Introduction
Ischemia-reperfusion injury (IRI) refers to the
phenomenon in which the injury to tissues or organs is aggravated
subsequent to restoration of the blood or oxygen supply based on
tissue or organ ischemia. The degree of IRI experienced in liver
surgery directly affects the function of the liver and its
viability following surgery, affecting clinical prognosis;
therefore, the pathophysiological changes of hepatic IRI have
consistently been the focus of studies (1,2).
Tubulin folding cofactor B (TBCB) is an important
member of the TBC family in cells. It is important for the proper
folding of β-tubulin and the formation of α/β-tubulin heterodimers,
which are critical for the normal growth of mammalian cells
(3). Cell microtubules predominantly
exist in the cytoplasm. As a component of the cellular spindle,
eukaryotic cilium, centrosomes and other organelles, cell
microtubules participate in the maintenance of cell morphology,
cell polarity, cell motility, cell division, and intracellular
transport, along with other cell biological functions, such as
cytoskeleton formation (4).
Hypoxia-ischemia is a common clinical pathological
process that causes extensive cell injury. The pathological process
of this injury is extremely complex and is associated with a
variety of factors. Changes in the cytoskeleton are significant in
the occurrence and development of hypoxic-ischemic injury (5,6). However,
although TBCs may be involved in tumorigenesis, there are few
studies on the expression of TBCs in IRI. In the current study, a
hepatic ischemia-reperfusion model was established by clamping of
the hepatic hilum of the mice in order to restore blood perfusion
(7). TBCB expression in the liver at
certain time-points and the association between the changes in TBCB
expression and liver function under such pathological conditions
were observed to investigate the pathophysiological changes in
hepatic IRI from a novel perspective, and to provide a theoretical
basis for its prevention and treatment.
Materials and methods
Reagents and instruments
TRIzol reagent was purchased from Invitrogen (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) kits
[SYBR® Premix Ex Taq™II (cat. no. DRR820A) and Prime
Script RT Reagent kit (cat. no. RR047A)] were purchased from Takara
Biotechnology Co., Ltd. (Dalian, China). Enzyme-linked
immunosorbent assay (ELISA) kits (Mouse IL-6 Quantikine ELISA kit;
cat. no. M6000B) for interleukin 6 (IL-6) and tumor necrosis
factor-α (TNF-α) were purchased from R&D Systems, Inc.
(Minneapolis, MN, USA). A biochemical analyzer was purchased from
Olympus and the ABI Prism 7300 Real-Time PCR System was purchased
from Applied Biosystems (Thermo Fisher Scientific, Inc.).
Animals
A total of 48 healthy, male C57BL/6 mice (body
weight, 18–20 g) were purchased from and reared at the Experimental
Animal Center of Guilin Medical College (Guilin, China). All
animals were used in accordance with institutional guidelines and
the current experiments were approved by the Animal Ethics
Committee of Guilin Medical College. The mice were randomly divided
into a control group (Sham, n=6) and an ischemia-reperfusion group
(n=42). The mice in the ischemia-reperfusion group were further
divided into 6 subgroups according to the different time durations
following reperfusion (2, 4, 6, 8, 12 and 24 h), with 7 mice per
subgroup (ischemia and reperfusion group at six time points, n=7
per group, and n=6 in the corresponding control group). Prior to
the experiment, the mice were fasted for 12 h, with free access to
drinking water. According to Pringle's method (8) (hepatic portal occlusion), a model of
total hepatic IRI (hepatic portal occlusion for 30 min) was
established. The mice were sacrificed at 2, 4, 6, 8, 12 and 24 h
after reperfusion, and the specimens were subsequently collected.
Blood samples (600 days per mouse, from the inferior vena cava)
were collected from the inferior vena cava of the mice and placed
in a tube without an anticoagulant. After standing for 30 min, the
blood was centrifuged (1,000 × g for 5 min) at room temperature and
stored in a freezer at −80°C. After blood sample collection, normal
saline perfusion to the liver via the portal vein was quickly
performed. The liver was then excised and divided into two equal
sections (size, 1.0×0.5×0.8 cm), with one section stored at −80°C
in a freezer, and the other section immersed in 10% formaldehyde
solution and then embedded in paraffin, followed by conventional
sectioning for pathological and immunohistochemical detection
assays.
Biochemical test
Serum aspartate aminotransferase (AST) and alanine
aminotransferase (ALT) levels were measured using an Olympus AU2000
automatic biochemical analyzer. Serum IL-6 and TNF-α levels were
determined by competitive inhibition ELISA. The procedures were
performed according to the ELISA kit manufacturer instructions.
Immunohistochemistry (IHC) assay
The liver tissue samples were fixed in 10%
formaldehyde solution, embedded in paraffin, and sliced into
sections with a thickness of 5 µm. Following dehydration in an
ethanol gradient that was replaced with xylene, hematoxylin and
eosin (H&E) staining was performed to observe the morphological
changes under a light microscope. Pathological scores were
determined according to the degree of tissue injury. The TBCB (cat.
no. ab96101), IL-6 (cat. no. ab7737) and TNF-α (cat. no. ab6671)
antibodies were all purchased from Abcam (Cambridge, MA, USA).
Subsequent to dewaxing with xylene and dehydration through an
ethanol gradient, the sections were incubated with 0.3% hydrogen
peroxide for 10 min to remove the endogenous peroxidase, followed
by a phosphate-buffered saline rinse, antigen repair in
ethylenediaminetetraacetic acid solution at high pressure, and
blocking with horse serum (cat. no. ZLI-9023; ZSGB-BIO, Beijing,
China). The sections were incubated with primary antibody (TBCB,
1:300; IL-6, 1:200; TNF-α, 1:50) for 1 h and then with
biotin-labeled secondary antibody (cat. no. KIT-5920; MaxVisionTM2
kit goat anti-mouse/rabbit IHC kit; Maixin, Fujian, China) for 1 h
at 37°C. After adding 100 µl freshly prepared diaminobenzidine
solution, color development was terminated in a timely manner
(10–20 min) and observations were made under an Olympus CX41
microscope. This was followed by rinsing with distilled water,
counterstaining with hematoxylin and rinsing with tap water for
blue color recovery. The sections were then dried through an
ethanol gradient, cleared by xylene and mounted with neutral
balsam. Five non-overlapping visual fields were randomly selected.
The positive-expression regions in the fields were observed at a
magnification of ×400, with density scanning performed using a
quantitative digital pathology image analysis system (cat. no.
H9-HMIAS-2000; Xuzhou City Technology Co., Ltd., Xuzhou, China) to
detect the absorbance in each field for statistical analysis.
Western blot analysis
To prepare the protein lysate, 1 ml
radioimmunoprecipitation assay buffer and 10 µl
phenylmethylsulfonyl fluoride were added to 100-mg tissue samples,
followed by homogenization on ice. After the protein concentration
was measured using the bicinchoninic acid method, the protein
samples (sham, 2.77 µl; 2 h, 3.23 µl; 4 h, 3.15 µl; 6 h, 3.4 µl; 8
h, 3.31 µl; 12 h, 3.4 µl; 24 h, 3.72 µl) were subjected to 10%
sodium dodecyl sulphate-polyacrylamide gel electrophoresis,
transferred to a polyvinylidene difluoride membrane (at 80 V for 60
min) and blocked with 5% skimmed milk. The membrane was then
incubated with the primary antibodies (TBCB, IL-6 and TNF-α;
dilution, 1:1,000) overnight and then with the secondary
antibodies, peroxidase-conjugated rabbit anti goat IgG (H+L)
[dilution, 1:5,000 (cat. no. ZB-2306); ZSGB-BIO] and
peroxidase-conjugated mouse anti goat IgG (H+L) [dilution, 1:5,000
(cat. no. ZB-230); ZSGB-BIO] at 37°C for 2 h. Enhanced
chemiluminescence chromogenic substrate was added followed by X-ray
exposure in a darkroom.
RT-qPCR
Total RNA was extracted from the liver tissue
samples using TRIzol, and 2 µg total RNA was used to synthesize
cDNA with the RT kit (Prime Script RT Reagent kit). The primer
sequences were as follows: Forward, 5′-AGTAGCGTTTCCCATTCAC-3′ and
reverse, 5′-ACTCACAGATTTCAAGCCA' for TBCB; forward,
5′-GCATGGAGTCCTGTGGCAT-3′, and reverse, 5′-CTAGAAGCATTTGCGTGG-3′
for β-actin. The concentrations of each of the primers were 100
nmol/l. PCR amplification was performed using the fluorescence qPCR
kit (SYBR® Premix Ex Taq™ II). The cycle threshold
values were obtained from the PCR curve, and the relative
expression levels of the target genes were calculated, with β-actin
serving as the internal reference (9).
Statistical analysis
The experimental data were analyzed using SPSS 18.0
software (SPSS, Inc., Chicago, IL, USA). The data were in line with
the normal distribution in the normal test and are represented as
means ± standard deviations. One-way analysis of variance (ANOVA)
was used for inter-group comparisons, and ANOVA with repeated
measurements was used for intra-group comparisons. P<0.05 was
considered to indicate a statistically significant difference and
pairwise t-tests were used to compare quantitative data.
Results
Hepatic IRI model established using
Pringle's method
Not including the the group in which mice were
sacrificed at 2 h after perfusion, the mice were awake and had free
access to food and drinking water within 4 h of surgery. No
abdominal infection was detected and all of the mice survived. In
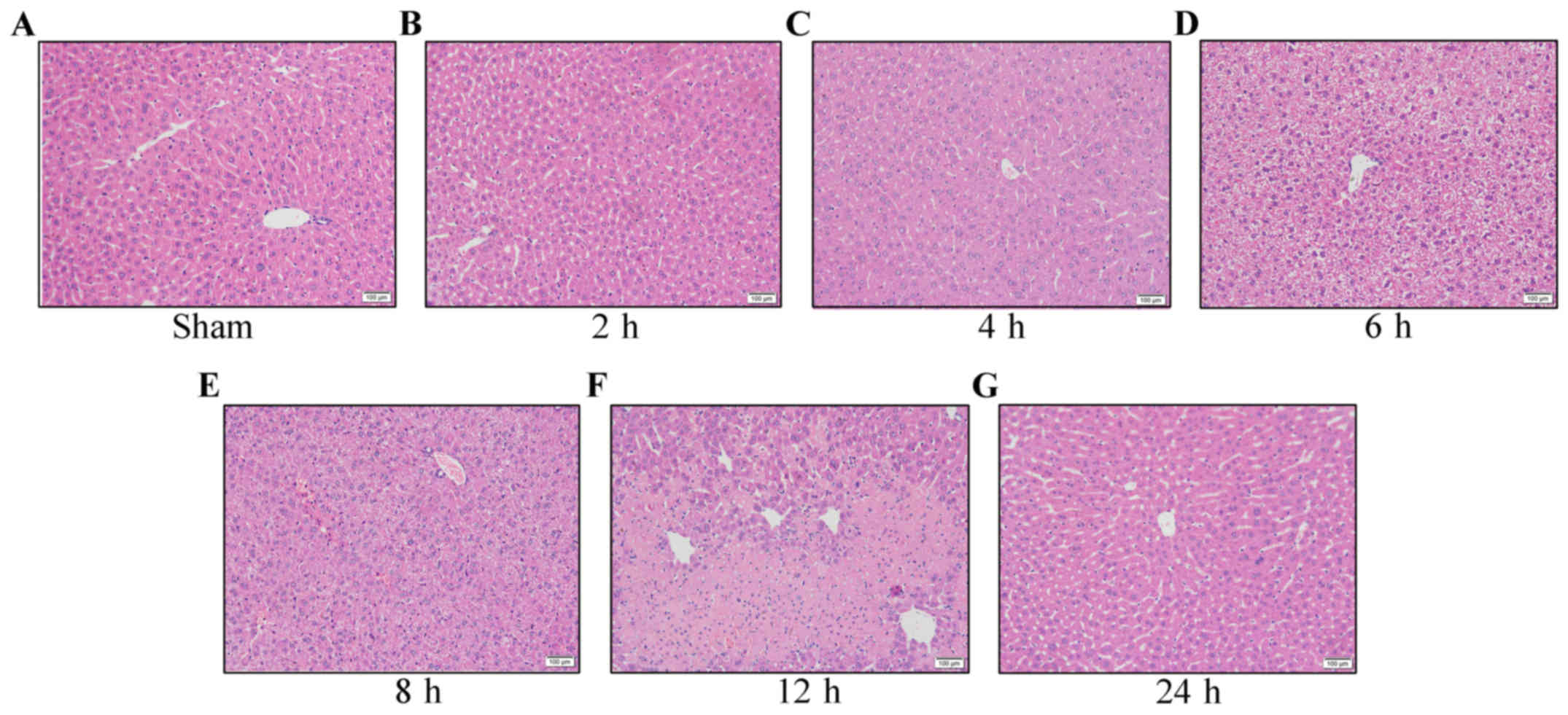
the control group (Fig. 1A), the
morphology of the hepatic cells was observed under a light
microscope and appeared normal, with no obvious oedema. At 2 h
after ischemia-reperfusion (Fig. 1B)
the changes were mild and at 4 h after reperfusion (Fig. 1C), obvious oedema was apparent in the
hepatic cells. Six hours after reperfusion (Fig. 1D), the oedema in the hepatic cells was
further aggravated and at 8 h after perfusion (Fig. 1E), point or flaky necrosis, or large
zones of necrosis were observed in the liver samples. At 12 h after
reperfusion (Fig. 1F), the necrosis
was most severe, showing sheet necrosis, hepatic sinusoidal
pressure and no obvious liver cord structure, with a large quantity
of infiltrated lymphocytes in the portal area, demonstrating the
most obvious changes in cell morphology. At 24 h after reperfusion
(Fig. 1G), compensatory recovery of
hepatic cells was observed, with mild oedema in the hepatic
cells.
Expression levels of ALT, AST, IL-6
and TNF-α were upregulated in hepatic IRI
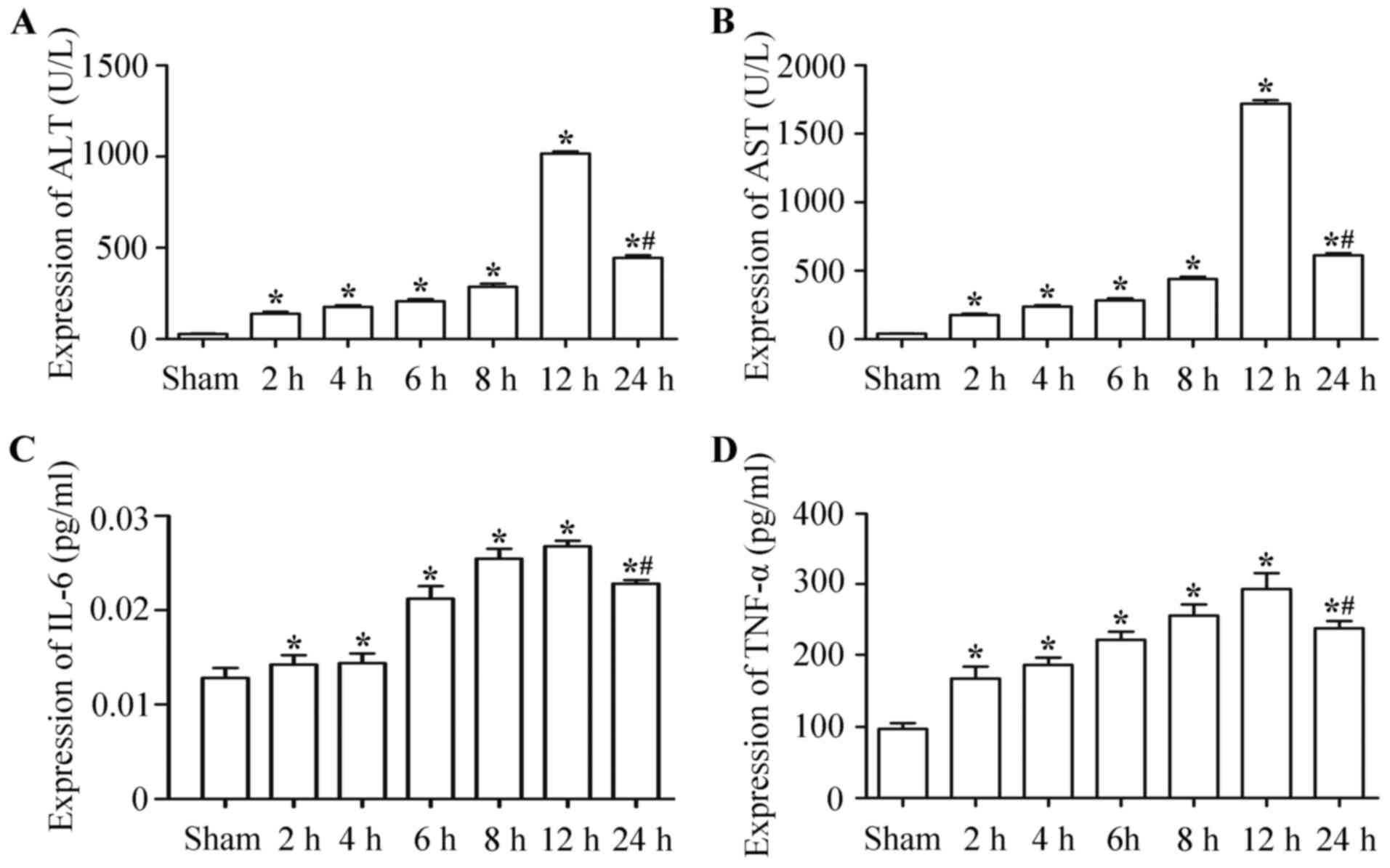
ALT (Fig. 2A) and AST
(Fig. 2B) levels in the serum at 2, 4,
6, 8, 12 and 24 h following ischemia-reperfusion were significantly
higher than those in the control group. At 12 h post-surgery, the
expression levels were the highest, but decreased by 24 h
post-surgery and the differences were statistically significant
(P<0.05).
IL-6 and TNF-α serum levels of the
ischemia-reperfusion group were significantly higher than those of
the control group (P<0.05). The IL-6 (Fig. 2C) and TNF-α (Fig. 2D) expression levels at 2, 4, 6, 8, 12
and 24 h after hepatic ischemia-reperfusion were significantly
higher than those of the control group at the corresponding
time-points after ischemia-reperfusion. At 12 h post-surgery, the
expression levels were the highest, but decreased by 24 h
post-surgery; the differences were statistically significant
(P<0.05).
TBCB expression level was upregulated
in hepatic IRI
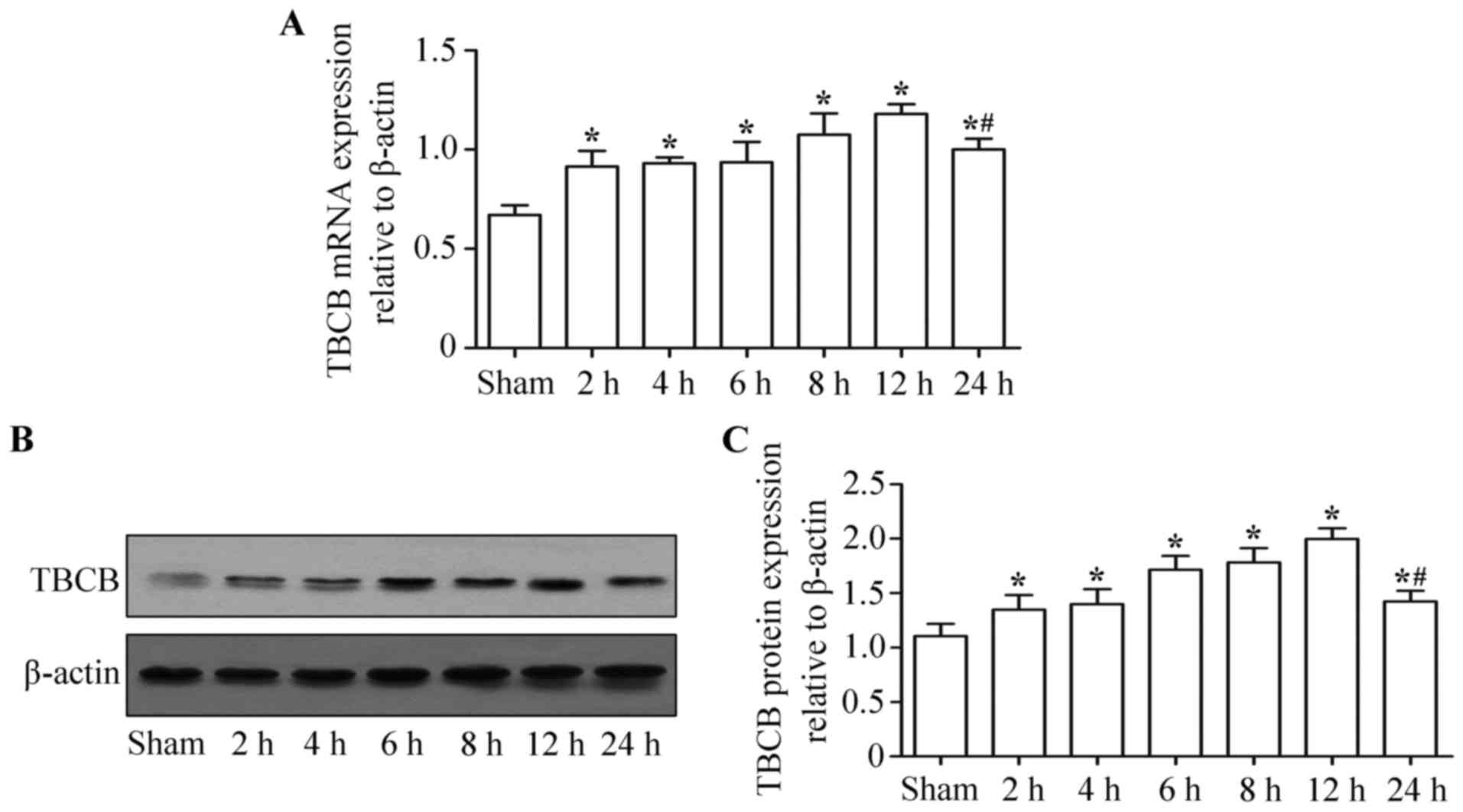
TBCB expression levels (mRNA and protein) at 2, 4,
6, 8, 12 and 24 h following ischemia-reperfusion were significantly
higher than those of the control group (P<0.05). There were no
significant differences in TBCB mRNA (Fig.
3A) and protein (Fig. 3B and C)
expression levels among the different subgroups in the ischemia
reperfusion group (P>0.05). The TBCB expression level was
highest at 12 h post-surgery, but had decreased by 24 h
post-surgery (P<0.05).
Upregulated expression levels of IL-6,
TNF-α and TBCB in hepatic IRI were correlated
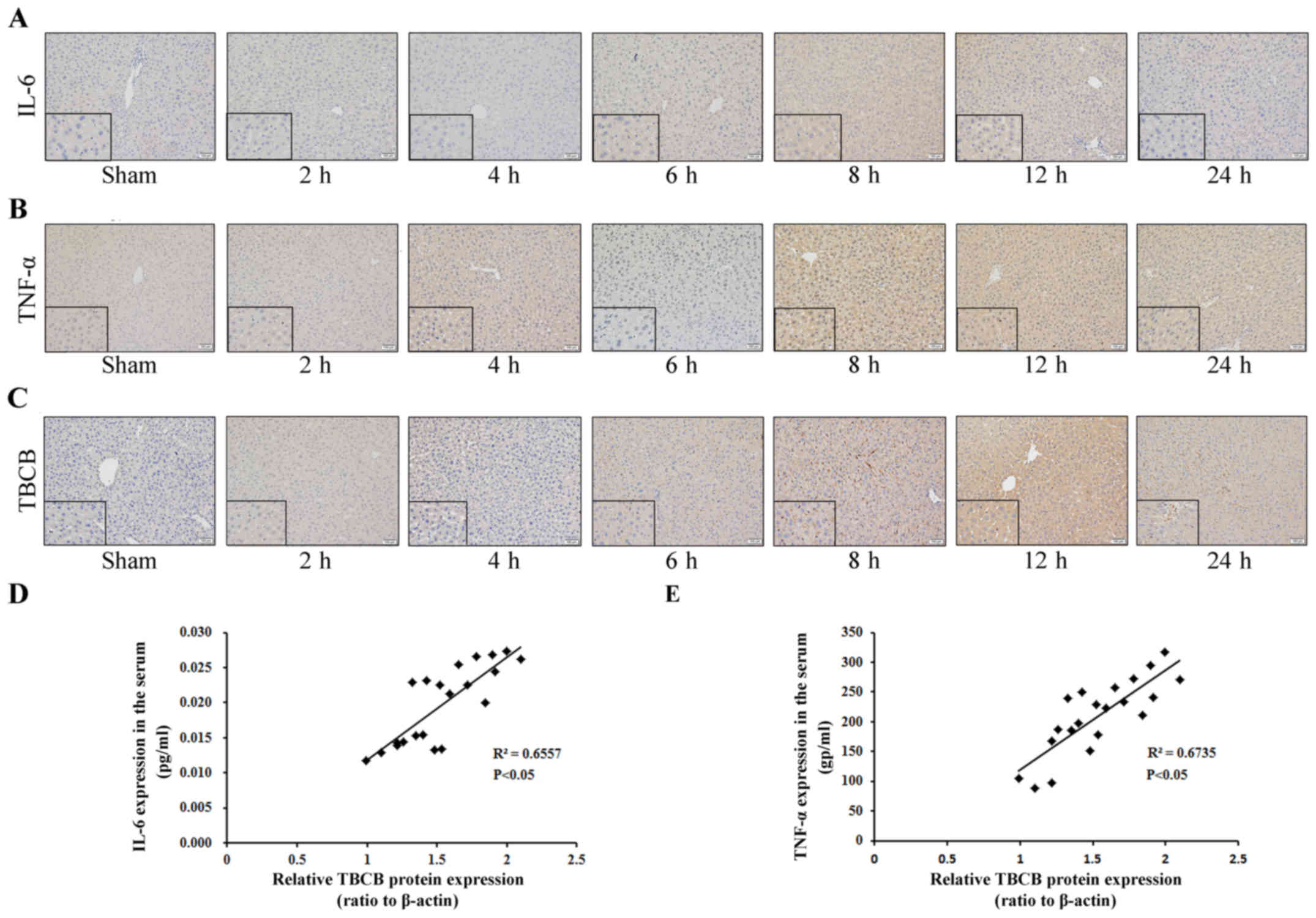
Expression levels of IL-6, TNF-α and TBCB at
different time-points following hepatic IRI were detected by IHC.
The positive expression of IL-6 in hepatic cells at 6, 8, 12 and 24
h following ischemia-reperfusion was observed. IL-6 expression
gradually increased from 6 h to its highest level at 12 h, but
decreased by 24 h (Fig. 4A). Compared
with the control group, positive TNF-α expression in the hepatic
cells at 4, 6, 8, 12 and 24 h following ischemia-reperfusion was
observed. TNF-α expression gradually increased to its highest level
at 12 h, but decreased by 24 h (Fig.
4B). TBCB expression levels at 6, 8, 12 and 24 h following
ischemia-reperfusion in the ischemia-reperfusion group were
significantly higher compared with the control group, the
expression gradually increased from 6 h to its highest level at 12
h, but decreased by 24 h (Fig.
4C).
To determine whether any correlation existed between
TBCB and IL-6 and TNF-α expression during hepatic IRI of mice, the
correlation between the level of TBCB protein expression in hepatic
tissue, and serum IL-6 and TNF-α expression levels was evaluated.
TBCB expression was identified to be positively correlated with
IL-6 (Fig. 4D) and TFN-α (Fig. 4E).
Discussion
IRI is a common pathophysiological process in liver
surgery and is inevitable in surgical procedures, including shock
resuscitation, liver transplantation and liver lobectomy. IRI often
markedly affects the clinical prognosis. Furthermore, the
pathophysiology of hepatic IRI is very complex and has not been
fully elucidated. Therefore, IRI prevention or mitigation is a
current research focus.
The cytoskeleton is a complex three-dimensional
network structure composed of protein filaments in the eukaryotic
cytoplasm that consists of cell microtubules, microfilaments and
intermediate fibers. The main role of the cytoskeleton is to
stabilize and maintain the cell morphology, and to support the
mutual association between the cellular and nuclear membranes. In
addition, the cytoskeleton is involved in cell movement, cell
polarity, cell division, and cytoplasmic transport, with an
important significance in signal transduction (4).
Cell microtubules are one of the main components of
the cytoskeleton. Microtubules primarily exist in the cytoplasm as
a component of the cellular spindle, eukaryotic cilium, centrosomes
and other organelles, and are composed of α- and β-tubulin (each
with molecular weights of ~55 kDa). Microtubule formation is a
complex process involving multiple molecules, such as TBCs (TBCA,
TBCB, TBCC, TBCD and TBCE) and multiple microtubule-associated
proteins. A heterodimer is formed with α- and β-tubulin, and 13
heterodimers arrange in a round coil to form a microtubule with a
relatively stable tubular structure and a diameter of ~25 nm
(10). The majority of the microtubule
fibers are in a dynamic state of assembly and disassembly, which is
a necessary process for performing their functions. Microtubules
constitute the intracellular network scaffold to maintain cell
morphology, which is associated with cell motility. In addition,
microtubules are involved in the regulation of the L-calcium
current on the cell membrane and are closely associated with
cellular electrophysiological activities. Ischemia-reperfusion
causes injuries to the microtubules and damages the network
structure of the cytoplasm, leading to the loss of support in the
cell membrane and increased cell fragility (11–13).
TBCs were initially demonstrated to be folding
proteins. The current study demonstrated that TBCs are
predominantly involved in the folding and degradation of the
tubulin complex, playing a significant role in the functional
diversity and dynamic equilibrium of microtubules (14). TBCB, one of the important members of
the TBC family, is very important in the proper folding of
β-tubulin and the formation of α/β-tubulin (3). Studies have found that abnormal
expression levels of TBCB and TBCE may directly cause microtubule
abnormalities (15). Furthermore, it
has been confirmed that TBCB is closely associated with
tumorigenesis and tumor metastasis. For example, the level of TBCB
expression in breast cancer tissues was significantly upregulated,
and TBCB overexpression may increase the degree of malignancy in
breast cancer cells (16).
Currently, the majority of studies have suggested
that IRI is associated with the excessive production of oxygen free
radicals, calcium overload, inflammatory response, energy
metabolism disorders and apoptosis (17–19).
Apoptosis is an important cause of severe liver damage and organ
dysfunction during ischemia-reperfusion (20,21).
Apoptosis is a multifactorial, multi-step, and multi-path complex
process, while oxygen free radicals, energy metabolism disorders,
intracellular calcium overload, cytokines, caspases, and the B-cell
lymphoma 2 family all induce apoptosis. In the implementation of
cell apoptosis, the significance of the role of caspase proteins is
often above that of cytoskeleton proteins. By activating and
blocking certain specific substrates (i.e., proteins involved in
cytoskeletal regulation), caspase proteins indirectly reconstitute
the structure of cells, resulting in the morphological changes
observed in apoptosis. Furthermore, after hypoxic-ischemic injury,
the intracellular Ca2+ concentration may alter. The
binding of Ca2+ and calmodulin activates a series of
protein kinases to react on cytoskeleton proteins, thus leading to
cytoskeletal disruption or recombination (22).
The alterations in the AST and ALT expression levels
in the model group were significantly higher than those in the sham
surgery group. ALT and AST are important indicators of liver
injury. In the modeling process of hepatic IRI, these enzymes are
effective indicators that demonstrate the success of modeling and
the degree of liver injury. In IRI, numerous inflammatory mediators
are released to activate the complement system. Kupffer cells,
neutrophils, monocytes and eosinophils in the liver exhibit signs
of infiltration and chemotaxis towards the ischemic region, thus
activating the NADPH/NADH oxidase system and generating a large
quantity of oxygen free radicals, also known as respiratory burst,
resulting in hepatic cell injury (23). Numerous studies have confirmed that
TNF-α participates in the pathophysiology of hepatic IRI (24,25). TNF-α
causes the release of cytokines, such as IL-1β, IL-6, and IL-8
(26). In the present study, during
the process of hepatic ischemia-reperfusion, the IL-6 and TNF-α
expression levels were significantly higher than those of the sham
surgery group, which was consistent with the findings in the
pathological H&E staining, thus indicating that TNF-α and IL-6
participate in the process of hepatic IRI, and are important in the
IRI-induced inflammatory response.
In the current study, the pathological changes of
hepatic IRI were simulated by establishing a hepatic IRI model in
mice. TBCB expression levels in injured liver tissue samples, at
different time-points subsequent to reperfusion, were investigated.
The TBCB expression level was identified to be significantly higher
in injured liver tissue samples when compared with tissue samples
from the sham surgery group, indicating that TBCB may be involved
in hepatic IRI, indicating that early detection, diagnosis and
prevention of liver diseases in clinical treatment are possible.
However, the underlying mechanism of TBCB in hepatic IRI remains
unclear. Therefore, further studies regarding TBCB expression
levels and the signal transduction pathways involving TBCB have
important clinical significance for improving IRI, organ
transplantation and gene therapy.
Acknowledgements
The present study was supported in part by The
National Natural Science Foundation of China (grant nos. 81430014,
81360367 and 81560393), the Natural Science Foundation of Guangxi
(grant nos. 2014GXNSFDA118019 and 2015jjDA40010), the Scientific
Research and Technology Development Project for Guilin (grant nos.
20140310-2-2, 20140127-3 and 20150133-6), the Guangxi Regional
High-risk Tumors Early Prevention and Control of Key Laboratory
Open Research Project (grant no. GK2014-TKF01), the Guangxi Science
Fund for Distinguished Young Scholars Program (grant no.
2016GXNSFFA380003), and the Guangxi Health and Family Planning
Commission ‘139’ Leading Talents Training Plan.
References
|
1
|
Fahrner R, Trochsler M, Corazza N,
Graubardt N, Keogh A, Candinas D, Brunner T, Stroka D and Beldi G:
Tumor necrosis factor-related apoptosis-inducing ligand on NK cells
protects from hepatic ischemia-reperfusion injury. Transplantation.
97:1102–1109. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guan LY, Fu PY, Li PD, Li ZN, Liu HY, Xin
MG and Li W: Mechanisms of hepatic ischemia-reperfusion injury and
protective effects of nitric oxide. World J Gastrointest Surg.
6:122–128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Archer JE, Vega LR and Solomon F: Rbl2p, a
yeast protein that binds to beta-tubulin and participates in
microtubule function in vivo. Cell. 82:425–434. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Inoué S: Microtubule dynamics in cell
division: Exploring living cells with polarized light microscopy.
Annu Rev Cell Dev Biol. 24:1–28. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He XL, Bazan JF, McDermott G, Park JB,
Wang K, Tessier-Lavigne M, He Z and Garcia KC: Structure of the
Nogo receptor ectodomain: A recognition module implicated in myelin
inhibition. Neuron. 38:177–185. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wojciak-Stothard B, Tsang LY and Haworth
SG: Rac and Rho play opposing roles in the regulation of
hypoxia/reoxygenation-induced permeability changes in pulmonary
artery endothelial cells. Am J Physiol Lung Cell Mol Physiol.
288:L749–L760. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mota-Filipe H, Sepodes B, McDonald MC,
Cuzzocrea S, Pinto R and Thiemermann C: The novel PARP inhibitor
5-aminoisoquinolinone reduces the liver injury caused by ischemia
and reperfusion in the rat. Med Sci Monit. 8:BR444–BR453.
2002.PubMed/NCBI
|
|
8
|
Pringle JH: V. Notes on the Arrest of
Hepatic Hemorrhage Due to Trauma. Ann Surg. 48:541–549. 1908.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Livak KJ1 and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)). Methods Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lewis SA, Tian G and Cowan NJ: The alpha-
and beta-tubulin folding pathways. Trends Cell Biol. 7:479–484.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ganote CE and Heide RS Vander:
Cytoskeletal lesions in anoxic myocardial injury. A conventional
and high-voltage electron-microscopic and immunofluorescence study.
Am J Pathol. 129:327–344. 1987.PubMed/NCBI
|
|
12
|
Gómez AM, Kerfant BG and Vassort G:
Microtubule disruption modulates Ca(2+) signaling in rat cardiac
myocytes. Circ Res. 86:30–36. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jovanović S and Jovanović A: Diadenosine
tetraphosphate-gating of cardiac K(ATP) channels requires intact
actin cytoskeleton. Naunyn Schmiedebergs Arch Pharmacol.
364:276–280. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lopez-Fanarraga M, Carranza G, Bellido J,
Kortazar D, Villegas JC and Zabala JC: Tubulin cofactor B plays a
role in the neuronal growth cone. J Neurochem. 100:1680–1687.
2007.PubMed/NCBI
|
|
15
|
Kortazar D, Fanarraga ML, Carranza G,
Bellido J, Villegas JC, Avila J and Zabala JC: Role of cofactors B
(TBCB) and E (TBCE) in tubulin heterodimer dissociation. Exp Cell
Res. 313:425–436. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hamel E, Sackett DL, Vourloumis D and
Nicolaou KC: The coral-derived natural products eleutherobin and
sarcodictyins A and B: Effects on the assembly of purified tubulin
with and without microtubule-associated proteins and binding at the
polymer taxoid site. Biochemistry. 38:5490–5498. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Choi E, Cha MJ and Hwang KC: Roles of
Calcium Regulating MicroRNAs in Cardiac Ischemia-Reperfusion
Injury. Cells. 3:899–913. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Demiryilmaz I, Turan MI, Kisaoglu A,
Gulapoglu M, Yilmaz I and Suleyman H: Protective effect of
nimesulide against hepatic ischemia/reperfusion injury in rats:
effects on oxidant/antioxidants, DNA mutation and COX-1/COX-2
levels. Pharmacol Rep. 66:647–652. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qiu Z, Zhou D and Sun D: Effects of human
umbilical cord mesenchymal stem cells on renal
ischaemia-reperfusion injury in rats. Int Braz J Urol. 40:553–561.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sindram D, Porte RJ, Hoffman MR, Bentley
RC and Clavien PA: Synergism between platelets and leukocytes in
inducing endothelial cell apoptosis in the cold ischemic rat liver:
A Kupffer cell-mediated injury. FASEB J. 15:1230–1232.
2001.PubMed/NCBI
|
|
21
|
Sindram D, Porte RJ, Hoffman MR, Bentley
RC and Clavien PA: Platelets induce sinusoidal endothelial cell
apoptosis upon reperfusion of the cold ischemic rat liver.
Gastroenterology. 118:183–191. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kothakota S, Azuma T, Reinhard C, Klippel
A, Tang J, Chu K, McGarry TJ, Kirschner MW, Koths K, Kwiatkowski
DJ, et al: Caspase-3-generated fragment of gelsolin: Effector of
morphological change in apoptosis. Science. 278:294–298. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shirasugi N, Wakabayashi G, Shimazu M,
Oshima A, Shito M, Kawachi S, Karahashi T, Kumamoto Y, Yoshida M
and Kitajima M: Up-regulation of oxygen-derived free radicals by
interleukin-1 in hepatic ischemia/reperfusion injury.
Transplantation. 64:1398–1403. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hernandez-Alejandro R, Zhang X, Croome KP,
Zheng X, Parfitt J, Chen D, Jevnikar A, Wall W, Min WP and Quan D:
Reduction of liver ischemia reperfusion injury by silencing of
TNF-α gene with shRNA. J Surg Res. 176:614–620. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mahmoud MF, El Shazly SM and Barakat W:
Inhibition of TNF-α protects against hepatic ischemia-reperfusion
injury in rats via NF-κB dependent pathway. Naunyn Schmiedebergs
Arch Pharmacol. 385:465–471. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Klebanoff SJ, Vadas MA, Harlan JM, Sparks
LH, Gamble JR, Agosti JM and Waltersdorph AM: Stimulation of
neutrophils by tumor necrosis factor. J Immunol. 136:4220–4225.
1986.PubMed/NCBI
|