were freshly made with different
D-(+)-trehalose concentrations (1.25, 1,0.6 or 0.3 M) or with a
medium containing 90% fetal bovine serum (FBS) and 10%
Me2SO. Dilutions were prepared using serum-free medium
(IMDM) from a 2.5-M trehalose stock solution in IMDM. The freezing
media and cryogenic vials (NalgeNunc International, Penfield, NY,
USA) were cooled prior to use.
Freezing media preparation for cryopreservation
of human CD34+ cells with different cryoprotectants for
short- and long-term cryopreservation
Five freezing media were freshly prepared as
follows: 10% Me2SO + 90% FBS; 1M trehalose; 1M trehalose
+ 10% Me2SO; 1M trehalose + 100 µg/ml catalase; and 1 M
trehalose + 100 µg/ml catalase + 10% Me2SO. The freezing
media and cryogenic vials were cooled prior to use. The trehalose
and catalase were freshly prepared in IMDM.
Cryopreservation of human CD34+
cells
The optimal concentration of CD34+ cells
(200,000) were frozen in cryogenic vials in a Nalgene®
controlled-rate freezing container (NalgeNunc International) by a
gradual addition of freezing medium in a final volume of 200 µl.
The freezing container contained 100% isopropyl alcohol that
provided a 1°C/min cooling rate when stored at-80°C in a controlled
freezer for the initial freezing cycle prior to placement in a
liquid nitrogen freezer (−196°C) until final use. The
CD34+ cells were cryopreserved for a short (20 days) and
longer (3 months) duration.
Thawing of human CD34+ cells
Frozen cells were rapidly thawed in a water bath at
37°C, gently shaken and removed when the ice crystals dissolved.
Subsequently cell viability and the ability to differentiate into
megakaryocytes (MKs) were evaluated. Cell viability was assessed
using the dye exclusion test with trypan blue and counts were
performed in triplicate. Percentages of viable cells after thawing
were normalized to non-cryopreserved cells (NT, 100% viability)
using the following equation: Cell viability (%) = number of
recovered cells after thawing ×100/number of non-cryopreserved
cells.
MK unilineage cultures
MK differentiation was induced by growing
CD34+ for 14 days in BIT 9500 Serum Substitute in the
presence of human LDL and 100 ng/ml thrombopoietin. Cells were
incubated at 37°C for 14 days in a fully humidified atmosphere of
5% CO2. The MK differentiation stage was evaluated after
thawing by flow cytometry of typical anti-CD61+ PE
antibody (dilution, 1:10).
Statistical analysis
Data were analyzed using Student's t-test, and the
results were expressed as the mean ± standard deviation of separate
experiments. P<0.05 was considered to indicate a statistically
significant difference.
Results
The aim of the current study was to develop an
effective cryopreservation technique for pure HSPCs obtained from
PB to replace the standard method that uses Me2SO. HSPCs
were isolated from healthy individuals after acquiring informed
consent. The expression levels of the CD34 surface stem marker was
evaluated by flow cytometry obtaining a percentage of
CD34+ cells between 89 and 98%. Morphological
observations subsequent to May-Grünwald-Giemsa staining correlated
with the flow cytometry data.
As the quality of the cryopreserved cells is also
cell concentration-dependent (19),
human CD34+ were initially frozen at different cell
concentrations (100,000, 150,000 and 200,000) with a medium
containing FBS and Me2SO. Subsequent to thawing, cell
viability was assessed using the dye exclusion test with trypan
blue. The results revealed that the optimal concentration to
cryopreserve CD34+ was 200,000 (data not shown).
Subsequently, the effect of cryopreservation on
CD34+ cells treated with four different trehalose
concentrations compared with Me2SO was evaluated
(Fig. 1). The findings indicated that
freezing with 1M trehalose provided improved cryoprotection to
CD34+ when compared with the standard freezing procedure
using Me2SO.
Furthermore, as it has been reported in previous
studies (20,21) that freezing with trehalose in
combination with an antioxidant (catalase) or with Me2SO
could improve cell viability, CD34+ cells were
cryopreserved for a short (20 days) and long (3 months) duration,
with a medium containing different combinations of catalase,
Me2SO and trehalose. The current data indicate that,
after thawing, the number and viability of the CD34+
cells was higher in all the samples cryopreserved with only 1M
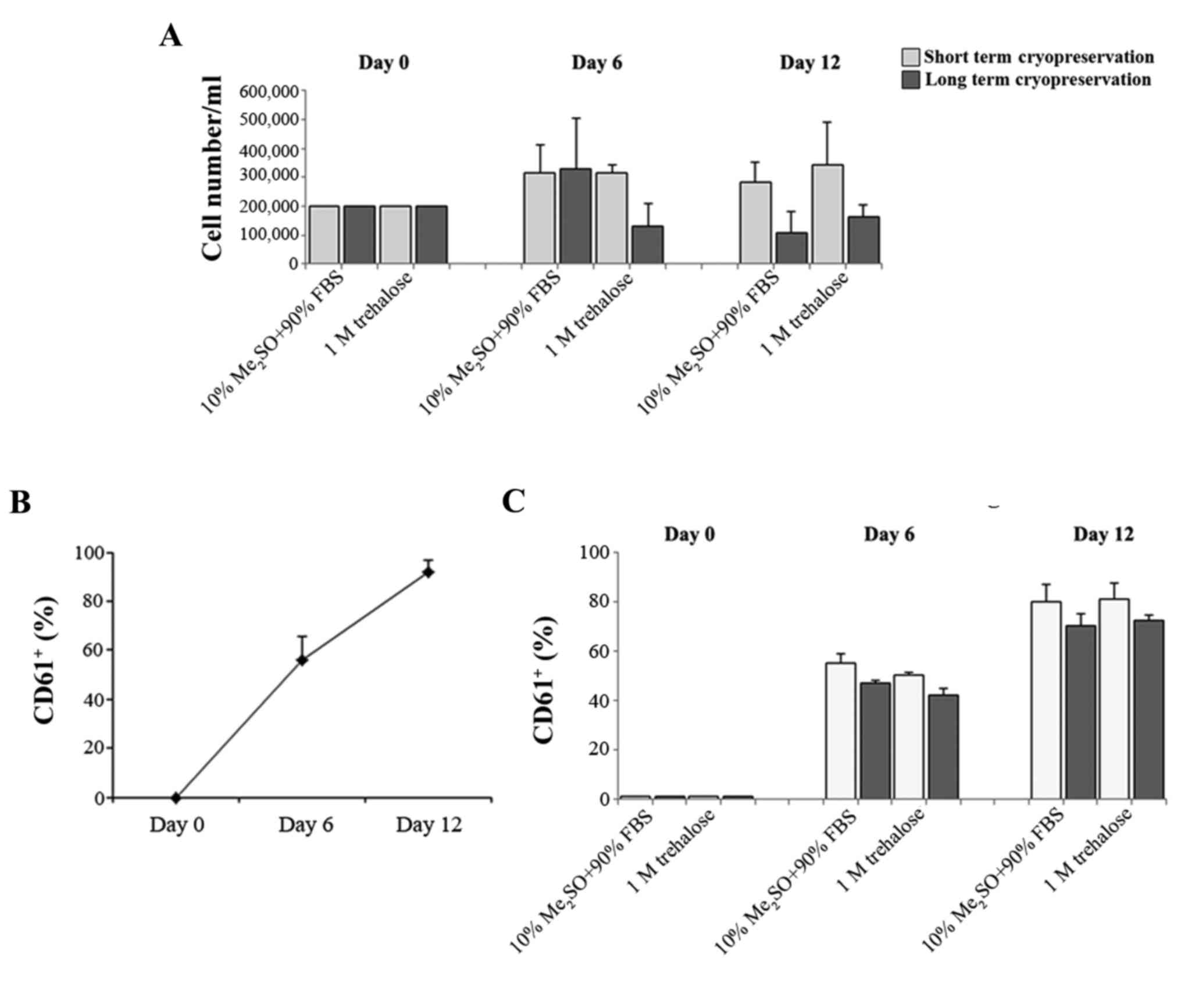
trehalose (Fig. 2).
The hematopoietic stem cell is also responsible for
the formation of differentiated cells in the blood that must be
continually replaced through the proliferation and differentiation
of immature forms. Megakaryopoiesis is a process of hematopoietic
differentiation previously investigated by the current authors
(22). Therefore, the ability of the
fresh CD34+ cells and those obtained after thawing to
proliferate and differentiate in MKs was compared. These cells were
previously cryopreserved with 1M trehalose or Me2SO for
short (20 days) and long (3 months) cryopreservation durations. MK
differentiation was induced by growing CD34+ for 14 days
and evaluated by flow cytometry of the typical
anti-CD61+ antibody. CD34+ cells that were
previously frozen with trehalose retained the ability to form MK
subsequent to thawing (Fig. 3A). In
addition the percentage of CD61+ cells obtained after
thawing using the two different methods (Fig. 3C) is similar to that of MK produced
from fresh CD34+ cells (Fig.
3B).
Discussion
Hematopoietic stem cells enable investigation of the
underlying mechanisms of hematopoietic development for the ability
to differentiate into different cell types. In addition, they may
be used for drug development and as model systems to evaluate
certain diseases for which there are no effective treatments
currently available.
Cryopreservation allows cell preservation overlong
periods by exposure to extremely low temperatures. Although this
procedure causes suspension of metabolic reactions, during the
freezing and thawing phases considerable physical changes occur,
which can be detrimental to cell survival. As a result of
cryopreservation, there is marked loss of cell viability and
function. Therefore, the addition of a cryoprotectant to the cells
is important. Among the cryoprotectants used, trehalose, which is a
sugar that is utilized in cell cryopreservation, is significant
(10–15). Trehalose is utilized in many fields,
such as in the pharmaceutical industry (contained in various
commercially available therapeutic products), the food industry (in
confectionary products) and in the cosmetics industry (for example,
in bath oils, creams and lotions) (23). Furthermore, the efficacy of trehalose
in clinical cryopreservation of human stem cells from cord blood
(24–26), bone marrow and mobilized PBSCs
(18) has been widely demonstrated.
Conversely, in the present study trehalose was used only for
research purposes to cryopreserve pure and not mobilized PBSCs, in
order to identify a more efficient protocol, which avoids cell loss
that occurs during the normal course of cryopreservation. It was
identified that 1M trehalose resulted in improved cryoprotection to
CD34+ when compared with the standard freezing procedure
that uses Me2SO. Furthermore, freezing with trehalose in
combination with catalase or Me2SO did not increase cell
viability. In addition, maintenance of the viability and ability of
CD34+ cells to differentiate into MKs after thawing at
short and long-term of cryopreservation was observed.
In conclusion the data indicate that the use of
trehalose allows a greater number of hematopoietic stem cells to be
obtained after thawing. The basis of a suitable cryopreservation
protocol was established in the current study. However, future
investigations are required to assess the repeatability on a
commercial scale.
Acknowledgements
The authors would like to thank Mr. Gabriele
Anastasi for his technical assistance.
References
|
1
|
Eaves CJ: Hematopoietic stem cells:
Concepts, definitions, and the new reality. Blood. 125:2605–2613.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Berz D, McCormack EM, Winer ES, Colvin GA
and Quesenberry PJ: Cryopreservation of hematopoietic stem cells.
Am J Hematol. 82:463–472. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Valeri CR and Pivacek LE: Effects of the
temperature, the duration of frozen storage, and the freezing
container on in vitro measurements in human peripheral blood
mononuclear cells. Transfusion. 36:303–308. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cilloni D, Garau D, Regazzi E, Sammarelli
G, Savoldo B, Caramatti C, Mangoni L, Rizzoli V and Carlo-Stella C:
Primitive hematopoietic progenitors within mobilized blood are
spared by uncontrolled rate freezing. Bone Marrow Transplant.
23:497–503. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McGann LE: Differing actions of
penetrating and nonpenetrating cryoprotective agents. Cryobiology.
15:382–390. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abrahamsen JF, Bakken AM and Bruserud Ø:
Cryopreserving human peripheral blood progenitor cells with
5-percent rather than 10-percent DMSO results in less apoptosis and
necrosis in CD34+ cells. Transfusion. 42:1573–1580.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jain NK and Roy I: Trehalose and protein
stability. Curr Protoc Protein Sci. Chapter 4, Unit 4.9. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Behm CA: The role of trehalose in the
physiology of nematodes. Int J Parasitol. 27:215–229. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sano F, Asakawa N, Inoue Y and Sakurai M:
A dual role for intracellular trehalose in the resistance of yeast
cells to water stress. Cryobiology. 39:80–87. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wolkers WF, Walker NJ, Tablin F and Crowe
JH: Human platelets loaded with trehalose survive freeze-drying.
Cryobiology. 42:79–87. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pellerin-Mendes C, Million L,
Marchand-Arvier M, Labrude P and Vigneron C: In vitro study of the
protective effect of trehalose and dextran during freezing of human
red blood cells in liquid nitrogen. Cryobiology. 35:173–186. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen Y, Foote RH and Brockett CC: Effect
of sucrose, trehalose, hypotaurine, taurine, and blood serum on
survival of frozen bull sperm. Cryobiology. 30:423–431. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eroglu A, Toner M and Toth TL: Beneficial
effect of microinjected trehalose on the cryosurvival of human
oocytes. Fertil Steril. 77:152–158. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Beattie GM, Crowe JH, Lopez AD, Cirulli V,
Ricordi C and Hayek A: Trehalose: A cryoprotectant that enhances
recovery and preserves function of human pancreatic islets after
long-term storage. Diabetes. 46:519–523. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Erdag G, Eroglu A, Morgan J and Toner M:
Cryopreservation of fetal skin is improved by extracellular
trehalose. Cryobiology. 44:218–228. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Motta JP, Paraguassú-Braga FH, Bouzas LF
and Porto LC: Evaluation of intracellular and extracellular
trehalose as a cryoprotectant of stem cells obtained from umbilical
cord blood. Cryobiology. 68:343–348. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang XB, Li K, Yau KH, Tsang KS, Fok TF,
Li CK, Lee SM and Yuen PM: Trehalose ameliorates the
cryopreservation of cord blood in a preclinical system and
increases the recovery of CFUs, long-term culture-initiating cells,
and nonobese diabetic-SCID repopulating cells. Transfusion.
43:265–272. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Scheinkönig C, Kappicht S, Kolb HJ and
Schleuning M: Adoption of long-term cultures to evaluate the
cryoprotective potential of trehalose for freezing hematopoietic
stem cells. Bone Marrow Transplant. 34:531–536. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alencar S, Garnica M, Luiz RR, Nogueira
CM, Borojevic R, Maiolino A and Dutra HS: Cryopreservation of
peripheral blood stem cell: The influence of cell concentration on
cellular and hematopoietic recovery. Transfusion. 50:2402–2412.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Limaye LS and Kale VP: Cryopreservation of
human hematopoietic cells with membrane stabilizers and
bioantioxidants as additives in the conventional freezing medium. J
Hematother Stem Cell Res. 10:709–718. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sasnoor LM, Kale VP and Limaye LS: A
combination of catalase and trehalose as additives to conventional
freezing medium results in improved cryoprotection of human
hematopoietic cells with reference to in vitro migration and
adhesion properties. Transfusion. 45:622–633. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vicari L, Martinetti D, Buccheri S,
Colarossi C, Aiello E, Stagno F, Villari L, Cavalli M, Di Raimondo
F, Gulisano M, et al: Increased phospho-mTOR expression in
megakaryocytic cells derived from CD34+ progenitors of
essential thrombocythaemia and myelofibrosis patients. Br J
Haematol. 159:237–240. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ohtake S and Wang YJ: Trehalose: Current
use and future applications. J Pharm Sci. 100:2020–2053. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rodrigues JP, Paraguassú-Braga FH,
Carvalho L, Abdelhay E, Bouzas LF and Porto LC: Evaluation of
trehalose and sucrose as cryoprotectants for hematopoietic stem
cells of umbilical cord blood. Cryobiology. 56:144–151. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Motta JP, Gomes BE, Bouzas LF,
Paraguassú-Braga FH and Porto LC: Evaluation of bioantioxidants in
cryopreservation of umbilical cord blood using natural
cryoprotectants and low concentrations of dimethylsulfoxide.
Cryobiology. 60:301–307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen G, Yue A, Ruan Z, Yin Y, Wang R, Ren
Y and Zhu L: comparison of the effects of different cryoprotectants
on stem cells from umbilical cord blood. Stem Cells Int.
2016:13967832016. View Article : Google Scholar : PubMed/NCBI
|