Introduction
Persistent liver injury resembles the chronic wound
healing response, which consequently leads to liver fibrosis. Liver
fibrosis is defined as an excessive accumulation of extracellular
matrix (ECM) proteins in the tissue arising in a wide spectrum of
disorders including viral hepatitis, metabolic and alcohol
consumption related diseases (1,2). In spite of
different possible mechanisms behind the establishment of the liver
fibrosis, some dimension of phenomena needs to be defined more.
Among liver resident cells, hepatic stellate cells
(HSCs) have a profound role in fibrosis development, which is
demonstrated by ECM production and deposition. The persistence
activation of HSCs following tissue injury is attributed as the
most essential event underlying liver fibrogenesis (3). During the fibrosis progress, quiescent
HSCs transform into myofibroblast-like cells, which acquire
modified phenotype with plentiful secretion of ECM proteins
(4).
Leptin, a 16-kDa hormone has direct effects on
angiogenesis, immunity and hematopoiesis as well as the metabolism
of liver cells. This protein has been employed by numerous groups
for experimental liver fibrogenesis (5). In vitro fibrosis studies
demonstrated that serum starvation and leptin treatment together
are sufficient to activate HSC model cells, LX-2, due to
significant elevated ECM and α-smooth muscle actin level in culture
(6,7).
Moreover, several studies have shown that leptin serves an
important role in inflammation and related pathogenesis in liver
particularly in fibrosis process. The plasma level of leptin has
been reported to be increased in cirrhosis and steatohepatitis
suffering patients (8,9). The results suggested leptin as a reliable
fibrosis induction molecule with the propensity of inflammation
establishment (5,10).
Interleukin (IL)-24/melanoma differentiation
associated gene (mda)-7 is a member of the IL-10 cytokine family
that performs a unique antitumor activity. Historically, mda-7 was
discovered using the subtraction hybridization method on cDNA
libraries from melanoma cells, that its reduced expression was
significant. (11,12). It serves an important role in
fibroproliferative diseases, including pulmonary fibrosis, chronic
kidney diseases, inflammatory bowel diseases and cardiovascular
diseases (13).
The cytokine exhibits different site-dependent
activities regardless of its family common properties. Whereas the
pro-inflammatory role is almost supposed as the main function of
IL-24/mda7, the anti-proliferative features and differentiation
induction impact was also assigned to it. IL-24/mda-7 is expressed
mainly in cells like NK cells, melanocytes, B cells, dendritic
cells and monocytes (14).
The cytokine has two heterodimeric receptors,
IL-22R1/IL-20R2 and IL-20R1/IL-20R2, which, like other cytokines,
is triggered through the Jak-STAT pathway (15). As IL-24/mda-7 exhibits characteristics
of apoptosis induction in transforming cells, its utility for
cancer gene therapy approaches in heavy is considered by several
groups (16,17). Furthermore, a recent study has
indicated that the IL-10 cytokine family can be effective in ECM
production with a pivotal role in fibrosis, but the underlying
mechanism by which IL-24/mda-7 may inhibit ECM production, is still
unknown (13).
In spite of studies regarding the role of
IL-24/mda-7 in psoriasis and inflammatory bowel diseases, the
possible role in other diseases needs to be investigated. As an
inflammatory-related disease, fibrosis involved cells may also be
affected by the expression level of this cytokine. So it is
noteworthy to clarify if the expression levels of IL-24/mda-7
protein and cognate receptors in HSC cells are changed following
activation. The aims of the study were to assess whether the
fibrogenic actions of leptin leaves significant changes in
IL-24/mda-7 secretion and cognate receptor expression in the LX-2
cell line, a human-derived HSCs.
Materials and methods
Cell culture
LX-2, an immortalized human HSC line, was a gift
provided by Professor Friedman (Mount Sinai School of Medicine, New
York, NY, USA). All the details of the generation of this unique
line have been described previously (18). The cell line was cultured in Dulbecco's
modified Eagle's medium (DMEM) complemented with 5% fetal bovine
serum (FBS), 1% non-essential amino acids, 100 U/ml penicillin and
100 mg/ml streptomycin. Cells were distributed in six-well plates
in 200,000 cells/well and treatment was performed when their
confluence reached 80%. They were incubated under humidified
standard condition, at 37°C and 5% CO2. The activation
procedure, leptin addition and serum starvation concomitant were
applied on LX-2 cells for 24 h. For serum starvation conditions, 1%
DMEM was employed instead of 5% DMEM. The purified Leptin was used
at concentrations of 25–100 ng/ml.
Real-time PCR analysis
Total cellular RNA was isolated from inactive LX-2
as control and active LX-2 (treated with leptin) by RNA extraction
kit (CinnaGen Inc., Tehran, Iran). The quantity and quality of
obtained RNA were checked by measuring the ratio of optical density
of 260/280 nm using Nanodrop™ spectrophotometer
(Nanodrop; Thermo Fisher Scientific, Wilmington, DE, USA) and then
was stored at −80°C until cDNA synthesis. The cDNA was then
synthesized using 1,000 ng total RNA in a first-strand cDNA
synthesis reaction by the help of RevertAid™ First
Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc., Waltham,
MA, USA). Reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) was performed using the ABI Biosystems StepOne
and the RealQ Plus 2x Master Mix Green (Ampliqon A/S, Odense,
Denmark). In each reaction, 200 nM of each primer (Table I) was added to target the specific
sequence. Specific primers targeting IL-24/mda7, IL-20R2, IL-22R1,
collagen (Col)-I, TIMP metalloproteinase inhibitor-1 (TIMP-1) and
transforming growth factor (TGF)-β were designed as shown in
Table I. The PGK housekeeping gene was
also used as internal control of qPCR reactions. The qPCR
conditions were set for 10 min at 94°C followed by 40 cycles of 15
sec at 94°C, 60 sec at 58°C and final extension of 7 min at 72°C.
The amplification signals of different samples were normalized to
PGK cycle threshold (Ct), and then 2−∆∆Cq method was
applied for comparing mRNA levels of activated vs. the control
(normal LX-2), which represented as fold-change in data analysis
(19).
 | Table I.Primers used in the present study. |
Table I.
Primers used in the present study.
| Gene name | Primer Sequence
(5′-3′) | Size (bp) |
|---|
| IL-24/mda-7 | Forward:
TGTGAAAGACACTATGCAAGCTC | 224 |
|
| Reverse:
GTGACACGATGAGAACAAAGTTG |
|
| IL-20R2 | Forward:
AGGCCCAGACATTCGTGAAG | 138 |
|
| Reverse:
CGACCACAAGGATCAGCATGA |
|
| IL-22R1 | Forward:
TGCTGACCATCTTGACTGTG | 181 |
|
| Reverse:
TCCCTCTCTCCGTACGTCTTAT |
|
| TGF-β | Forward:
AAGGCGAAAGCCCTCAATTT | 136 |
|
| Reverse:
CAGCAACAATTCCTGGCGATA |
|
| Col-I | Forward:
GAGGGCCAAGACGAAGACATC | 140 |
|
| Reverse:
CAGATCACGTCATCGCACAAC |
|
| TIMP-1 | Forward:
TACTTCCACAGGTCCCACAAC | 202 |
|
| Reverse:
GTTTGCAGGGGATGGATAAAC |
|
| PGK | Forward:
TAAAGCCGAGCCAGCCAAAA | 116 |
|
| Reverse:
CTCCTACCATGGAGCTGTGG |
|
ELISA
At ~48 h following LX-2 cell activation with leptin
and serum starvation, the culture supernatants were accumulated for
measurement of the released TGF-β by a human-mouse TGF-β ELISA kit
(eBioscience Inc., San Diego, CA, USA,) according to the
manufacturer's instructions. The final absorbance was read at 450
nm by the ELISA reader with using a FLUOstar OMEGA microplate
reader and software (BMG Labtech Ltd., Aylesbury, UK). and the
concentration of TGF-β protein was evaluated comparing with a
standard curve.
In addition, the culture supernatants from the
leptin-treated and untreated LX-2 cells were collected following 24
and 48 h of incubation. Then they were evaluated using an ELISA
kit, IL-24/mda-7 human ELISA kit (Abcam, Cambridge, MA, USA; cat.
no. ab171345). The final absorbance was read at 450 nm and the
concentration of IL-24/mda-7 protein was evaluated comparing with a
standard curve.
Statistical analysis
Data were expressed as the mean ± standard error of
the mean and were analyzed by independent samples t-test, using
GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference between group means.
Results
Leptin treatment activates LX-2
fibrotic phenotype
To complete the activation process of LX-2, leptin
treatment, concomitant with serum starvation was applied on culture
media. Leptin treatment with serum starvation could induce
conversion of LX-2 cells to activate a phenotype characterized by
veiled morphology in the culture plate (20) and expression of pro-fibrogenic genes,
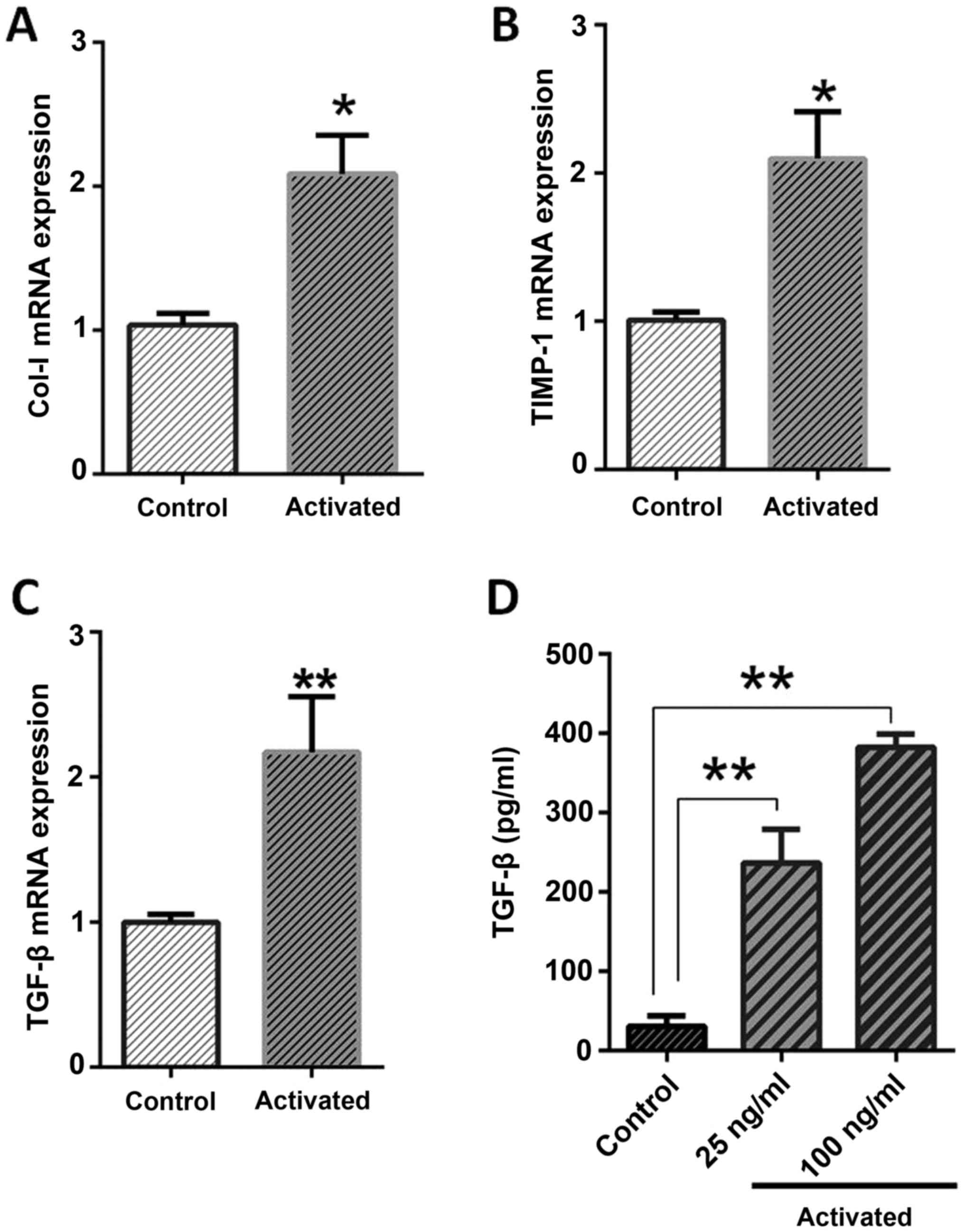
including Col-I, TIMP-1 and TGF-β.
The expression analysis of pro-fibrotic genes
indicated that, in activated LX-2, the expression pattern of TGF-β
(P=0.0061), Col-I (P=0.012) and TIMP-1 (P=0.0047) were
significantly higher than normal cells, as depicted in Fig. 1. The ELISA results were also in
agreement with gene expression, as the concentration of TGF-β
protein in the culture supernatant was significantly higher than in
normal cells (P<0.0001). The TGF-β protein concentrations were
determined near to 382.5 pg/ml, 237 pg/ml for 100 ng and 25 ng
leptin treatments, respectively, in comparison to 30.5 pg/ml for
normal cells (Fig. 1).
Expression of IL-24/mda-7 in LX-2
cells
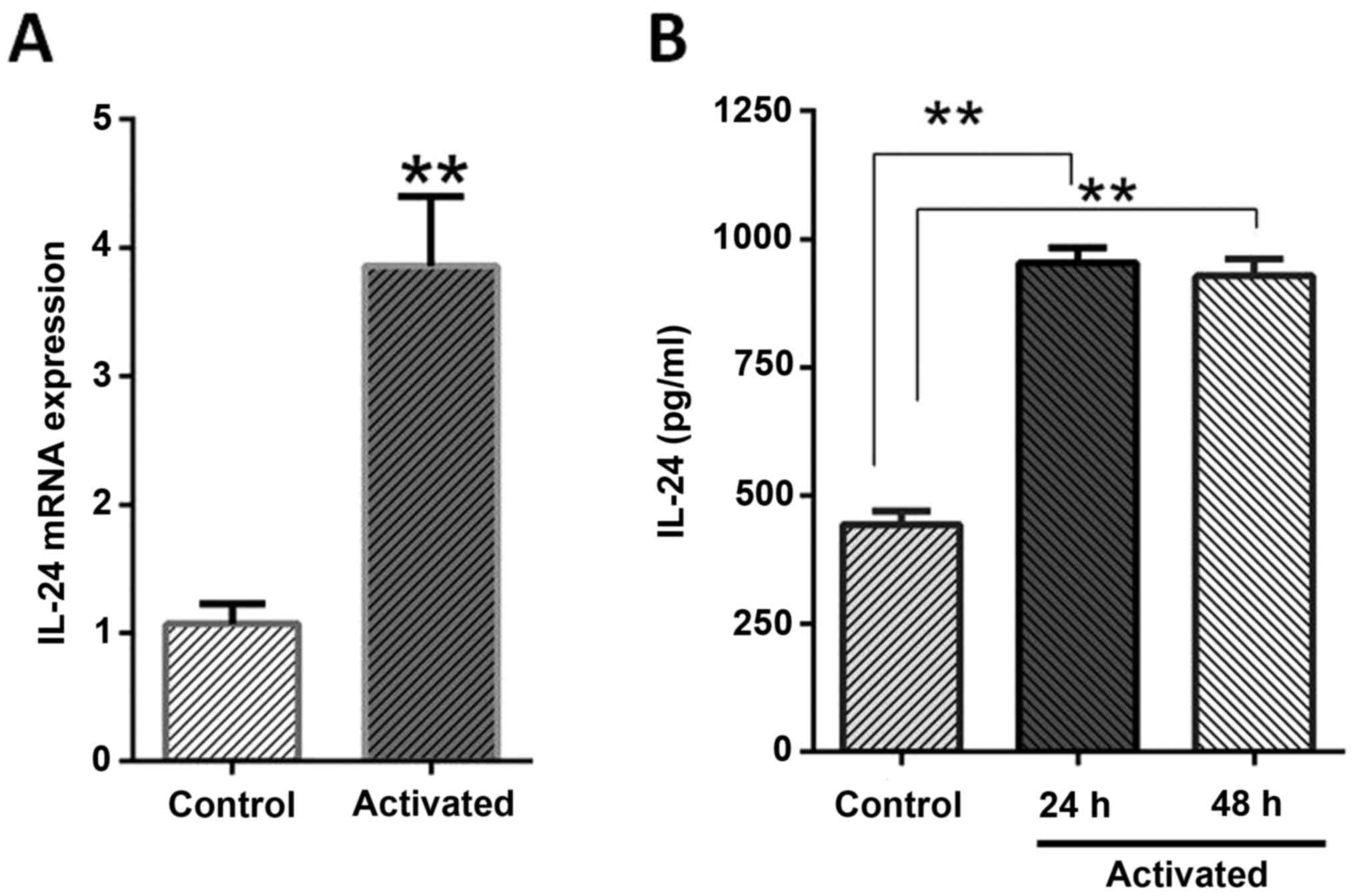
When the expression pattern of IL-24/mda-7 was
evaluated in mRNA and protein levels, it revealed that LX-2 cells
normally expressed this protein as detected by RT-qPCR and ELISA
methods. It was also identified that activation phenotype was
associated with a two-folds increment in IL-24/mda-7 mRNA when
compared to normal cells (P=0.0002). The ELISA results were also in
agreement with gene expression as the concentration of IL-24/mda-7
protein in culture supernatant was significantly higher than normal
cells (P<0.01). The IL-24/mda-7 protein concentration was
determined near to 961.1 pg/ml, 918.1 pg/ml and 438.5 pg/ml for 24
and 48 h treatments and for normal cells, respectively (Fig. 2).
Expression of IL-24/mda-7 receptors in
LX-2 cell lines
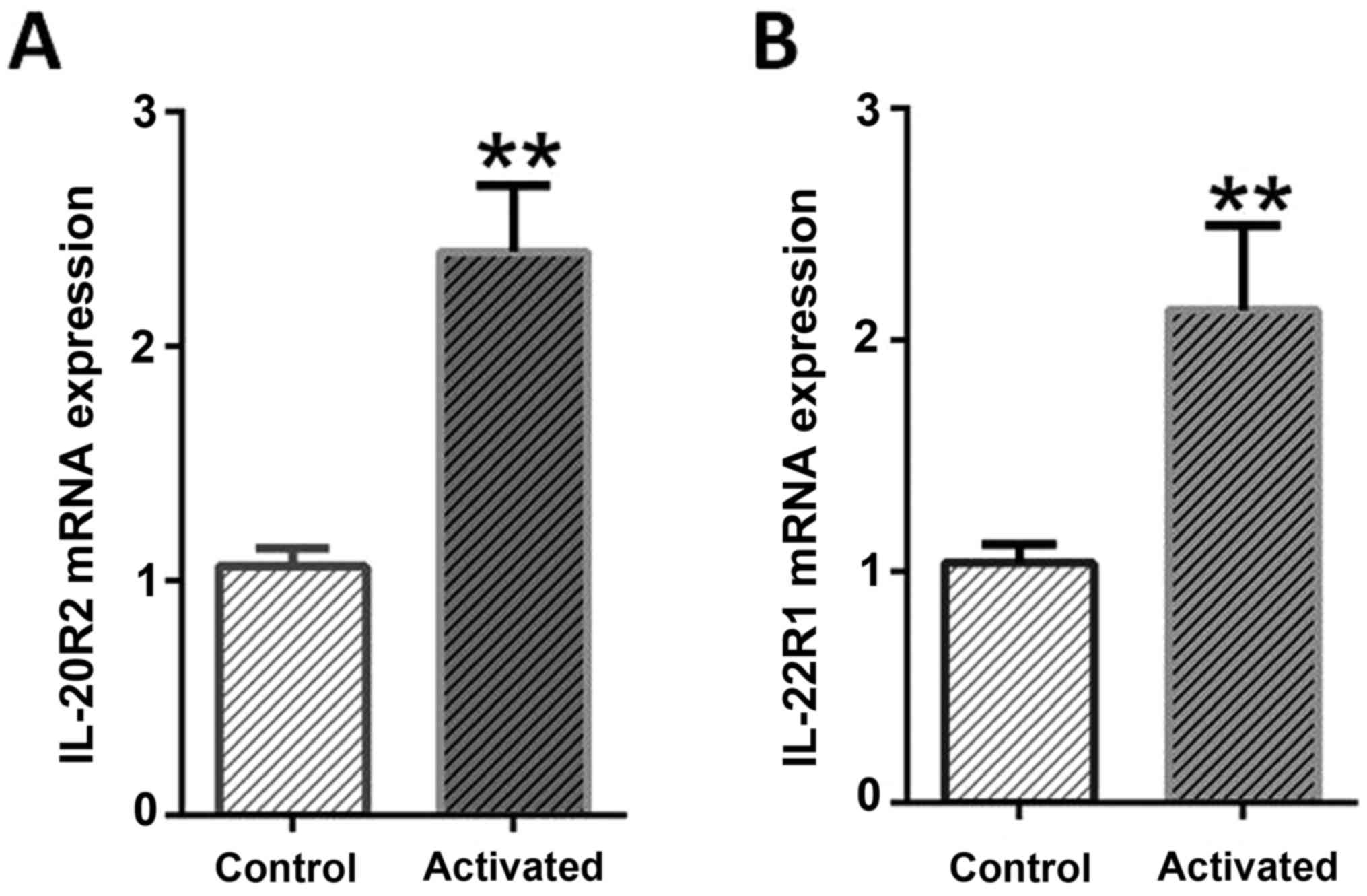
When the expression level of two different
IL-24/mda-7 receptors mRNAs (IL-20R1 and IL-22R1) were monitored
through RT-qPCR, these were demonstrated that in normal cells, both
receptors were expressed at least in mRNA level. Hence, in
activated LX-2, a significant elevation of IL-20R1 (P<0.0001)
and IL-22R1 receptors with more than >2-fold changes (P=0.0079)
were detectable when compared to normal cells. While the expression
changes of both receptors were similar, IL-20R2 elicited more than
IL-22R following activation process. These results suggested that
stress condition (leptin and serum starvation) as expected, lead to
a significant increase in the expression pattern of both
investigated IL-24/mda-7 receptors (Fig.
3).
Discussion
The pathophysiology of fibrosis as a durable
response remained unresolved in some fields. The possible role of
IL-10 family members such as IL-20, IL-22 and, more recently,
IL-24/mda-7, started to be delineated more in fibrosis development
(13). Even though the general player
molecules involved in HSC cell activation and transition into
myofibroblasts have been studied deeply, the possible role of
IL-24/mda7 needs to be investigated in liver fibrosis. Based on the
authors' knowledge, this is the first report considering
IL-24/mda-7 importance in HSC activation.
The increased expression of IL-24/mda-7 in relevance
to the renovation of the kidney was recently reported (21). As mentioned, liver injury is similar to
other chronic wound healing responses, which consequently leads to
liver fibrosis. In this context, the aims of the present study were
to evaluate whether IL-24/mda-7 and cognate receptor (IL-22R1 and
IL-20R2) subunits were expressed in LX2 cells and what will happen
to their expression level in response to in vitro
activation. The results indicated that stress conditions
encompassing leptin treatment and starvation induce LX2 cell
activation, confirmed by the higher expression of TGF-β, TIMP-1 and
collagen profibrotic genes as described in a previous study
(22). The data were correlated with
previous reports that claimed leptin to prompt fibrosis signaling
pathways (6,23). The results demonstrated that the LX-2
cells have been activated.
Based on the first findings, it was shown that
IL-24/mda7 was expressed in measurable levels even before
activation, as mRNA and protein were detectable by RT-qPCR and
ELISA. Besides, it was indicated that, in activated cells,
IL-24/mda-7 expression was significantly noticeable compared to
baseline expression of normal LX-2. The expression levels of IL-24
in activated LX-2 cells are steady state around 24 and 48 h. This
data correlated with the increases seen in hepatic IL-22 mRNA
levels. IL-22 has an important role in the liver regeneration and
this cytokine has functional similarities to IL-24/mda-7 (24,25).
The reports considering IL-24/mda-7 expression in
human HSCs are limited. Although, expression level of IL-20 and
IL-22 were evaluated in human and mice stellate cells, comparing
IL-24/mda-7 expression in normal or activated HSCs is rare
(13,26). New research revealed that IL-22,
another member of the IL-10 cytokine family, promotes HSC
proliferation and activation, prevents HSC apoptosis, induces the
overexpression of HSC growth factors and fibrosis markers including
Col-IV, laminin and hyaluronic acid (27). But unlike IL-22, no definite role in
liver fibrosis was assigned for IL-24/mda-7, in spite of various
studies that have reported its anticancer properties (24,28).
It must be noted that LX-2 is accepted as a
semi-activated cell, so the level of IL-24/mda-7 in the current
experiments may not independently reflect the state of completely
normal human stellate cells. Previously, Imaeda et al
(26) detected a significant level of
expression for IL-24/mda-7 in pancreatic stellate cells. They
supposed that this cytokine may have a role in the pathology of
chronic pancreatitis cases. Whereas the exact mechanism responsible
for IL-24/mda-7 expression is not described before, an involvement
of MAPK pathway and STAT3 activation is under consideration
(29,30).
It is reported that IL-24/mda-7 is expressed by
various cell types including B cells, dendritic cells, natural
killer cells, melanocytes and monocytes (31). Despite the ambiguous role in immunity,
increased expression severely correlates with differentiation state
of the cells. Regarding TGF-β in fibrosis development, it may be
proposed that IL-24/mda-7 acts in a similar manner. In other words,
an increase in IL-24/mda-7 expression may be a controlling feedback
in early fibrosis that thereafter exacerbate the responsible
mechanisms (32). Conversely, to
compare with other cell lines such as HepG2, it was indicated that
IL-24/mda-7 has higher expression in the LX-2 cell line, which
reveals the important role of this protein in activation of LX-2
cell line (33). The mouse
IL-24/mda-7-like molecule, termed FISP (IL-4-induced secreted
protein) and the rat IL-24/mda-7-like molecule, termed C49A,
increased in wound repair (34). This
observation led to the hypothesis that expression of IL-24/mda-7
in vivo may exhibit impact on the fibrogenesis process.
However, some other properties are not similar among species
indicating their principle differences. It may be predicted that
the investigation of IL-24/mda7 in other species may mislead
scientists. It should be noted that evaluation of fibrotic gene
expression for mediating treatment responses to IL-24/mda-7 will be
the subject of ongoing study in animal models. Also, assessment of
the expression level for this protein and cognate receptors should
be performed by other methods such as western blotting,
immunohistochemistry or immunoblotting, a limitation of the current
study.
In order to perform its autocrine function,
IL-24/mda-7 binds and signals through the heterodimeric
IL-22R1/IL-20R2 and IL-20R1/IL-20R2 receptors, following by STAT-3
phosphorylation. The effectiveness of IL-24/mda-7 is dependent on
the presence of these receptors on target cells. The finding
revealed that IL-20R2 and IL-22R1 receptors were not only expressed
on the LX-2 cells, but were also upregulated following cell
activation. One of the reasons that the authors focused on
IL-22R1/IL-20R2 instead of the other heterodimers is because of the
interaction between IL-22R1/IL-20R2 and IL-24 receptor complexes is
a key mediator for the wound healing response, a process that
mimics fibrosis in the liver (35).
In addition, Imaeda et al demonstrated that
these receptors were detectable in chronic pancreatitis tissue of
suffering patients (26). The
expression of IL-20R2 and IL-22R1 was also investigated elsewhere,
and IL-22R1 expression was reported to be distributed among the
kidney, liver, pancreas and skin (13).
Furthermore, in another study, it was revealed that
IL-20R1 and IL-20R2 were expressed in synovial cells (RASCs), and
the results indicated that RASCs had constitutive expression of
IL-20R1 and IL-20R2 (36).
Furthermore, Kunz et al identified that IL-20R2 expression
levels in keratinocytes increased followed by STAT3 activation
(30). Conversely, Sarkar et al
(34) analyzed 12 mycoplasma-free
melanoma cell lines finding that lack IL-24/mda-7 receptors in some
mycoplasma-free melanoma cell lines disrupted Jak/STAT pathway.
However, the expression of IL-24/mda-7 receptors in a liver cell
line, such as HepG2, is still not well understood. Results of the
current study have provided evidence that IL-22RA1 and IL-20R2 were
consistently expressed in active and normal LX-2 cell lines. In
future study, more reliable results can be achieved by analyzing
IL22R1/IL-20R2 protein expression with western blotting.
In conclusion, expression of IL-24/mda-7 and its
cognate receptors in the normal LX-2 cell line emphasized their
possible impact on fibrogenesis process. In addition, leptin could
increase the expression of IL-24/mda-7 and its receptors implying
that IL-24/mda-7 ought to be investigated more as a new target for
fibrosis therapy. The unraveling of the unknown mechanism underling
IL-24/mda-7 function during fibrosis may also be useful to
understand the pathology of the disease and to open a path for new
therapeutic drugs.
References
|
1
|
Rockey DC: Current and future
anti-fibrotic therapies for chronic liver disease. Clin Liver Dis.
12:939–962. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bataller R and Brenner DA: Liver fibrosis.
J Clin Invest. 115:209–218. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kisseleva T and Brenner DA: Role of
hepatic stellate cells in fibrogenesis and the reversal of
fibrosis. J Gastroenterol Hepatol. 22 Suppl 1:S73–S78. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang JX and Török NJ: Liver injury and
the activation of the hepatic myofibroblasts. Curr Pathobiol Rep.
1:215–223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Loffreda S, Yang SQ, Lin HZ, Karp CL,
Brengman ML, Wang DJ, Klein AS, Bulkley GB, Bao C, Noble PW, et al:
Leptin regulates proinflammatory immune responses. FASEB J.
12:57–65. 1998.PubMed/NCBI
|
|
6
|
Cao Q, Mak KM and Lieber CS: Leptin
represses matrix metalloproteinase-1 gene expression in LX2 human
hepatic stellate cells. J Hepatol. 46:124–133. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yegorov YE, Akimov SS, Hass R, Zelenin AV
and Prudovsky IA: Endogenous beta-galactosidase activity in
continuously nonproliferating cells. Exp Cell Res. 243:207–211.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Uygun A, Kadayifci A, Yesilova Z, Erdil A,
Yaman H, Saka M, Deveci MS, Bagci S, Gulsen M, Karaeren N and
Dagalp K: Serum leptin levels in patients with nonalcoholic
steatohepatitis. Am J Gastroenterol. 95:3584–3589. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saxena NK, Saliba G, Floyd JJ and Anania
FA: Leptin induces increased alpha2(I) collagen gene expression in
cultured rat hepatic stellate cells. J Cell Biochem. 89:311–320.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Crespo J, Rivero M, Fábrega E, Cayón A,
Amado JA, García-Unzeta MT and Pons-Romero F: Plasma leptin and
TNF-alpha levels in chronic hepatitis C patients and their
relationship to hepatic fibrosis. Dig Dis Sci. 47:1604–1610. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pestka S, Krause CD, Sarkar D, Walter MR,
Shi Y and Fisher PB: Interleukin-10 and related cytokines and
receptors. Annu Rev Immunol. 22:929–979. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang H, Lin JJ, Su ZZ, Goldstein NI and
Fisher PB: Subtraction hybridization identifies a novel melanoma
differentiation associated gene, mda-7, modulated during human
melanoma differentiation, growth and progression. Oncogene.
11:2477–2486. 1995.PubMed/NCBI
|
|
13
|
Sziksz E, Pap D, Lippai R, Béres NJ,
Fekete A, Szabó AJ and Vannay Á: Fibrosis related inflammatory
mediators: Role of the IL-10 cytokine family. Mediators Inflamm.
2015:7646412015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Patani N, Jones A Douglas, Mansel R, Jiang
W and Mokbel K: Tumour suppressor function of MDA 7/IL 24 in human
breast cancer. Cancer Cell Int. 10:292010.PubMed/NCBI
|
|
15
|
Wang M, Tan Z, Zhang R, Kotenko SV and
Liang P: Interleukin 24 (MDA-7/MOB-5) signals through two
heterodimeric receptors, IL-22R1/IL-20R2 and IL-20R1/IL-20R2. J
Biol Chem. 277:7341–7347. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang EY, Madireddi MT, Gopalkrishnan RV,
Leszczyniecka M, Su Z, Lebedeva IV, Kang D, Jiang H, Lin JJ,
Alexandre D, et al: Genomic structure, chromosomal localization and
expression profile of a novel melanoma differentiation associated
(mda-7) gene with cancer specific growth suppressing and apoptosis
inducing properties. Oncogene. 20:7051–7063. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hosseini E, Hosseini SY, Hashempour T,
Fattahi MR and Sadeghizadeh M: Effect of RGD coupled MDA-7/IL-24 on
apoptosis induction in a hepatocellular carcinoma cell line. Mol
Med Rep. 15:495–501. 2017.PubMed/NCBI
|
|
18
|
Yang C, Zeisberg M, Mosterman B, Sudhakar
A, Yerramalla U, Holthaus K, Xu L, Eng F, Afdhal N and Kalluri R:
Liver fibrosis: Insights into migration of hepatic stellate cells
in response to extracellular matrix and growth factors.
Gastroenterology. 124:147–159. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2-ΔΔCq method. Μethods. 25:402–408. 2001.
|
|
20
|
Hosseini SY, Kalantar K, Shahin K, Ghayour
M, Rajabibazl M, Fattahi MR, et al: Comparison of the in vitro
antifibrogenic effects of silymarin, silybin A and 18α glycyrrhizin
on activated hepatic stellate cells. Jundishapur J Nat Pharm Prod
Inpress. e402852016.
|
|
21
|
Pap D, Sziksz E, Kiss Z, Rokonay R,
Veres-Székely A, Lippai R, Takács IM, Kis É, Fekete A, Reusz G, et
al: Microarray analysis reveals increased expression of matrix
metalloproteases and cytokines of interleukin-20 subfamily in the
kidneys of neonate rats underwent unilateral ureteral obstruction:
A potential role of IL-24 in the regulation of inflammation and
tissue remodeling. Kidney Blood Press Res. 42:16–32. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Khanizadeh S, Ravanshad M, Hosseini S,
Davoodian P, Zadeh A Nejati and Sarvari J: Blocking of SMAD4
expression by shRNA effectively inhibits fibrogenesis of human
hepatic stellate cells. Gastroenterol Hepatol Bed Bench. 8:262–269.
2015.PubMed/NCBI
|
|
23
|
Marra F: Leptin and liver fibrosis: A
matter of fat. Gastroenterology. 122:1529–1532. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tian H, Zhang DF, Zhang BF, Li HZ, Zhang
Q, Li LT, Pei DS and Zheng JN: Melanoma differentiation associated
gene-7/interleukin-24 induces caspase-3 denitrosylation to
facilitate the activation of cancer cell apoptosis. J Interferon
Cytokine Res. 35:157–167. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ren X, Hu B and Colletti LM: IL-22 is
involved in liver regeneration after hepatectomy. Am J Physiol
Gastrointest Liver Physiol. 289:G74–G80. 2009.
|
|
26
|
Imaeda H, Nishida A, Inatomi O, Fujiyama Y
and Andoh A: Expression of interleukin-24 and its receptor in human
pancreatic myofibroblasts. Int J Mol Med. 28:993–999.
2011.PubMed/NCBI
|
|
27
|
Gao Y, Ren H, Meng F, Li J, Cheung E, Li
H, Zhao J, Liu H, Liu Z and Zhang M: Pathological roles of
interleukin-22 in the development of recurrent hepatitis C after
liver transplantation. PLoS One. 11:e01544192016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu CW, Yin GF, Wang XR, Ren BW, Zhang WG,
Bai QL, Lv YM, Li WL and Zhao WQ: IL-24 induces apoptosis via
upregulation of RNA-activated protein kinase and enhances
temozolomide-induced apoptosis in glioma cells. Oncol Res.
22:159–165. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shefler I, Pasmanik-Chor M, Kidron D,
Mekori YA and Hershko AY: T cell-derived microvesicles induce mast
cell production of IL-24: Relevance to inflammatory skin diseases.
J Allergy Clin Immunol. 133:217–224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kunz S, Wolk K, Witte E, Witte K, Doecke
WD, Volk HD, Sterry W, Asadullah K and Sabat R: Interleukin
(IL)-19, IL-20 and IL-24 are produced by and act on keratinocytes
and are distinct from classical ILs. Exp Dermatol. 15:991–1004.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mirzaei MH and Esmaeilzadeh A:
Overexpression of MDA-7/IL-24 as an anticancer cytokine in gene
therapy of thyroid carcinoma. J Med Hypotheses Ideas. 8:7–13. 2014.
View Article : Google Scholar
|
|
32
|
Jiang H, Su ZZ, Lin JJ, Goldstein NI,
Young CS and Fisher PB: The melanoma differentiation associated
gene mda-7 suppresses cancer cell growth. Proc Natl Acad Sci USA.
93:9160–9165. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xiao CW, Xue XB, Zhang H, Gao W, Yu Y,
Chen K, Zheng JW and Wang CJ: Oncolytic adenovirus-mediated
MDA-7/IL-24 overexpression enhances antitumor activity in
hepatocellular carcinoma cell lines. Hepatobiliary Pancreat Dis
Int. 9:615–621. 2010.PubMed/NCBI
|
|
34
|
Sarkar D, Su ZZ, Lebedeva IV, Sauane M,
Gopalkrishnan RV, Dent P and Fisher PB: mda-7 (IL-24): Signaling
and functional roles. Biotechniques. 33:30–39. 2002.
|
|
35
|
Kirsch S: The effect of cytokines of the
Interleukin-10-family on cutaneous wound healing. Freie
Universität; Berlin: 2013, (In German).
|
|
36
|
Sakurai N, Kuroiwa T, Ikeuchi H, Hiramatsu
N, Maeshima A, Kaneko Y, Hiromura K and Nojima Y: Expression of
IL-19 and its receptors in RA: Potential role for synovial
hyperplasia formation. Rheumatology (Oxford). 47:815–820. 2008.
View Article : Google Scholar : PubMed/NCBI
|