Introduction
Lung cancer has taken over from liver cancer as the
leading cause of death in patients with malignant tumors in China
(1). At least 30% of non-small cell
lung cancer (NSCLC) patients missed the opportunity to have an
operation when first visiting the doctor; therefore, their
prognosis became worse, due to lack of effective therapy (2). EGFR-targeted therapy is a promising
strategy for the treatment of NSCLC; some randomized trials have
demonstrated a significantly higher tumor remission rate and longer
progression free survival (PFS) in patients with EGFR mutation
treated with first-line TKI (2–7). The
frequency of the EGFR mutation in NSCLC in the Asian population is
~30%, while the white population is ~20% (8,9). In clinical
subgroups, the frequency of mutation in Asian males and smokers is
lower than that of Asian females and non-smokers (5,10).
Despite the initial success of these drugs in all
patients, the median progression free survival was 12–16 months
(4,7,11). Acquired
resistance to EGFR-TKIs has been attributed to several molecular
mechanisms, although the resistance of patients with unknown
etiology is ~35% (12). The most
common causes of resistance are the development of the T790M
mutation (13), amplification of MET
(14,15)
and, in rare cases, transformation to small cell histology
(16). Despite clinical evidence for
progress in the treatment of EGFR-TKI, continued EGFR inhibition
seems to provide sustained clinical benefit (17,18).
Carcinoembryonic antigen (CEA), recognized as a
NSCLC marker, is also can be used for detecting adenocarcinoma with
~60% sensitivity and 50% specificity (19,20), whereas
the sensitivity and specificity is ~25 to 40% and 25%,
respectively, for squamous cell carcinoma (21,22).
According to previous reports, CEA was a significant predictor of
sensitivity and survival in patients treated with gefitinib
(23,24)
However, the CEA level as a predictive marker of response to
EGFR-TKIs has not been extensively evaluated. The authors assumed
that the level of CEA could predict the extent of benefit from
EGFR-TKIs, and tumor patients with high serum CEA level may benefit
more from EGFR-TKIs than those with low serum CEA level.
Patients and methods
Patients
Patients with the clinic diagnosis of NSCLC treated
at the Department of Thoracic Surgery of the Fourth Affiliated
Hospital, Harbin Medical University (Harbin, China) were recruited
between February 2012 and May 2015. Inclusion criteria comprised:
Patients with stages IIIB to IV NSCLC who had received palliative
surgical resection were confirmed for EGFR mutations (either exon
19 deletions or L858R in exon 21) and received either erlotinib 150
mg/day or gefitinib 250 mg/day orally (clinical stage was
determined by the 7th edition of tumor, node, metastasis
classification) (25). The patients
were enrolled for palliative care with EGFR-TKIs if they had an
Eastern Cooperative Oncology Group performance status of 0–2 and
life expectancy >3 months. Patients were appraised by a review
of data from a prospective re-biopsy protocol of patients with
acquired resistance to EGFR tyrosine kinase inhibitors. Patients
with a previous history of malignancy were excluded. The study was
approved by the institutional review boards of the Fourth
Affiliated Hospital of Harbin Medical University (Harbin, China).
Informed consent was acquired from each patient prior to the
commencement of treatment.
Methods
Serum CEA was measured by an enzyme immunoassay
within one week before starting the erlotinib or gefitinib
treatment. Measurement was performed at the Clinical Laboratory of
the Fourth Affiliated Hospital of Harbin Medical University using a
sequential chemiluminescence immunoassay (Immulite 2000 Immunoassay
System; Siemens Healthineers, Erlangen, Germany). The total
included 100 patients were categorized into three groups according
to their serum CEA level: High-CEA group (H), CEA baseline levels
>10 ng/ml, normal-CEA group (N), CEA baseline levels <5.0
ng/ml and low-CEA group (L), CEA baseline levels between 5 ng/ml
and 10 ng/ml. The authors analyzed the correlation between serum
CEA level and clinical benefits in the patients receiving EGFR-TKI
treatment.
Statistical analyses
The primary endpoint, progression-free survival was
calculated from the first day of treatment to the date of the first
disease progression or, under the condition without disease
progression, the last follow-up or death. The overall survival (OS)
rate was calculated from the first day of treatment to the date of
mortality due to any cause or the date of the last follow-up. The
secondary endpoints contained objective response rate (ORR), OS and
safety. The PFS and OS rates were estimated using the Kaplan-Meier
life-table method and the survival curves were compared using the
log-rank test. Comparison of ORRs in different groups was performed
using χ2 tests. Independent predictive factors
associated with PFS were evaluated in multivariate analysis using a
Cox regression model. All P-values were based on two-sided testing
and statistical analyses were carried out using SAS statistical
software (version 9.2; SAS Institute, Cary, NC, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
Between February 2012 and May 2015, a total of 320
patients with the diagnosis of advanced NSCLC were screened for
EGFR mutations before the start of EGFR-TKIs. A total of 100
patients were completely consistent with the inclusion criteria. Of
the 100 patients, 54 were female and 46 men, with a mean age of 55
years (range, 23–84). In the 100 patients, 80 were non-smokers and
20 past or current smokers; 93 had adenocarcinoma, 6 squamous cell
carcinoma and one adenosquamous carcinoma; according to TNM
classification, 13 were in stage IIIB and 87 in stage IV. A total
of 49 patients (49%) with a baseline CEA level (>10 ng/ml) were
recruited with median CEA of 222.8 ng/ml (group H) (range, 10–6,840
ng/ml). A total of 21 patients with a baseline CEA level between 5
and 10 ng/ml were recruited with median CEA of 6.8 ng/ml (group L).
A total of 30 patients with a baseline CEA level (<5.0 ng/ml)
were in the normal group (group N). The characteristics of all
patients are shown in Table I. Sex and
smoking status were well balanced among these groups.
 | Table I.Patient demographics and clinical
characteristics. |
Table I.
Patient demographics and clinical
characteristics.
| Characteristic | Group H | Group L | Group N | P-value |
|---|
| Age (years) |
|
|
| 0.823 |
|
Median | 54.5 | 51.5 | 56 |
|
|
Range | 27–84 | 37–69 | 23–75 | 0.927 |
| Gender |
|
|
|
|
|
Women | 29 | 12 | 13 |
|
| Men | 20 | 9 | 17 |
|
| Smoking history |
|
|
| 0.752 |
|
Non-smoker | 42 | 18 | 20 |
|
|
Smoker | 7 | 3 | 10 |
|
| ECOG |
|
|
| 0.798 |
| 0 | 18 | 9 | 6 |
|
| 1 | 28 | 10 | 18 |
|
| 2 | 3 | 2 | 6 |
|
| Clinical stage |
|
|
| 0.893 |
|
IIIB | 6 | 3 | 4 |
|
| IV | 43 | 18 | 26 |
|
| Histology |
|
|
| 0.532 |
|
Non-adenocarcinoma | 2 | 1 | 3 |
|
|
Adenocarcinoma | 47 | 20 | 27 |
|
| Site of metastatic
disease |
|
|
| 0.634 |
|
Lung | 22 | 17 | 19 |
|
|
Brain | 5 | 2 | 3 |
|
|
Bone | 13 | 9 | 10 |
|
| Lymph
node | 23 | 17 | 22 |
|
|
Visceral (liver, spleen) | 4 | 2 | 3 |
|
| Baseline CEA |
|
|
| 0.000 |
|
Median | 222.8 | 6.8 | 3.7 |
|
|
Range | 10–6,840 | 5–10 | 0.8–5 |
|
| Oral medicine |
|
|
| 0.758 |
|
Gefitinib (250 mg/day) | 22 | 25 | 19 |
|
|
Erlotinib (150 mg/day) | 12 | 11 | 11 |
|
| EGFR mutation
type |
|
|
| 0.354 |
| Exon 19
deletion | 25 | 22 | 23 |
|
| Exon 21
L858R | 9 | 11 | 10 |
|
| Resistance
mechanism |
|
|
| 1.000 |
|
T790M | 13 | 12 | 10 |
|
| MET
amplification | 2 | 1 | 3 |
|
| Small
cell histology | 1 | 1 | 1 |
|
|
Unknown | 7 | 4 | 5 |
|
Efficacy and toxicities
Between February 2012 and May 2015, 100 patients
commenced erlotinib or gefitinib treatment (Table II). The last follow-up was carried out
on June 30, 2016, and median follow-up duration was 20.5 months
(range, 2.5–48.0). Disease progression occurred in 93 patients. The
median PFS indicated statistical significance among the three
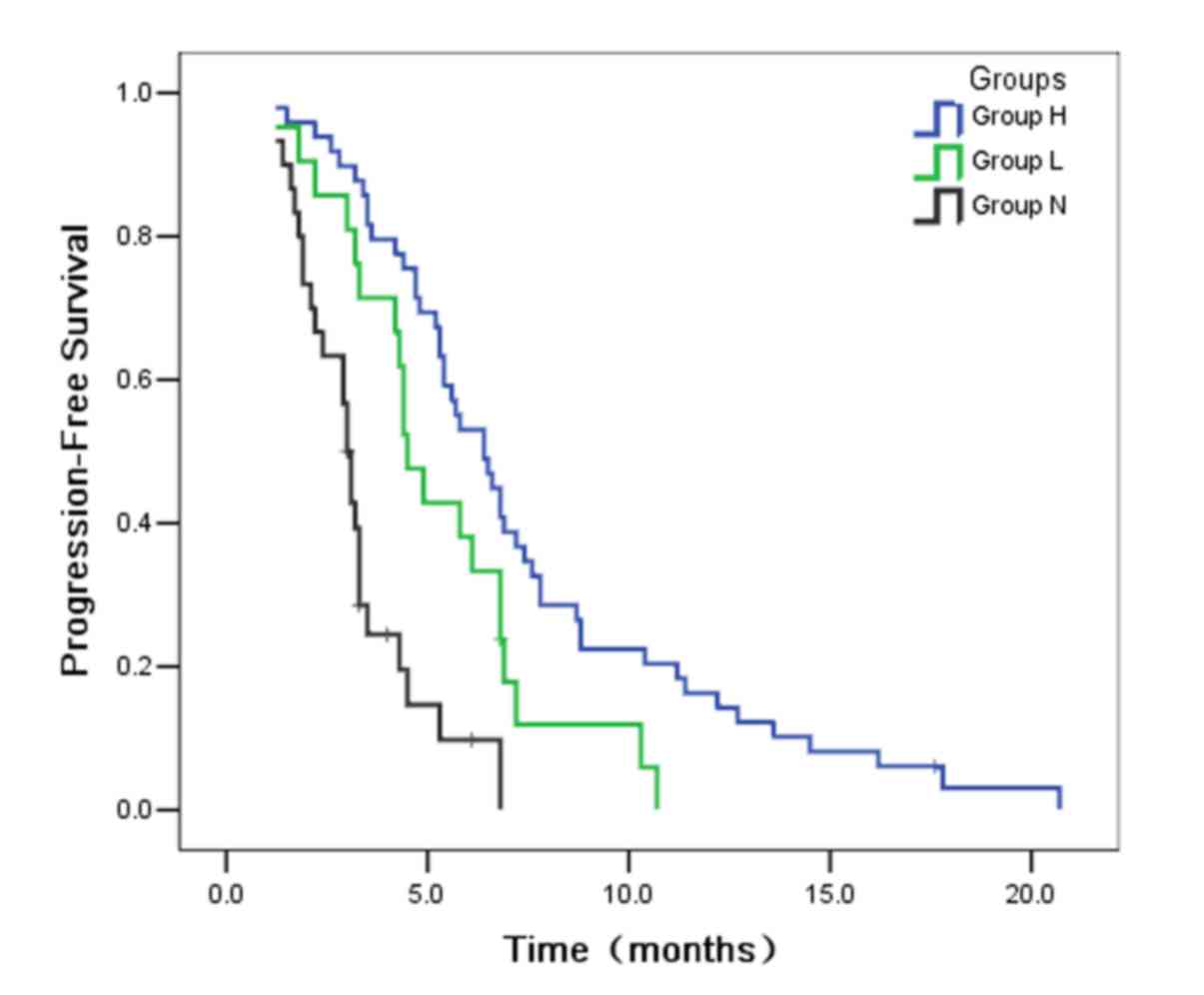
groups (P<0.001; Fig. 1). The
median PFS was significantly longer in group H (6.4 months) than in
group L (4.5 months; P=0.027). Furthermore, the median PFS was also
statistically longer in group L than in group N (3.0 months;
P=0.002). From the results of PFS, the authors could suggest that
the patients with high serum CEA level were able to benefit more
from gefitinib therapy than those with low serum CEA. In a
multivariate Cox equilibrium regression model, patients were
grouped by CEA level, gender, smoking status and pathology as
concomitant variables, and results displayed that group H was an
independent positive predictive factor for PFS [hazard ratio (HR),
1.25; 95% confidence interval (CI), 1.09–1.39].
 | Table II.Efficacy of erlotinib or gefitinib
treatment. |
Table II.
Efficacy of erlotinib or gefitinib
treatment.
| Efficacy | Group H | Group L | Group N | P-value |
|---|
| PFS (median,
months) | 6.4 | 4.5 | 3.0 | 0.000 |
| OS (median,
months) | 11.9 | 9.4 | 7.8 | 0.000 |
| Tumor response |
|
|
| 0.010 |
| CR | 2 | 1 | 1 |
|
| PR | 30 | 7 | 9 |
|
| SD | 14 | 8 | 12 |
|
| PD | 3 | 5 | 8 |
|
The ORRs were 65.3, 38 and 33.3%, respectively, in
groups H, L and N. ORR were significantly higher in group H than in
groups N and L (P=0.006 and 0.035, respectively), while no
statistical difference was observed between groups L and N
(P=0.726). A total of 80 patients had not died at the last
follow-up date. The median OS of patients were 11.9, 9.4 and 7.8
months, respectively, in groups H, L and N. Similar with ORR, the
median overall survivals (OS) was significantly longer in group H
than in groups N and L (P<0.001 and P=0.022, respectively;
Fig. 2), while no statistical
difference was observed between groups L and N (P=0.115). These
results suggest that the patients with pre-therapeutic high serum
CEA level have better response to gefitinib or erlotinib and longer
OS than those with low or normal serum CEA level.
The most common adverse events were skin rash and
anorexia (75 and 51% in the current study, respectively), and there
were no significant differences in their incidences among the three
groups. Most patients showed only grade 1/2 adverse events. Grade 3
rash was observed in nine patients, and no dose reduction or
discontinuation was performed in any patients due to intolerable
toxicities. No interstitial lung disease occurred.
Acquired resistance to EGFR-TKIs
Of 100 samples, 76 developed acquired resistance to
EGFR-TKIs. A total of 60 patients were identified that received
re-biopsy at the time of development of acquired resistance
successfully. The resistance mechanism was similar in three groups,
with the acquired T790M mutation being most common, followed by MET
amplification and small cell histologic transformation.
Discussion
The role of CEA as a prognostic factor has been well
established in colon cancer and is now part of the routine
follow-up evaluation recommended by the current NCCN guidelines
(26–29). In NSCLC, a number of studies evaluating
CEA and prognosis have been written with contrasting results in the
perioperative setting, some showing its role as a prognostic value
(30,31)
and others not confirming it (22,32,33). To the best of the authors' knowledge,
no serum marker for EGFR-mutated NSCLC has been reported to predict
the efficacy of EGFR-TKIs; the present study may be the first to
demonstrate that the pre-therapeutic serum CEA level may predict
the extent of benefits from EGFR-TKIs in advanced NSCLC patients
carrying the EGFR mutation. The research results revealed
significantly different median PFS between these groups
(P<0.001), suggesting that patients with high serum CEA level
may benefit more than those with low serum CEA in spite of partial
overlap of 95% CIs.
EGFR mutations occur most frequently in female,
non-smoking, East Asian and adenocarcinoma patients (34). Therefore, tumor patients carrying EGFR
activating mutations demonstrated a better response to EGFR-TKI
treatment than those without EGFR mutations (9). However, the effectiveness of TKIs
treatment is not the same in the patients carrying EGFR mutations.
Some patients had dramatic response to EGFR-TKIs treatment, while
others did not exhibit any response.
The predictive and prognostic value of CEA level
have been assessed in advanced NSCLC patients fully treated with
gefitinib and erlotinib, due to conflicting results have been
reported higher levels of CEA and the response is directly related
to EGFR-TKI, but its effectiveness has not yet been established
(35). On the other hand, Okamoto
et al (24) and Jung et
al (23) reported that patients
treated with EGFR-TKI with higher CEA levels had a longer survival
and a better response than those with low CEA levels. Shoji et
al (36) reported that the rate of
EGFR gene mutation is significantly increased as the levels of CEA
increases (for the levels of CEA of <5, ≥5 but <20 and ≥20
the rate of EGFR gene mutation was 35, 55 and 87.5%, respectively;
P=0.040). Their study presented a significant association between
EGFR gene mutations and the levels of CEA in patients with lung
adenocarcinomas. To the best of the authors' knowledge, little is
known about the function of CEA. Wirth et al (37) reported that CEA can inhibit the
apoptosis and has prometastatic roles in colon cancer cells, and
Ordonez et al (38) also
reported that the overexpression of CEA can protect tumor cells
from apoptosis and inhibit cell death. EGFR mutations were detected
within an ATP binding pocket with catalytic domain, and the mutants
also had an enhanced tyrosine kinase activity in response to the
ligand. In addition, the present studies have demonstrated that
such downstream molecules as Akt and STAT3 serve a crucial role in
the antiapoptotic pathways of EGFR mutations in tumor cells
(39). Moreover, the mutated EGFRs are
autophosphorylated in the absence of interleukin-3 without EGF
stimulation, and their expression leads to the STAT5 activation and
the upregulation of the extracellular signal-regulated kinase 1 or
2 (Erk1 or 2), Erk5 and Akt (40). It
is hypothesized that this continuous signal of the mutant EGFR can
stimulate antiapoptotic activity in a ligand-independent manner.
Thus, overexpression of the CEA protein as antiapoptotic may be
observed in patients with EGFR mutants.
In the present study, the median OS of patients were
11.9, 9.4 and 7.8 months, respectively, in groups H, L and N.
Similar to the ORR, the difference in the median OS between groups
H and N and between groups H and L were significant (P<0.001 and
P=0.022, respectively), whereas these between groups L and N were
not (P=0.115). In addition, the multivariate analysis revealed that
group H was an independent positive predictive factor for PFS (HR,
1.25; 95% CI, 1.09–1.39). Considering histologic heterogeneity in
NSCLC, the authors hypothesized that the serum CEA level in
patients with pretreated lung cancer partly represents the extent
of the mutant EGFR component in the lung cancer. This hypothesis
may partially explain why the effect durations were not as long as
expected in some patients with EGFR mutations. By determining the
serum CEA level, one could select the patients with high serum CEA
levels for EGFR-TKIs treatment to guarantee the best therapy
effect. Importantly, more attention should be paid to patients with
low serum CEA levels while making therapeutic strategies, it is
necessary for them to give combined strategies, rather than single
administration of EGFR-TKIs.
With regard to the potential benefit of learning
about a small cell histologic transformation, as well as the
prognostic value of EGFR T790M mutation, the authors biopsy
patients at the time of development of acquired resistance as part
of routine consideration. These data demonstrate that there were no
differences between the patients with low serum CEA and the
patients who had high serum CEA. It is presumed that the level of
CEA could not predict acquired resistance to EGFR-TKIs.
In summary, the present study suggests that the
relative pre-therapeutic CEA level can predict the extent of
benefits from EGFR-TKIs, but can not predict the acquired drug
resistance to EGFR-TKIs therapy in patients with EGFR mutations.
However, the current study is believed to possess some limitations.
Firstly, the current study was the limited sample size of 100
patient cases. Secondly, it is uncertain whether the serum CEA
level actually represents a rich mutant EGFR component in each
patient. Further basic research is needed to clarify the possible
molecular mechanisms behind this association.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Han JY, Park K, Kim SW, Lee DH, Kim HY,
Kim HT, Ahn MJ, Yun T, Ahn JS, Suh C, et al: First-SIGNAL:
First-line single-agent iressa versus gemcitabine and cisplatin
trial in never-smokers with adenocarcinoma of the lung. J Clin
Oncol. 30:1122–1128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al North-East Japan Study Group, : Gefitinib or chemotherapy for
non-small-cell lung cancer with mutated EGFR. N Engl J Med.
362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mitsudomi T, Morita S, Yatabe Y, Negoro S,
Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et
al West Japan Oncology Group, : Gefitinib versus cisplatin plus
docetaxel in patients with non-small-cell lung cancer harbouring
mutations of the epidermal growth factor receptor (WJTOG3405): An
open label, randomised phase 3 trial. Lancet Oncol. 11:121–128.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rosell R, Carcereny E, Gervais R,
Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R,
Pallares C, Sanchez JM, et al Spanish Lung Cancer Group in
collaboration with Groupe Français de Pneumo-Cancérologie and
Associazione Italiana Oncologia Toracica, : Erlotinib versus
standard chemotherapy as first-line treatment for European patients
with advanced EGFR mutation-positive non-small-cell lung cancer
(EURTAC): A multicentre, open-label, randomised phase 3 trial.
Lancet Oncol. 13:239–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kawaguchi T, Matsumura A, Fukai S, Tamura
A, Saito R, Zell JA, Maruyama Y, Ziogas A, Kawahara M and Ou SH
Ignatius: Japanese ethnicity compared with Caucasian ethnicity and
never-smoking status are independent favorable prognostic factors
for overall survival in non-small cell lung cancer: A collaborative
epidemiologic study of the National Hospital Organization Study
Group for Lung Cancer (NHSGLC) in Japan and a Southern California
Regional Cancer Registry databases. J Thorac Oncol. 5:1001–1010.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rosell R, Moran T, Queralt C, Porta R,
Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M,
et al Spanish Lung Cancer Group, : Screening for epidermal growth
factor receptor mutations in lung cancer. N Engl J Med.
361:958–967. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Saijo N, Takeuchi M and Kunitoh H: Reasons
for response differences seen in the V15-32, INTEREST and IPASS
trials. Nat Rev Clin Oncol. 6:287–294. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang JC, Shih JY, Su WC, Hsia TC, Tsai CM,
Ou SH, Yu CJ, Chang GC, Ho CL, Sequist LV, et al: Afatinib for
patients with lung adenocarcinoma and epidermal growth factor
receptor mutations (LUX-Lung 2): A phase 2 trial. Lancet Oncol.
13:539–548. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sequist LV, Waltman BA, Dias-Santagata D,
Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger
S, Cosper AK, et al: Genotypic and histological evolution of lung
cancers acquiring resistance to EGFR inhibitors. Sci Transl Med.
3:75ra262011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kobayashi S, Boggon TJ, Dayaram T, Jänne
PA, Kocher O, Meyerson M, Johnson BE, Eck MJ, Tenen DG and Halmos
B: EGFR mutation and resistance of non-small-cell lung cancer to
gefitinib. N Engl J Med. 352:786–792. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Engelman JA, Zejnullahu K, Mitsudomi T,
Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen
J, et al: MET amplification leads to gefitinib resistance in lung
cancer by activating ERBB3 signaling. Science. 316:1039–1043. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bean J, Brennan C, Shih JY, Riely G, Viale
A, Wang L, Chitale D, Motoi N, Szoke J, Broderick S, et al: MET
amplification occurs with or without T790M mutations in EGFR mutant
lung tumors with acquired resistance to gefitinib or erlotinib.
Proc Natl Acad Sci USA. 104:pp. 20932–20937. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zakowski MF, Ladanyi M and Kris MG;
Memorial Sloan-Kettering Cancer Center Lung Cancer OncoGenome
Group, : EGFR mutations in small-cell lung cancers in patients who
have never smoked. N Engl J Med. 355:213–215. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chmielecki J, Foo J, Oxnard GR, Hutchinson
K, Ohashi K, Somwar R, Wang L, Amato KR, Arcila M, Sos ML, et al:
Optimization of dosing for EGFR-mutant non-small cell lung cancer
with evolutionary cancer modeling. Sci Transl Med. 3:90ra592011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chaft JE, Oxnard GR, Sima CS, Kris MG,
Miller VA and Riely GJ: Disease flare after tyrosine kinase
inhibitor discontinuation in patients with EGFR-mutant lung cancer
and acquired resistance to erlotinib or gefitinib: Implications for
clinical trial design. Clin Cancer Res. 17:6298–6303. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bergman B, Brezicka FT, Engström CP and
Larsson S: Clinical usefulness of serum assays of neuron-specific
enolase, carcinoembryonic antigen and CA-50 antigen in the
diagnosis of lung cancer. Eur J Cancer. 29A:198–202. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kleisbauer JP, Castelnau O, Thomas P,
Ramirez J, Lanteaume A and Roux F: Prognostic value of
pre-therapeutic levels of carcino-embryonic antigen in primary
bronchial carcinoma]. Bull Cancer. 82:1019–1024. 1995.(In French).
PubMed/NCBI
|
|
21
|
Kulpa J, Wójcik E, Reinfuss M and
Kołodziejski L: Carcinoembryonic antigen, squamous cell carcinoma
antigen, CYFRA 21-1, and neuron-specific enolase in squamous cell
lung cancer patients. Clin Chem. 48:1931–1937. 2002.PubMed/NCBI
|
|
22
|
Nisman B, Lafair J, Heching N, Lyass O,
Baras M, Peretz T and Barak V: Evaluation of tissue polypeptide
specific antigen, CYFRA 21-1, and carcinoembryonic antigen in
nonsmall cell lung carcinoma: Does the combined use of cytokeratin
markers give any additional information? Cancer. 82:1850–1859.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jung M, Kim SH, Hong S, Kang YA, Kim SK,
Chang J, Rha SY, Kim JH, Kim DJ and Cho BC: Prognostic and
predictive value of carcinoembryonic antigen and cytokeratin-19
fragments levels in advanced non-small cell lung cancer patients
treated with gefitinib or erlotinib. Yonsei Med J. 53:931–939.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Okamoto T, Nakamura T, Ikeda J, Maruyama
R, Shoji F, Miyake T, Wataya H and Ichinose Y: Serum
carcinoembryonic antigen as a predictive marker for sensitivity to
gefitinib in advanced non-small cell lung cancer. Eur J Cancer.
41:1286–1290. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Marshall HM, Leong SC, Bowman RV, Yang IA
and Fong KM: The science behind the 7th edition Tumour, Node,
Metastasis staging system for lung cancer. Respirology. 17:247–260.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brundage MD, Davies D and Mackillop WJ:
Prognostic factors in non-small cell lung cancer: A decade of
progress. Chest. 122:1037–1057. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Desch CE, Benson AB III, Somerfield MR,
Flynn PJ, Krause C, Loprinzi CL, Minsky BD, Pfister DG, Virgo KS
and Petrelli NJ; American Society of Clinical Oncology, :
Colorectal cancer surveillance: 2005 update of an American Society
of Clinical Oncology practice guideline. J Clin Oncol.
23:8512–8519. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin JK, Lin CC, Yang SH, Wang HS, Jiang
JK, Lan YT, Lin TC, Li AF, Chen WS and Chang SC: Early
postoperative CEA level is a better prognostic indicator than is
preoperative CEA level in predicting prognosis of patients with
curable colorectal cancer. Int J Colorectal Dis. 26:1135–1141.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee WS, Baek JH, Kim KK and Park YH: The
prognostic significant of percentage drop in serum CEA post
curative resection for colon cancer. Surg Oncol. 21:45–51. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kozu Y, Maniwa T, Takahashi S, Isaka M,
Ohde Y and Nakajima T: Prognostic significance of postoperative
serum carcinoembryonic antigen levels in patients with completely
resected pathological-stage I non-small cell lung cancer. J
Cardiothorac Surg. 8:1062013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Okada M, Nishio W, Sakamoto T, Uchino K,
Yuki T, Nakagawa A and Tsubota N: Prognostic significance of
perioperative serum carcinoembryonic antigen in non-small cell lung
cancer: Analysis of 1,000 consecutive resections for clinical stage
I disease. Ann Thorac Surg. 78:216–221. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Foa P, Fornier M, Miceli R, Seregni E,
Santambrogio L, Nosotti M, Cataldo I, Sala M, Caldiera S and
Bombardieri E: Tumour markers CEA NSE, SCC, TPA and CYFRA 21.1 in
resectable non-small cell lung cancer. Anticancer Res. 19(4C):
3613–3618. 1999.PubMed/NCBI
|
|
33
|
Reinmuth N, Brandt B, Semik M, Kunze WP,
Achatzy R, Scheld HH, Broermann P, Berdel WE, Macha HN and Thomas
M: Prognostic impact of Cyfra21-1 and other serum markers in
completely resected non-small cell lung cancer. Lung Cancer.
36:265–270. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shigematsu H, Lin L, Takahashi T, Nomura
M, Suzuki M, Wistuba II, Fong KM, Lee H, Toyooka S, Shimizu N, et
al: Clinical and biological features associated with epidermal
growth factor receptor gene mutations in lung cancers. J Natl
Cancer Inst. 97:339–346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu YL, Zhong WZ, Li LY, Zhang XT, Zhang L,
Zhou CC, Liu W, Jiang B, Mu XL, Lin JY, et al: Epidermal growth
factor receptor mutations and their correlation with gefitinib
therapy in patients with non-small cell lung cancer: A
meta-analysis based on updated individual patient data from six
medical centers in mainland China. J Thorac Oncol. 2:430–439. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shoji F, Yoshino I, Yano T, Kometani T,
Ohba T, Kouso H, Takenaka T, Miura N, Okazaki H and Maehara Y:
Serum carcinoembryonic antigen level is associated with epidermal
growth factor receptor mutations in recurrent lung adenocarcinomas.
Cancer. 110:2793–2798. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wirth T, Soeth E, Czubayko F and Juhl H:
Inhibition of endogenous carcinoembryonic antigen (CEA) increases
the apoptotic rate of colon cancer cells and inhibits metastatic
tumor growth. Clin Exp Metastasis. 19:155–160. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ordoñez C, Screaton RA, Ilantzis C and
Stanners CP: Human carcinoembryonic antigen functions as a general
inhibitor of anoikis. Cancer Res. 60:3419–3424. 2000.PubMed/NCBI
|
|
39
|
Cappuzzo F, Magrini E, Ceresoli GL,
Bartolini S, Rossi E, Ludovini V, Gregorc V, Ligorio C, Cancellieri
A, Damiani S, et al: Akt phosphorylation and gefitinib efficacy in
patients with advanced non-small-cell lung cancer. J Natl Cancer
Inst. 96:1133–1141. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jiang J, Greulich H, Jänne PA, Sellers WR,
Meyerson M and Griffin JD: Epidermal growth factor-independent
transformation of Ba/F3 cells with cancer-derived epidermal growth
factor receptor mutants induces gefitinib-sensitive cell cycle
progression. Cancer Res. 65:8968–8974. 2005. View Article : Google Scholar : PubMed/NCBI
|