Introduction
Transfusion-related acute lung injury (TRALI) is a
serious complication characterized by the acute onset of
non-cardiogenic pulmonary edema following transfusion of blood
products. Although its incidence decreased with modified
transfusion practices, TRALI was the leading cause of
transfusion-related fatalities in the United States from 2007
through to 2011 (1). Acute
pancreatitis-associated lung injury (APALI) is the most common and
a severe complication in patients with multisystem organ failure.
Previous studies indicated that neutrophils serve a critical role
in inflammatory reactions in acute lung injury (2,3).
Rapamycin is a new macrolide immunosuppressant.
Rapamycin exerts an immunosuppressive effect by blocking signal
transduction of various cytokine receptors, thereby preventing T
lymphocytes and other cells from going from the G1 phase to the S
phase of the cell cycle. A recent report has also indicated that
rapamycin can inhibit the inflammatory reaction induced by nuclear
factor (NF)-κB activation and neutrophils (2,3). Rapamycin
also reportedly exacerbates the severity of acute lung injury
induced by endotoxin and causes cell apoptosis (4).
It is unknown whether rapamycin has an effect on
TRALI caused by blood transfusion. Therefore, the authors
constructed Sprague-Dawley rat models of TRALI and APALI, and
observed the effect of rapamycin on the pathological process of
neutrophil apoptosis in the early stage of acute lung injury
induced by blood transfusion, in order to obtain experimental
evidence for future clinical treatment feasibility.
Materials and methods
Ethics statement
The current study was approved by the Shanghai Jiao
Tong University Affiliated Sixth People's Hospital of Medicine
Ethics Committee (Shanghai, China).
Animals
A total of 60 male Sprague-Dawley rats aged 6–7
weeks (weight, 250 g) were purchased from Shanghai SIPPR/BK
Experimental Animals, Inc. (Shanghai, China). The rats were reared
to be specific pathogen free, and standard feed, drinking water,
bedding, and all other items were aseptically processed. The rats
were maintained with a 12 h:12 h light cycle.
Instruments and reagents
The main instruments included a low-temperature
high-speed centrifuge (Allegra X-22R; Beckman Coulter, Inc., Brea,
CA, USA), and flow cytometer (Navios; Beckman Coulter, Inc.). The
main regents were as follows: Lipopolysaccharide (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany), L-arginine (Amresco, Inc.,
Framingham, MA, USA), kits for isolation of rat peripheral blood
neutrophils (cat. no. LZS1091), red blood cell lysis buffer (cat.
no. NH4CL2009), cell dilution (cat. no. 2010C1119), cell washing
liquid (cat. no. 2010X1118) and Annexin V-fluorescein
isothiocyanate (FITC)/propidium iodide (PI) cell apoptosis
detection kit (cat. no. 40302ES20; Shanghai Yi Sheng Biotechnology
Co., Ltd., Shanghai, China).
Plasma preparation
AB type human whole blood was randomly selected, and
2 ml blood samples were set aside. Following 21 days of storage,
plasma was obtained by centrifugation at 1,200 × g and then
preserved at −80°C. Before infusion, plasma was incubated at 56°C
for 30 min.
Preparation of animal models
A total of 60 Sprague-Dawley rats were randomly
divided into 6 groups (10 rats in each group): A normal control
group, a TRALI experimental group, an APALI experimental group, a
rapamycin intervention control group, a rapamycin intervention
TRALI experimental group, and a rapamycin intervention APALI
experimental group. In order to construct acute lung injury models,
the rats were treated as follows. The TRALI group rats received
intraperitoneal injection of LPS (2 mg/kg). At 1 h, the rats were
anesthetized by intraperitoneal injection of pentobarbital
anesthesia (40 mg/kg); femoral vessels were isolated and infused
with 50% heparin-saline mixture. Subsequently, 1 ml blood [~10% of
the capacity of whole blood was removed within 5–10 min, and the
equivalent AB human plasma was transfused (plasma infusion rate
less than 4 ml/h)] (5). The APALI
group rats received 3 intraperitoneal injections of 9% L-arginine
solution (dilute with physiological saline)(1 g/kg) at 1 h
intervals (5). The rapamycin
intervention experimental TRALI and APALI group rats underwent
intragastric administration of rapamycin (10 mg/kg, once a day for
3 days), and other experimental methods were as in the above
protocols. Finally, the rapamycin intervention control group rats
in the TRALI and APALI control groups were intraperitoneally
injected with rapamycin in saline (2 mg). Under anesthesia, 1 ml
blood was removed from the rats and 1 ml of normal saline was
transfused. The ensuing experimental operation for the control
group was the same as previously described for the TRALI and APALI
group.
Histopathological examination of lung
tissues
The rats were killed by right ventricular puncture,
and lung tissue specimens (1×1 cm) were collected. Hematoxylin and
eosin staining was used for histopathological examination. There
were 60 images randomly selected for analysis.
Isolation of neutrophils in peripheral
blood
As per the kit instructions: 1 ml fresh
anticoagulated blood was added onto the surface of a 1 ml isolation
liquid and centrifuged at 400–500 × g for 25 min. The centrifuge
tube will have been divided into 4 layers; the liquid layers rich
in neutrophils and red blood cells are drawn off. Following
washing, red blood cell lysis solution is used to completely
destroy the red blood cells in the rat peripheral blood. The
supernatant is discarded by centrifuging at ~400–500 × g for 5 min
and the precipitate is washed twice with a cell washing solution to
obtain useful neutrophils.
Quantitative detection of early stage
neutrophil apoptosis
The Annexin V and PI double-labeling method was used
according to manufacturer's instructions. The separation and
purification of neutrophils is suspended in 100 µl buffer, 5 µl
Annexin V-FLUOS, and 5 µl PI at room temperature, with dark
incubation for 10 min; 400 µl incubation buffer solution from the
Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI)
cell apoptosis detection kit (cat. no. 40302ES20; Shanghai Yi Sheng
Biotechnology Co., Ltd.)] was added to terminate the reaction. A
total of 10,000 cells are detected by dual-channel flow
cytometry.
Statistical analysis
The data were analyzed using SPSS software (version,
17.0; SPSS Inc., Chicago, IL, USA). Continuous variables were
described using mean ± standard deviation and the comparison
between groups was performed with a t-test or correlation analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Histopathological examination of lung
tissues
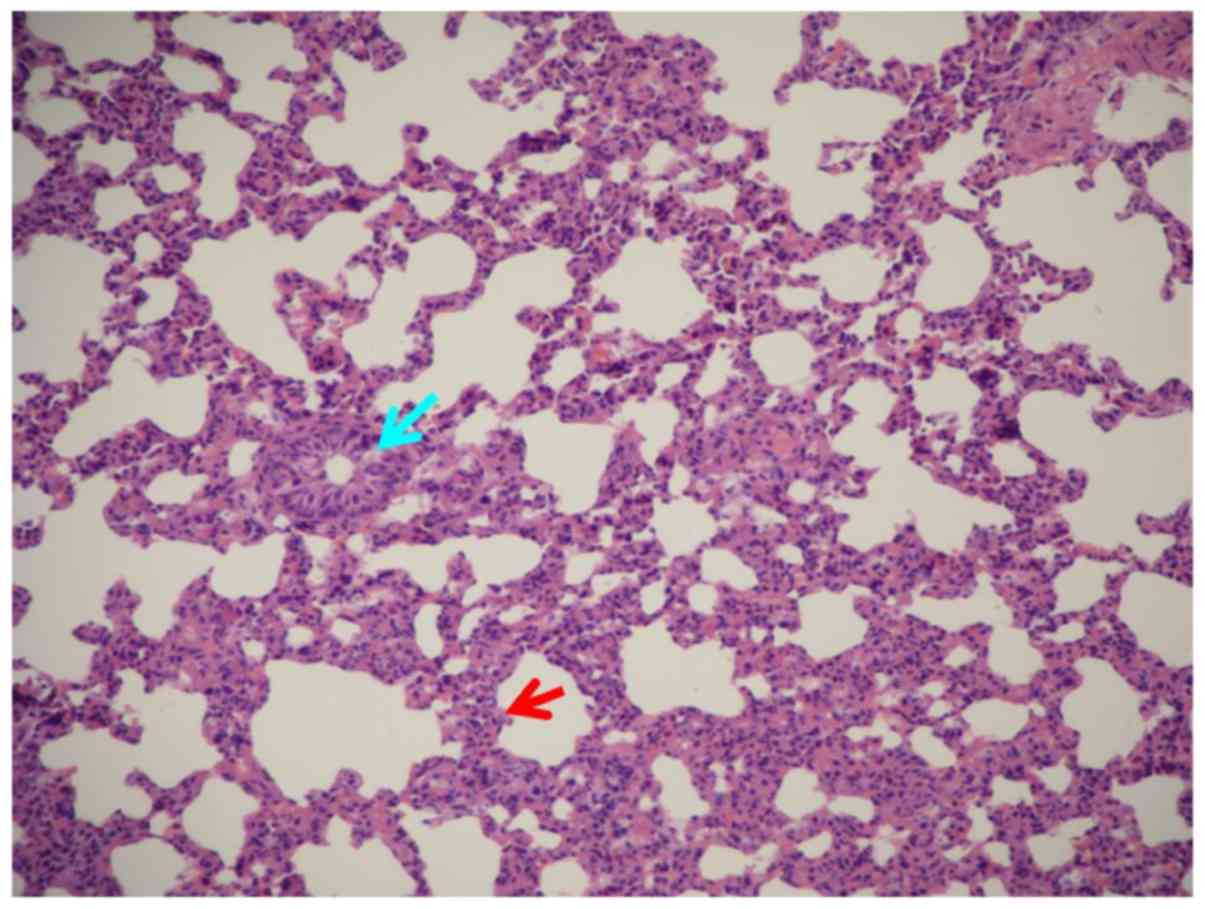
Lung tissue pathological changes in the TRALI and
APALI groups included significantly widened alveolar intervals,
damage and fracture of alveolar walls and capillaries, congestion,
erythrocyte effusion in organization gaps, a large number of
inflammatory cells in the alveolar spaces, and inflammatory
neutrophil infiltration in interstitial spaces (Figs. 1 and 2).
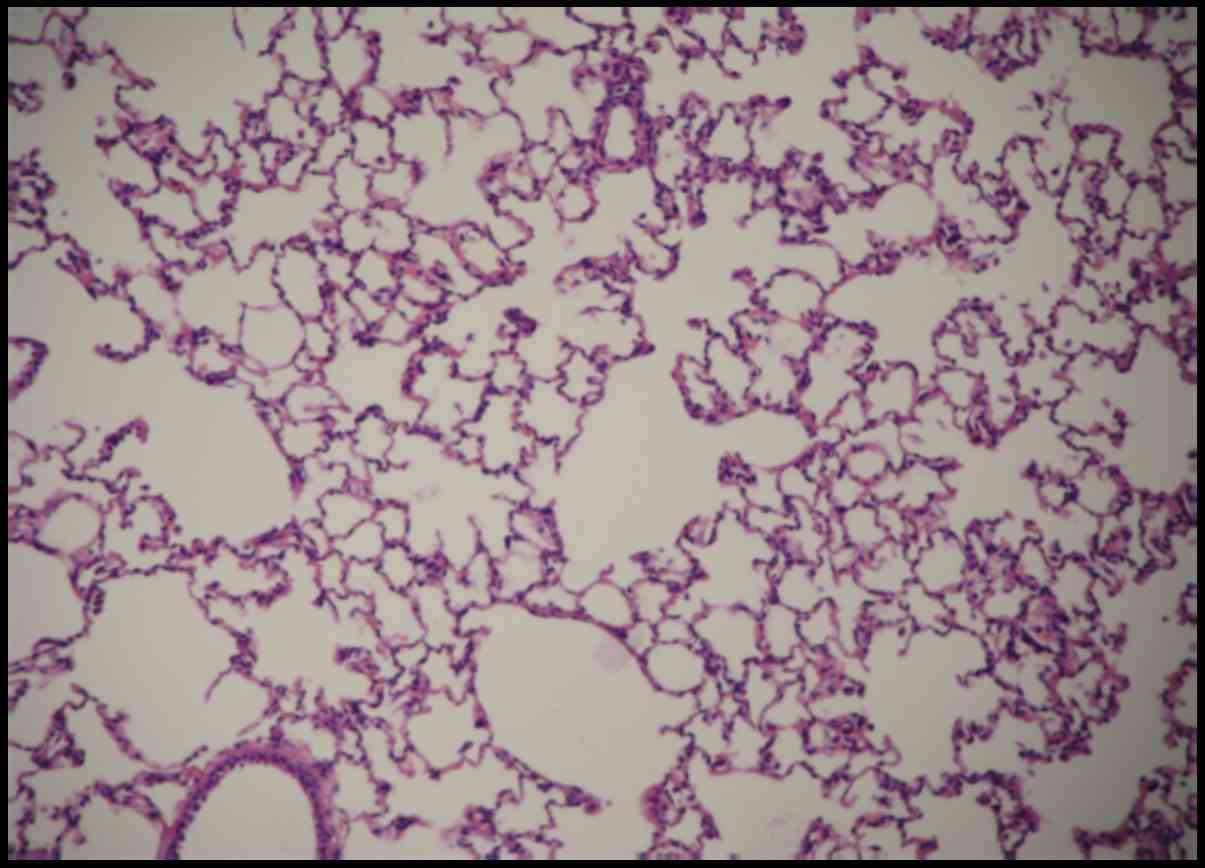
However, the control group reported no significant changes
(Fig. 3). Therefore, the rat models
were successfully developed.
Changes of early neutrophil apoptosis
in peripheral blood in rats with TRALI
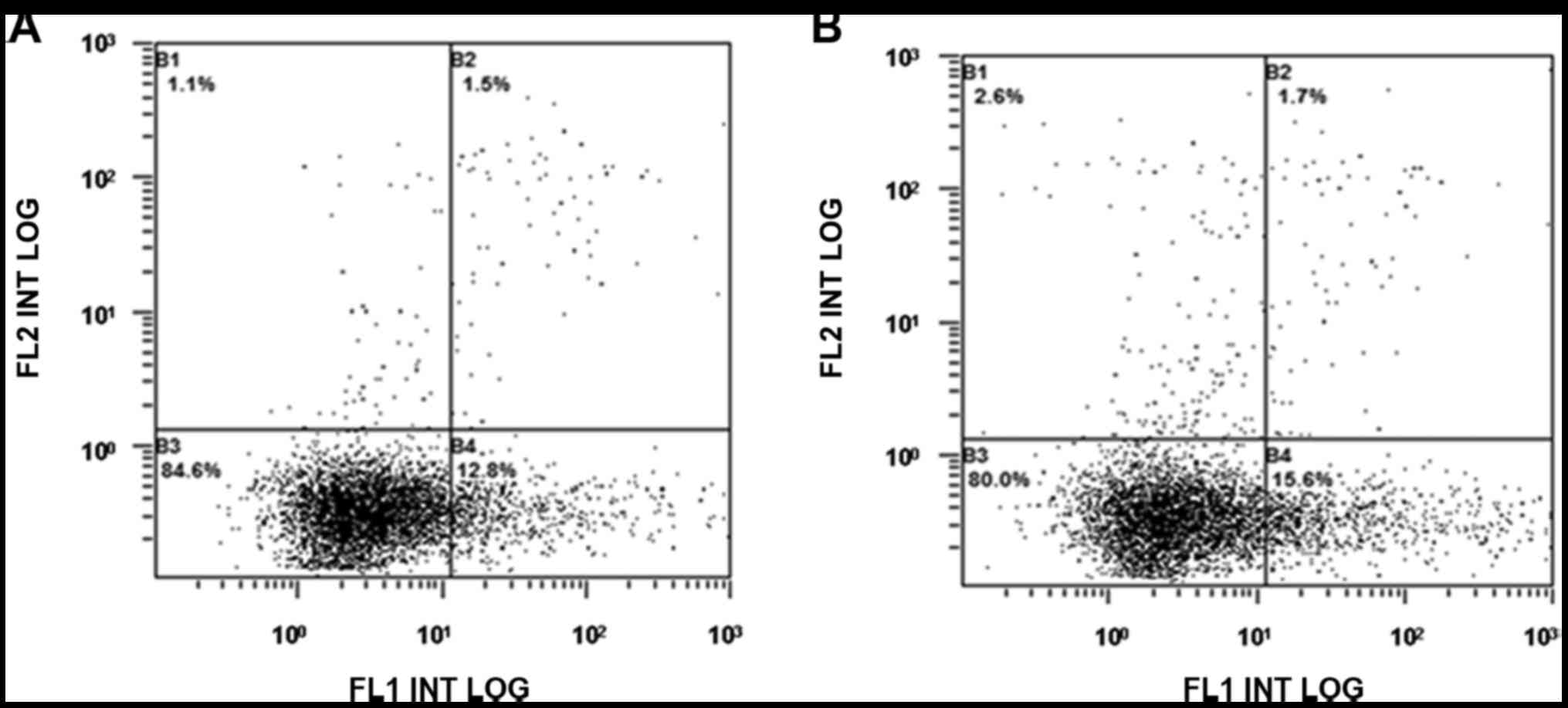
Compared with the normal control group, the early
peripheral blood neutrophil apoptosis rate in the TRALI
experimental group increased (P<0.01; Fig. 4).
There was no difference in the early peripheral
blood neutrophil apoptosis rate between the rapamycin intervention
TRALI group and the TRALI control group, while the early neutrophil
apoptosis rate in the TRALI rapamycin intervention group was higher
than that in the control group (P<0.01; Figs. 5 and 6).
The early apoptosis rate in the TRALI rapamycin
intervention experimental group was significantly higher than that
in the control group (P<0.01; Table
I).
 | Table I.Comparisons of the early apoptosis
rate of peripheral blood neutrophils in the rats. |
Table I.
Comparisons of the early apoptosis
rate of peripheral blood neutrophils in the rats.
| Group | Neutrophil apoptosis
rate (n=10) |
|---|
| Rapamycin
intervention TRALI |
18.84±1.94a |
| TRALI
experimental |
18.06±1.66a,c |
| Rapamycin
intervention APALI |
7.95±1.92b,c,d |
| APALI
experimental |
10.00±3.12b |
| Rapamycin
intervention control | 11.32±4.23 |
| Normal control | 13.35±3.68 |
Changes of early neutrophil apoptosis
in peripheral blood in rats with APALI
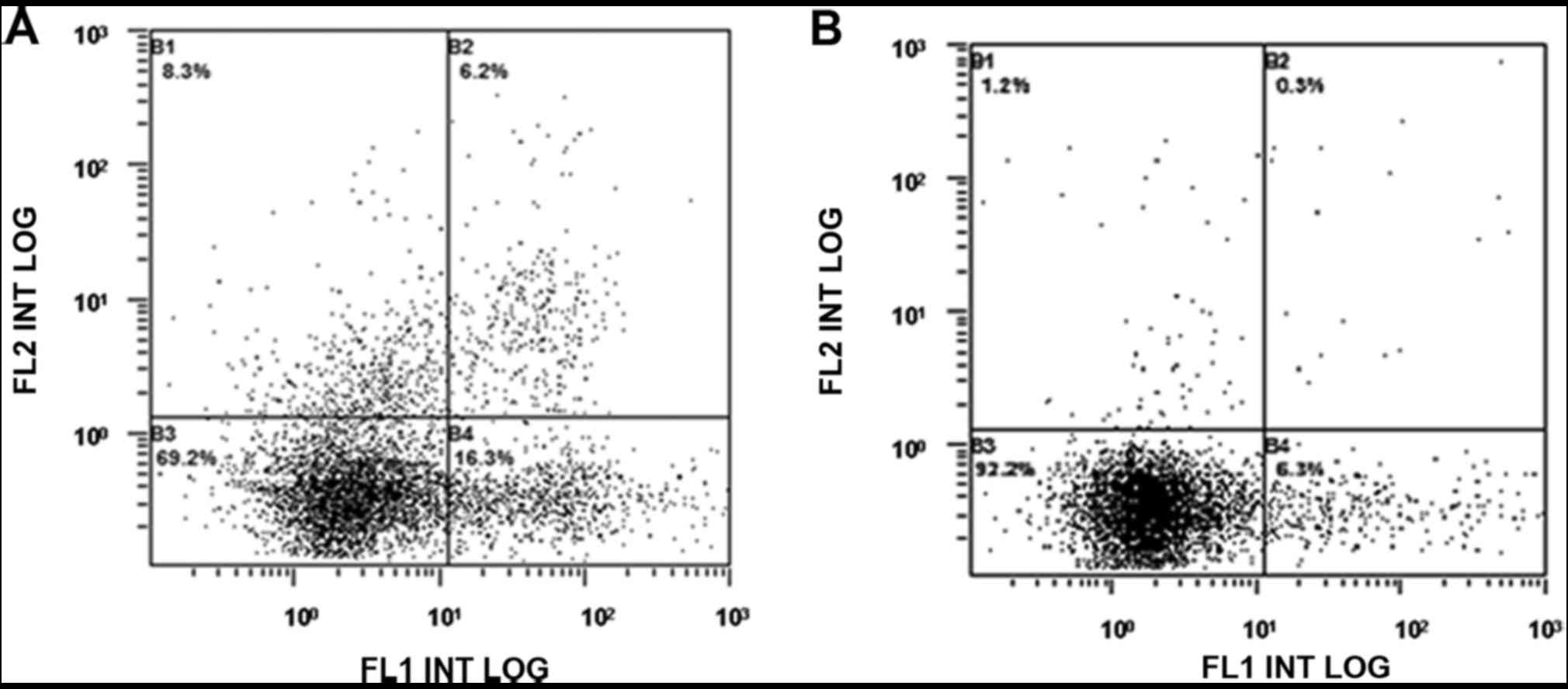
The early apoptosis rates in both the APALI
experimental group and rapamycin intervention APALI group were
lower than those in the normal control group (P<0.05; Fig. 4). There was no difference in the early
apoptosis rates between the rapamycin intervention APALI group and
the APALI experimental group (P>0.05).
The early apoptosis rate in the rapamycin
intervention APALI group was lower than that in the APALI
experimental group (P<0.05). There was no difference in the
early apoptosis rates of neutrophils between the rapamycin
intervention APALI group and the control group (P>0.05; Table I).
Comparison of the early apoptosis rates of
peripheral blood neutrophils in the TRALI experimental group and
APALI experimental group. Compared with that in the APALI group,
the early apoptosis rate of peripheral blood neutrophils
significantly increased in the TRALI group (P<0.05). Following
rapamycin intervention, the early apoptosis rate of peripheral
blood neutrophils in the TRALI experimental group was significantly
higher than that in the APALI experimental group (P<0.05;
Table I).
Another study (6) that
was performed by the authors indicated that 10 mg/kg rapamycin can
significantly reduce the expression of p-p70s6k, which confirmed
mTOR activation was inhibited in the experimental dose of rapamycin
in the current study.
Discussion
A specific inhibitor of the mammalian target of
rapamycin (mTOR) is a commonly used pharmacological tool for the
study of mTOR biology (7). As a key
controller of cell growth and survival, the mTOR can physically
interact with STAT1 and suppress the induction of STAT1-dependent
apoptosis genes (3). The mTOR can also
modify gene transcription by directly regulating transcription
factors. There are two functionally distinct mammalian targets of
rapamycin complexes (mTORCs), mTORC1 and mTORC2, with mTORC1
serving a central role in cell growth and cellular responses to
metabolic stress. mTORC1 participates in the regulation of
transcriptional as well as translational events for genes involved
in biosynthetic and metabolism pathways (6–9). In
contrast, mTORC2 controls the organization of the actin
cytoskeleton and is rapamycin insensitive (10).
Acute lung injury (ALI) is characterized by lung
inflammation and diffuses infiltration of neutrophils into the
alveolar space. The inhibition of neutrophil apoptosis has been
implicated in the pathogenesis of ALI. It has been proposed that
activated neutrophils in the lungs have an unusually prolonged
half-life of 8 h due to delayed phagocytosis (or apoptosis) by
macrophages (11). In a septic
patient, this delay appears to be related to the severity of sepsis
since a progressive decrease in neutrophil apoptosis has been
associated with the increased severity of sepsis (12). The inhibition of neutrophil apoptosis
may contribute to the development of ALI (13). It was reported that lung inflammation
could be ameliorated by enhancing the apoptosis of neutrophils
(14). Depletion of neutrophils
substantially mitigated the extent of lung injury, indicating a
pathomechanistic role for neutrophils in chest trauma-induced
septic ALI (15). Inflammasomes have
been reported to be involved in ALI in previous studies (16,17). Histone
deacetylase 9 inhibited autophagy in primary brain microvessel
endothelial cells that exacerbates brain ischemia injury (16). However, rapamycin can induce protective
autophagy in vascular endothelial cells exposed to oxygen-glucose
deprivation (17).
Rapamycin is approved by the U.S. Food and Drug
Administration for immunosuppression. It has been reported that
rapamycin decreased the severity of lung injury after intratracheal
LPS or PAM administration, as determined by diminished neutrophil
accumulation in the lungs and reduced interstitial pulmonary edema
(18). The downregulation of autophagy
may lead to systemic inflammation and ALI following sepsis. The
direct or indirect modification of autophagy using rapamycin,
respectively, resulted in improved survival. Enhancing or restoring
autophagy early after sepsis seems to be a potential strategy for
the treatment of sepsis-induced ALI (19). However, rapamycin can reduce
paraquat-induced ALI through inhibition of NF-κB activation, as
well as increased apoptosis of leukocytes and neutrophils (20).
The current study demonstrated that the early stage
apoptosis of neutrophils in Sprague-Dawley rats with APALI was
decreased, indicating that peripheral neutrophil apoptosis was
delayed, which occurs frequently in patients with burns, infection
and multi-organ failure (21), and
further increases the severity of lung injury (22).
However, early stage apoptosis of neutrophils in the
rapamycin intervention group did not change significantly compared
to that in the control group, and lung injury did not improve
following rapamycin intervention, suggesting that rapamycin did not
change the early stage apoptosis rate of neutrophils. In addition,
rapamycin did not affect early stage apoptosis of neutrophils in
the APALI group according to our results. Therefore, the authors
believe that rapamycin had limited protective effects on APALI, but
did not relieve APALI.
The early apoptosis rate of neutrophils in the TRALI
group was increased compared to that in the normal control group in
the current study, but rapamycin had little effect on neutrophil
apoptosis in TRALI. Compared with the APALI group, with or without
rapamycin intervention, delayed neutrophil apoptosis did not occur
in TRALI. The activation and inactivation of mTOR depend on the
different stimuli and different types of cells and the differential
regulations under the influence of these factors lead to the
different effects of rapamycin on mTOR (6). The authors believe that early stage
apoptosis of neutrophils in TRALI was different from that in APALI,
and rapamycin had a limited protective effect on TRALI and APALI in
Sprague Dawley rats. With future research, better treatments for
acute lung injury with more targeted effects may be developed.
Acknowledgements
The current study was funded by the Fourth Round
(2015–2017) Shanghai Public Health Priority Construction Project,
‘Transfusion Medicine’ (grant no.15GWZK0501), Shanghai Science and
Technology Commission Research Project Funding (grant no.
13140902903), and Shanghai Sixth People's Hospital Consortium
Subject (2015; grant no. 1324).
References
|
1
|
Menis M, Anderson SA, Forshee RA, McKean
S, Johnson C, Warnock R, Gondalia R, Mintz PD, Holness L, Worrall
CM, et al: Transfusion-related acute lung injury and potential risk
factors among the inpatient US elderly as recorded in Medicare
claims data, during 2007 through 2011. Transfusion. 54:2182–2193.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Abraham E: Neutrophils and acute lung
injury. Crit Care Med. 31:S195–S199. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fielhaber JA, Carroll SF, Dydensborg AB,
Shourian M, Triantafillopoulos A, Harel S, Hussain SN, Bouchard M,
Qureshi ST and Kristof AS: Inhibition of mammalian target of
rapamycin augments lipopolysaccharide-induced lung injury and
apoptosis. J Immunol. 188:4535–4542. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mossman JA, Biancani LM, Zhu CT and Rand
DM: Mitonuclear Epistasis for Development Time and Its Modification
by Diet in Drosophila. Genetics. 203:463–484. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sharif R, Dawra R, Wasiluk K, Phillips P,
Dudeja V, Kurt-Jones E, Finberg R and Saluja A: Impact of toll-like
receptor 4 on the severity of acute pancreatitis and
pancreatitis-associated lung injury in mice. Gut. 58:813–819. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yuan YE, Liwei L and Zhiqiang L: Effects
of rapamycin on expressions of m TOR down-stream proteins
p70s6k/p-p70s6k and pulmonary histopathological changes in
transfusion-related acute lung injury rat model. Chin J Blood
Transfus. 29:251–254. 2016.
|
|
7
|
Hay N and Sonenberg N: Upstream and
downstream of mTOR. Genes Dev. 18:1926–1945. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tee AR and Blenis J: mTOR, translational
control and human disease. Semin Cell Dev Biol. 16:29–37. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peng T, Golub TR and Sabatini DM: The
immunosuppressant rapamycin mimics a starvation-like signal
distinct from amino acid and glucose deprivation. Mol Cell Biol.
22:5575–5584. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jacinto E, Loewith R, Schmidt A, Lin S,
Rüegg MA, Hall A and Hall MN: Mammalian TOR complex 2 controls the
actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol.
6:1122–1128. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheretakis C, Dror Y and Glogauer M: A
noninvasive oral rinse assay to monitor engraftment, neutrophil
tissue delivery and susceptibility to infection following HSCT in
pediatric patients. Bone Marrow Transplant. 36:227–232.
2005.PubMed/NCBI
|
|
12
|
Fialkow L, Filho L Fochesatto, Bozzetti
MC, Milani AR, Filho EM Rodrigues, Ladniuk RM, Pierozan P, de Moura
RM, Prolla JC, Vachon E, et al: Neutrophil apoptosis: A marker of
disease severity in sepsis and sepsis-induced acute respiratory
distress syndrome. Crit Care. 10:R1552006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin WC, Lin CF, Chen CL, Chen CW and Lin
YS: Inhibition of neutrophil apoptosis via sphingolipid signaling
in acute lung injury. J Pharmacol Exp Ther. 339:45–53. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Perl M, Hohmann C, Denk S, Kellermann P,
Lu D, Braumüller S, Bachem MG, Thomas J, Knöferl MW, Ayala A, et
al: Role of activated neutrophils in chest trauma-induced septic
acute lung injury. Shock. 38:98–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li X, Li C, Liang W, Bi Y, Chen M and Dong
S: Protectin D1 promotes resolution of inflammation in a murine
model of lipopolysaccharide-induced acute lung injury via enhancing
neutrophil apoptosis. Chin Med J (Engl). 127:810–814.
2014.PubMed/NCBI
|
|
16
|
Shi W, Wei X, Wang Z, Han H, Fu Y, Liu J,
Zhang Y, Guo J, Dong C, Zhou D, et al: HDAC9 exacerbates
endothelial injury in cerebral ischaemia/reperfusion injury. J Cell
Mol Med. 20:1139–1149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Urbanek T, Kuczmik W, Basta-Kaim A and
Gabryel B: Rapamycin induces of protective autophagy in vascular
endothelial cells exposed to oxygen-glucose deprivation. Brain Res.
1553:1–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lorne E, Zhao X, Zmijewski JW, Liu G, Park
YJ, Tsuruta Y and Abraham E: Participation of mammalian target of
rapamycin complex 1 in Toll-like receptor 2- and 4-induced
neutrophil activation and acute lung injury. Am J Respir Cell Mol
Biol. 41:237–245. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yen YT, Yang HR, Lo HC, Hsieh YC, Tsai SC,
Hong CW and Hsieh CH: Enhancing autophagy with activated protein C
and rapamycin protects against sepsis-induced acute lung injury.
Surgery. 153:689–698. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen D, Ma T, Liu XW, Yang C and Liu Z:
Rapamycin reverses paraquat-induced acute lung injury in a rat
model through inhibition of NFκB activation. Int J Clin Exp Pathol.
8:4627–4638. 2015.PubMed/NCBI
|
|
21
|
Taneja R, Parodo J, Jia SH, Kapus A,
Rotstein OD and Marshall JC: Delayed neutrophil apoptosis in sepsis
is associated with maintenance of mitochondrial transmembrane
potential and reduced caspase-9 activity. Crit Care Med.
32:1460–1469. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
O'Neill S, O'Neill AJ, Conroy E, Brady HR,
Fitzpatrick JM and Watson RW: Altered caspase expression results in
delayed neutrophil apoptosis in acute pancreatitis. J Leukoc Biol.
68:15–20. 2000.PubMed/NCBI
|