Introduction
Impaired glucose tolerance, insulin resistance (IR)
and insulin secretion have been observed in hyperthyroidism and
hypothyroidism (1–6). Known factors responsible for abnormal
glucose tolerance include aberrant metabolic rate, endogenous
gluconeogenesis and glucose absorption, which are affected by
thyroid hormone (7,8).
The American Diabetic Association has recently
approved the use of glycated hemoglobin (HbA1c) as a screening
method, as well as a diagnostic test for diabetes mellitus.
However, previous studies identified that HbA1c is not a reliable
diagnostic test for diabetes in the presence of hyperthyroidism or
hypothyroidism (9,10).
The prevalent use of continuous glucose monitoring
(CGM) has provided details on glycemic variation that were
otherwise not possible with HbA1c or self-monitoring of blood
glucose (SMBG). Torimoto et al (11) reported the use of CGM in a patient with
Graves' disease (GD) complicated by diabetes mellitus. The authors
demonstrated that hyperthyroidism induces elevation of postprandial
blood glucose (PBG) and fasting blood glucose (FBG) levels due to
the dawn phenomenon, and that glycemic variation is alleviated with
improvement in thyroid function. However, in the aforementioned
case, the patient was diabetic and exogenous insulin was used,
which may have interfered with the blood glucose levels.
Radioiodine therapy is one of the most popular
treatment strategies for GD in China. The present study describes a
patient with GD, who presented with hyperthyroidism and
subsequently exhibited hypothyroidism following radioiodine
therapy. CGM was employed to observe the glycemic variation during
the hyperthyroidism and hypothyroidism states.
Case report
The patient was a 28-year-old man. He began to
experience heart palpitations, tremors and weight loss in 2012, and
was diagnosed with GD and treated with methiamazole. The patient
persisted with the treatment for 4 years; however, the thyroid
hormone levels did not return to normal (TSH 0.35–4.9 mIU/l and FT4
9–19 pmol/l). Therefore, the patient chose radioiodine therapy and
stopped the methiamazole treatment on August 2nd 2016. The dose of
methiamazole was 15 mg per day when the drug was withdrawn. There
was no family history of thyroid disease. He admitted to Nanjing
First Hospital on August 10th 2016. The signed informed consent of
radioiodine therapy and including in the present study was
obtained.
Prior to radioiodine therapy, the patient was 179.0
cm tall and weighed 71 kg [body mass index (BMI), 22.16
kg/m2). The patient's blood pressure was 120/80 mmHg and
heart rate was 88 bpm and he exhibited clear consciousness. Diffuse
goiter was noted on clinical examination. The respiratory and heart
sounds were normal, and the neurological examination was
normal.
Prior to radioiodine therapy, laboratory tests
exhibited normal hepatic and renal functions, as well as standard
normal serum electrolytes. Thyrotrophin receptor antibody was 6.38
IU/l (normal range, <1.75 IU/l). Thyroid uptake rates were 95.7%
at 2 h, 97.1% at 6 h and 95.4% at 24 h. The Tc-99m scan indicated
an enlarged thyroid and increased uptake rate.
Oral glucose tolerance tests (OGTTs) using 75 g
glucose (dissolved in 200 ml water) were performed prior to
radioiodine therapy. Serum samples were obtained before, and 30 and
120 min after oral administration of blood glucose, insulin,
C-peptide and glucagon determination. The ratio of glucagon/glucose
(GLA/GLU) and GLA/insulin (INS) were calculated to represent the
inhibition of glucose or insulin on glucagon secretion.
The patient was subjected to 3-day retrospective CGM
(Sof-sensor; CGMS-Gold, Medtronic Incorporated, Northridge, LA,
USA) in the presence of hyperthyroidism and hypothyroidism. During
the CGM period, the patient was instructed to maintain his usual
physical activity and received meals consisting of total caloric
intake of 25 kcal/kg/day. The ratio of carbohydrate, protein and
fats was ~55, 17 and 28%, respectively. The CGM was corrected 4
times per day by SMBG.
The patient was administered 13 m Ciradioactive
iodine (131I) on August 17th 2016. Thyroid function
tests were repeated every month after radioiodine therapy (Table I) and OGTT was performed again when the
thyroid hormone exhibited hypothyroidism on December 23rd 2016,
while blood lipid measurements and CGM were also performed.
 | Table I.Blood thyroid hormone levels. |
Table I.
Blood thyroid hormone levels.
| Hormone | August 8th, 2016 | September 16th,
2016 | October 19th,
2016 | December 16th,
2016 | January 20th,
2017 | Normal range |
|---|
| TSH (mIU/l) |
<0.007 |
<0.007 |
<0.007 | 82.42 | 37.27 | 0.35–4.94 |
| FT3 (pmol/l) | >46.08 | 10.96 | 7.33 | 3.26 | 4.03 | 2.63–5.70 |
| FT4 (pmol/l) |
38.36 | 27.55 | 23.79 | 5.15 | 10.67 |
9.00–19.00 |
OGTTs demonstrated increased blood insulin and
C-peptide levels 30 min after oral glucose administration in the
hypothyroidism state compared with in the hyperthyroidism state,
while the blood glucose levels, and fasting and 120 min-insulin
exhibited little change (Fig. 1A). The
blood glucagon level was suppressed when the patient exhibited
hyperthyroidism, and the glucagon secretion was improved following
radioiodine therapy (Fig. 1B). The
insulin area under the curve (AUC) was higher in the
hyperthyroidism state than in the hypothyroidism state (Fig. 1C). HbA1c decreased 0.2% following
radioiodine therapy. Although homeostatic model assessment of IR
(HOMA-IR) before and after radioiodine therapy was higher than
normal, the HOMA-IR decreased following radioiodine therapy
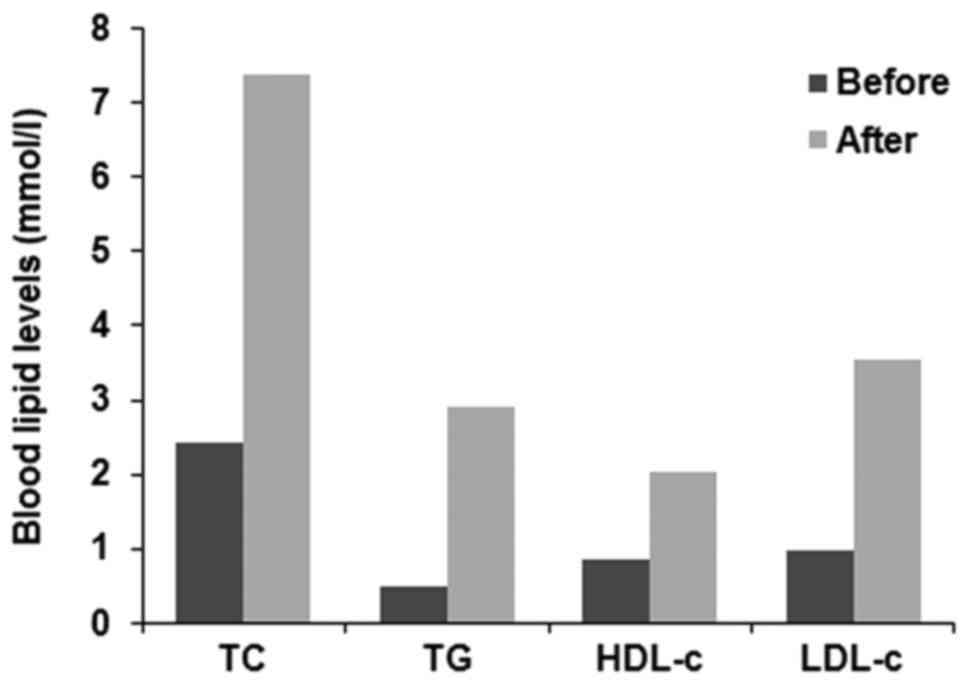
(Fig. 1C). Total cholesterol,
triglyceride, low-density lipoprotein cholesterol (LDL-c) and high
density lipoprotein cholesterol aberrantly increased when the
patient entered the hypothyroidism state (Fig. 2).
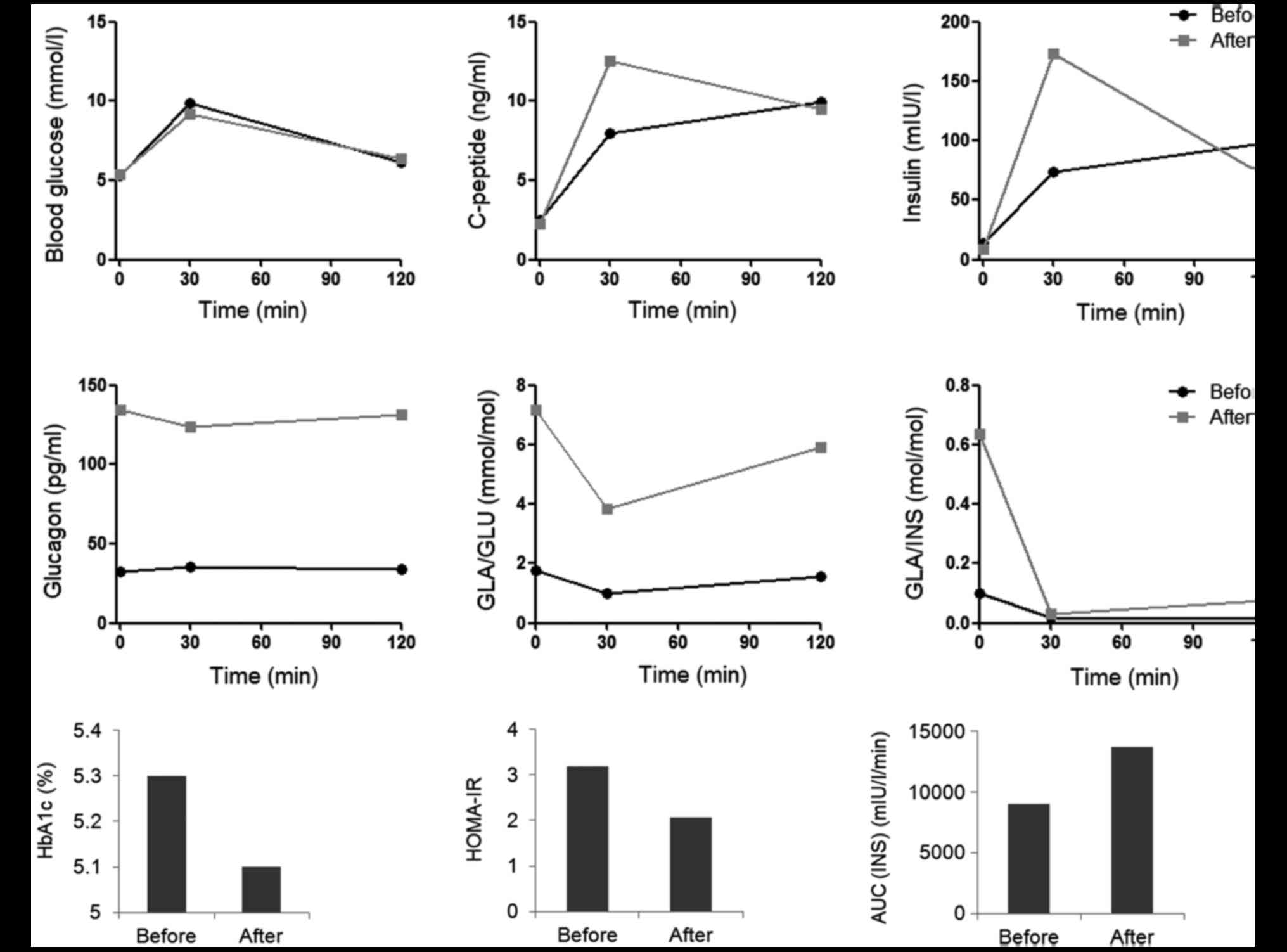 | Figure 1.Glucose metabolism of a patient with
Graves' disease before and after radioiodine therapy. (A) Blood
glucose, insulin and C-peptide before, and 30 and 120 min after
OGTT. (B) Blood glucagon levels and the inhibition of glucose and
insulin on glucagon secretion (GLA/GLU and GLA/INS) in OGTT. (C)
HbA1c, HOMA-IR and AUC (INS) before and after radioiodine therapy.
OGTT, oral glucose tolerance test; HbA1c, glycated hemoglobin; GLA,
glucagon; GLU, glucose; INS, insulin; HOMA-IR, homeostatic model
assessment of insulin resistance; AUC (INS), insulin area under the
curve. |
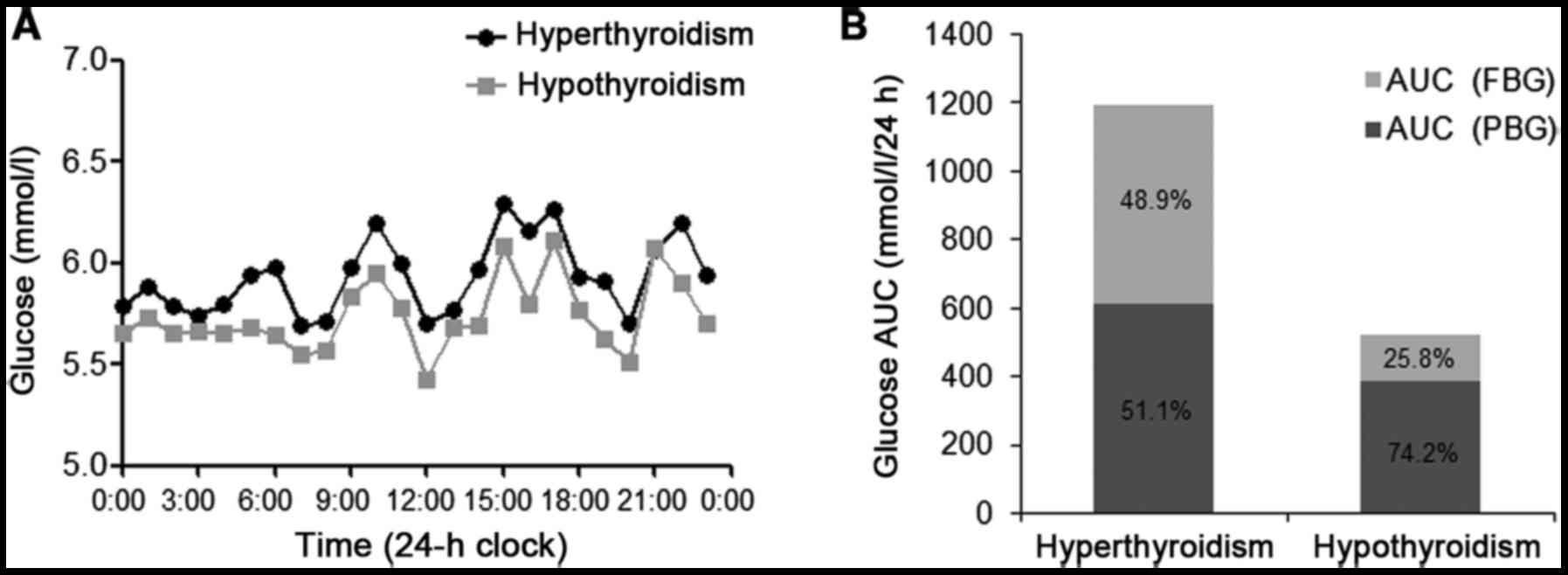
CGM was performed to assess glucose variability
onAugust 11th 2016 and December 23rd 2016 for 3 days. The patient
exhibited a reduction in 24-h mean glucose (MG) following
radioiodine therapy. No significant differences in the standard
deviation of the MG (SDMG) and the 24-h mean amplitude
of glycemic excursion (MAGE) between the hypothyroidism and
hyperthyroidism states (Table II).
Prior to radioiodine therapy, nocturnal hyperglycemia was observed
by CGM occurring at ~8:00 p.m. when the patient fell asleep, and
the blood glucose was maintained at a high level until before
breakfast (7:00 a.m.; Fig. 3A).
Nocturnal blood glucose, which was demonstrated by the AUC for
glucose during the night (8:00 p.m.-7:00 a.m.;
AUCnight), improved when the patient exhibited
hypothyroidism following radioiodine therapy (Table II). The dawn phenomenon (from 4:00 to
7:00 a.m.) was also observed prior to radioiodine therapy (Fig. 3A). The relative contributions of FBG
and PBG to 24-h hyperglycemia were calculated as described in a
previous study (12). Glucose AUC
above a glucose value of 5.6 mmol/l in 24 h was defined as
AUCtotal to represent overall hyperglycemia. The area
above FBG (AUCPPG) was defined as the contribution of
PBG to overall hyperglycemia over 24 h. The proportion of the
contributions of FBG and PBG to 24 h hyperglycemia was calculated
as
[(AUCtotal-AUCPBG)/AUCtotal]x100%
and (AUCPBG/AUCtotal)x100%, respectively. The
AUCtotal decreased and the contributions of FBG to 24 h
hyperglycemia decreased from 48.9 to 25.8%, along with a marked
decrease in the thyroid hormone level (Fig. 3B).
 | Table II.Parameters of glycemic variability
measured by continuous glucose monitoring. |
Table II.
Parameters of glycemic variability
measured by continuous glucose monitoring.
|
| Hyperthyroidism | Hypothyroidism |
|---|
|
|
|
|
|---|
| Variable | Day 1 | Day 1 | Day 3 | Day 1 | Day 1 | Day 3 |
| MG (mmol/l) | 6.93 | 6.40 | 5.86 | 4.90 | 5.30 | 5.00 |
| AUCnight
(mmol/l) | 3,552 | 3,371 | 3,583 | 2,718 | 3,440 | 2,911 |
| SDMG
(mmol/l) | 0.81 | 0.42 | 0.96 | 0.65 | 0.62 | 0.64 |
| MAGE (mmol/l) | 1.30 | 1.40 | 2.24 | 2.18 | 1.43 | 1.36 |
| %CV (%) | 11.74 | 6.64 | 16.39 | 13.33 | 11.61 | 12.82 |
| MODD (mmol/l) | 0.73 | 2.43 | 1.30 | 0.84 |
|
|
The patient exhibited no significant symptoms of
hypothyroidism following radioiodine therapy, although his weight
had increased to 76.5 kg (BMI, 23.88 kg/m2) by December
23rd 2016. Thereafter, the patient commenced sodium levothyroxine
treatment (50 µg/day). The levels of thyroid hormone (Table I) and blood lipids had returned to
normal by January 20th 2017.
Discussion
To the best of our knowledge, this is the first
study to describe the use of CGM to evaluate glucose variability in
a non-diabetic patient with hyperthyroidism. Though the patient
exhibited normal HbA1c and blood glucose levels in OGTT, his CGM
data showed abnormity in glucose metabolism. The 24-h MG before
radioiodine therapy was higher than the normal range for the
Chinese population (13) and was
higher than the 24-h MG (5.77 mmol/l) calculated by HbA1c using the
following equation: 24-h MG (mmol/l)=1.198 × HbA1c-0.582 (14). Following radioiodine therapy, the 24-h
MG fell to within the normal range, accompanied by decreased
thyroid hormone, while the blood glucose in OGTT and the HbA1c
value decreased slightly.
The current study demonstrated that the sensitivity
of CGM in patients with hyperthyroidism was higher than that of
HbA1c and OGTT. The reasons for low sensitivity of HbA1c in
patients with thyroid dysfunction remain unclear. One reason may be
the alteration of erythrocytes in circulation caused by thyroid
dysfunction (10,15). OGTT may not account for nocturnal
hyperglycemia, which was observed in the present report. A previous
study identified increased secretion of growth hormone in
thyrotoxicosis (16), which may
contribute to nocturnal hyperglycemia. Klieverik et al
(17) identified that following local
application of T3 to thyroid hormone sensitive neurons in the
paraventricular nucleus, an increased sympathetic outflow to the
liver significantly stimulated hepatic gluconeogenesis. Nocturnal
hyperglycemia in hyperthyroid patients may also be caused by
sympathetic overactivity. The relative contributions of FBG and PBG
to 24-h hyperglycemia were similar in hyperthyroidism, although the
contributions of FBG to 24-h hyperglycemia decrease markedly due to
the improvement of nocturnal hyperglycemia following radioiodine
therapy.
The patient in the present case exhibited high
HOMA-IR, which was attributed to a high fasting insulin level. The
result was similar to previous studies (18,19). In
addition, the HOMA-IR level decreased following radioiodine therapy
accompanied by decreased fasting and 120-min insulin levels.
However, the 30-min insulin level, which partially represented the
first phase of insulin secretion, increased significantly.
The present case demonstrated low glucagon levels in
thyrotoxicosis. The GLA/GLU and GLA/INS, which indicates the
inhibition of glucose and insulin on glucagon, were also lower than
after radioiodine therapy. Previous studies demonstrated
conflicting data regarding plasma glucagon levels of patients with
hyperthyroidism (20,21). Hepatic glycogen depletion in
hyperthyroidism may stimulate glucagon secretion and aggravate
glucose intolerance (22,23). However, high blood glucose and insulin
levels inhibit glucagon secretion (20). Furthermore, the metabolic clearance
rate of glucagon was significantly increased in hyperthyroidism
(21) and may explain the phenomenon
in the current report.
In this case MAGE and SDMG before and
after radioiodine therapy were within the normal ranges (MAGE,
<3.9 mmol/l and SDMG, <1.4 mmol/l) (24) and were not significantly different.
However, a previous study demonstrated that MAGE and
SDMG were increased in patients with diabetes and
hyperthyroidism, and could be improved when the thyroid function
normalized (11). Further case-control
studies are recommended for the glucose variability in patients
with thyroid dysfunction.
A previous study showed that the early onset of
hypothyroidism following radioiodine therapy in GD is particularly
common (57%) (25). Radioiodine
therapy causes oxidative stress (26)
and aggravates glucose intolerance. In the present case, glucose
metabolism was improved following radioiodine therapy, but
dyslipidemia was observed when the patient exhibited
hypothyroidism. The blood lipid levels returned to normal following
thyroid hormone replacement therapy without lipid-lowing therapy.
Hence, close monitoring of blood lipids and thyroid function
following radioiodine therapy is important.
In conclusion, to the best of our knowledge the
current study is the first to identify high 24-h MG and nocturnal
hyperglycemia using CGM in a non-diabetic patient with
hyperthyroidism. Thus, glucose metabolism disorder in patients with
hyperthyroidism, which is not identified by OGTT and HbA1c test,
may be more common than previously believed.
References
|
1
|
Ahrén B: Hyperthyroidism and glucose
intolerance. Acta Med Scand. 220:5–14. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fukuchi M, Shimabukuro M, Shimajiri Y,
Oshiro Y, Higa M, Akamine H, Komiya I and Takasu N: Evidence for a
deficient pancreatic beta-cell response in a rat model of
hyperthyroidism. Life Sci. 71:1059–1070. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Karbalaei N, Noorafshan A and Hoshmandi E:
Impaired glucose-stimulated insulin secretion and reduced β-cell
mass in pancreatic islets of hyperthyroid rats. Exp Physiol.
101:1114–1127. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ozdemir D, Dagdelen S and Usman A: Serum
Adiponectin Levels and Changes in Glucose Metabolism before and
after Treatment for Thyroid Dysfunction. Intern Med. 54:1849–1857.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maratou E, Hadjidakis DJ, Peppa M,
Alevizaki M, Tsegka K, Lambadiari V, Mitrou P, Boutati E, Kollias
A, Economopoulos T, et al: Studies of insulin resistance in
patients with clinical and subclinical hyperthyroidism. Eur J
Endocrinol. 163:625–630. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Maratou E, Hadjidakis DJ, Kollias A,
Tsegka K, Peppa M, Alevizaki M, Mitrou P, Lambadiari V, Boutati E,
Nikzas D, et al: Studies of insulin resistance in patients with
clinical and subclinical hypothyroidism. Eur J Endocrinol.
160:785–790. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Levin RJ and Smyth DH: The effect of the
thyroid gland on intestinal absorption of hexoses. J Physiol.
169:755–769. 1963. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dimitriadis GD and Raptis SA: Thyroid
hormone excess and glucose intolerance. Exp Clin Endocrinol
Diabetes. 109 Suppl 2:S225–S239. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang L, Shen X, Yan S, Yuan X, Lu J and
Wei W: HbA1c in the diagnosis of diabetes and abnormal glucose
tolerance in patients with Graves' hyperthyroidism. Diabetes Res
Clin Pract. 101:28–34. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Anantarapu S, Vaikkakara S, Sachan A,
Phaneendra BV, Suchitra MM, Reddy AP, Epuri S, Mukka A and Vemvakam
D: Effects of thyroid hormone replacement on glycated hemoglobin
levels in non diabetic subjects with overt hypothyroidism. Arch
Endocrinol Metab. 59:495–500. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Torimoto K, Okada Y, Arao T, Mori H,
Yamamoto S, Narisawa M, Kurozumi A and Tanaka Y: Glucose
variability before and after treatment of a patient with Graves'
disease complicated by diabetes mellitus: Assessment by continuous
glucose monitoring. Endocr J. 61:321–328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang JS, Tu ST, Lee IT, Lin SD, Lin SY, Su
SL, Lee WJ and Sheu WH: Contribution of postprandial glucose to
excess hyperglycaemia in Asian type 2 diabetic patients using
continuous glucose monitoring. Diabetes Metab Res Rev. 27:79–84.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou J, Li H, Ran X, Yang W, Li Q, Peng Y,
Li Y, Gao X, Luan X, Wang W, et al: Reference values for continuous
glucose monitoring in Chinese subjects. Diabetes Care.
32:1188–1193. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou J, Mo Y, Li H, Ran X, Yang W, Li Q,
Peng Y, Li Y, Gao X, Luan X, et al: Relationship between HbA1c and
continuous glucose monitoring in Chinese population: A multicenter
study. PLoS One. 8:e838272013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Messarah M, Saoudi M, Boumendjel A,
Boulakoud MS and Feki AE: Oxidative stress induced by thyroid
dysfunction in rat erythrocytes and heart. Environ Toxicol
Pharmacol. 31:33–41. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Iranmanesh A, Lizarralde G, Johnson ML and
Veldhuis JD: Nature of altered growth hormone secretion in
hyperthyroidism. J Clin Endocrinol Metab. 72:108–115. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Klieverik LP, Janssen SF, van Riel A,
Foppen E, Bisschop PH, Serlie MJ, Boelen A, Ackermans MT, Sauerwein
HP, Fliers E, et al: Thyroid hormone modulates glucose production
via a sympathetic pathway from the hypothalamic paraventricular
nucleus to the liver. Proc Natl Acad Sci USA. 106:5966–5971. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Al-Shoumer KA, Vasanthy BA and Al-Zaid MM:
Effects of treatment of hyperthyroidism on glucose homeostasis,
insulin secretion, and markers of bone turnover. Endocr Pract.
12:121–130. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Theodoropoulou A, Psyrogiannis A,
Metallinos IC, Habeos I, Vgenakis AG and Kyriazopoulou V: Ghrelin
response to oral glucose load in hyperthyroidism, before and after
treatment with antithyroid drugs. J Endocrinol Invest. 32:94–97.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tosi F, Moghetti P, Castello R, Negri C,
Bonora E and Muggeo M: Early changes in plasma glucagon and growth
hormone response to oral glucose in experimental hyperthyroidism.
Metabolism. 45:1029–1033. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dimitriadis G, Hatziagelaki E, Mitrou P,
Lambadiari V, Maratou E, Raptis AE, Gerich JE and Raptis SA: Effect
of hyperthyroidism on clearance and secretion of glucagon in man.
Exp Clin Endocrinol Diabetes. 119:214–217. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kabadi UM: Is hepatic glycogen content a
regulator of glucagon secretion? Metabolism. 41:113–115. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aranda A, Montoya E and Herrera E: Effects
of hypo- and hyper-thyroidism on liver composition, blood glucose,
ketone bodies and insulin in the male rat. Biochem J. 128:597–604.
1972. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou J, Li H, Ran X, Yang W, Li Q, Peng Y,
Li Y, Gao X, Luan X, Wang W, et al: Establishment of normal
reference ranges for glycemic variability in Chinese subjects using
continuous glucose monitoring. Med Sci Monit. 17:CR9–CR13. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vijayakumar V, Ali S, Nishino T and
Nusynowitz M: What influences early hypothyroidism after
radioiodine treatment for Graves' hyperthyroidism? Clin Nucl Med.
31:688–689. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rosário PW, Batista KC and Calsolari MR:
Radioiodine-induced oxidative stress in patients with
differentiated thyroid carcinoma and effect of supplementation with
vitamins C and E and selenium (antioxidants). Arch Endocrinol
Metab. 60:328–332. 2016. View Article : Google Scholar : PubMed/NCBI
|