Introduction
Atherosclerosis is an inflammatory disease of
arteries frequently leading to heart attacks and death (1). Investigators have previously suggested
that C-reactive protein (CRP) induce atherosclerosis via different
signaling pathways (2). Two isoforms
of CRP have been identified thus far: pentameric (p)CRP and
monomeric (m)CRP (3). Although the
role of these isoforms is debatable, the majority of studies
suggested anti-inflammatory and pro-inflammatory roles for pCRP and
mCRP, respectively (3–6).
One of the proposed mechanisms underlying CRP action
suggests that circulating CRPs initially bind to adherent platelets
at the endothelial level of the artery. Subsequently, these
activated platelets (7,8) whereby other cells (9) trigger the dissociation of pCRP into five
monomers on its membrane. mCRPs activate monocytes through the
inflammatory process, as previously described (10).
Both oxLDL and CRP (in its two isoforms) are
co-localized in human atherosclerotic plaques and form complexes
with glycoproteins (11). In
vitro, the complex formed between oxLDL, CRP and
lysophosphatidylcholine demonstrates a decreased pro-inflammatory
activity suggesting an ability to slow the progression of
atherosclerosis (12).
Macrophages in the plaque release different types of
cytokines including: interleukin (IL)-1β, IL-8 (13), IL-6 (14)
and tumor necrosis factor (TNF)-α (15) and produce reactive oxygen species (ROS)
(16) to induce inflammation. These
molecules affect formation, development and the destabilization of
atherosclerotic plaques (17).
Moreover, they activate adhesion molecules on endothelial cells
causing further recruitment of monocytes in the arteries (5,10).
Furthermore, ROS induces vascular diseases by causing endothelial
cell dysfunction, increasing inflammatory cell recruitment in the
arteries (18).
Previous studies have utilized different models to
investigate either the effect of CRP isoforms, or that of pCRP in
combination with various forms of LDL, on monocytes and macrophages
(6,19).
Interestingly, very few studies suggested a possible interaction of
these three molecules with plaque cells that may interfere with the
plaque development and/or rupture (11). Furthermore, studies investigating CRP
effects used CRP preserved with azide, which may cause misleading
results with regards to cytokine release (20–23).
In the present study, we investigated the single and
combined effects of azide-free CRP isoforms and oxLDL on the
release of inflammatory cytokines (IL-1β, IL-6, IL-8, TNF-α) and
ROS by U937-derived macrophages in order to obtain a better
understanding of the role of these important molecules in the
inflammatory process associated with atherosclerosis.
Materials and methods
CRP monomerization
Human mCRP was obtained by heating human pCRP
(catalog. no. 140-11-5; Lee Biosolutions, MO, USA) at 80°C for 70
min, as previously described (20).
CRP monomerization was confirmed by SDS-PAGE (12.5% polyacrylamide
gel; 20 µg protein/lane), which revealed the existence of a 40 kDa
band, thus verifying efficient monomerization (data not shown).
U937 cell culture, differentiation and
treatment
The human monocytic cell line, U937 (kindly provided
by Prof. Marwan El Sabban, American University of Beirut, Beirut,
Lebanon), was cultured in growth medium composed of RPMI-1640
medium supplemented with penicillin (100 U/ml), streptomycin (100
µg/ml), L-glutamine (2 mM) and 10% fetal bovine serum (FBS; all
from Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and maintained
at 37°C in a humidified 5% CO2 atmosphere. Growth medium
was replaced every two 2-3 days. U937 monocytes were induced to
differentiate into U937-derived macrophages by culturing cells in
the presence of 100 nM phorbol-myristate-acetate (PMA;
Sigma-Aldrich; Merck KGaA) for 24 h. Macrophages were washed with
phosphate buffered saline (PBS) and cultured for an additional 24 h
in growth medium. Following 24 h culture period, macrophages were
washed with PBS, detached via gentle scraping and finally collected
by centrifugation at 240 × g, 4°C, for 5 min. Cell viability was
evaluated by the trypan blue exclusion method (24) and was >80% in all experiments.
Macrophages were seeded at an initial density of 7×105
viable cells in 24-well plates and allowed to adhere for 24 h.
Macrophages were then cultured in growth medium supplemented with
polymyxin B (25 µg/ml, Sigma-Aldrich; Merck KGaA) and then either
left untreated or treated with 25 µg/ml of mCRP, pCRP, oxLDL
(medium oxidized low density lipoprotein, Kalen Biomedical, LLC,
Germantown, MD, USA) alone or in combination. Culture supernatants
were collected after 24 h and stored at −80°C for later cytokine
analysis.
Enzyme-linked immunosorbent assay
IL-1β (R&D Systems, Inc., Minneapolis, MN, USA),
IL-6, IL-8 and TNF-α (PeproTech, Inc., Rocky Hill, NJ, USA) levels
were assayed in culture supernatants according to the
manufacturers' instructions. Samples were analyzed in duplicates
and absorbance was measured via Epoch microplate reader (BioTek
Instruments, Inc., Winooski, VT, USA).
ROS assay
To evaluate intracellular ROS production,
U937-derived macrophages were collected, washed and incubated with
10 µM 2′,7′-dichlorodihydrofluorescein diacetate
(H2DCF-DA; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) for 45 min at 37°C in the dark. Cells were then washed and
finally resuspended in cell wash solution (BD Biosciences, Franklin
Lakes, NJ, USA). The mean fluorescence intensity of DCF was
measured within 30 min by FACSCalibur and CellQuest software,
version 5.1 (BD Biosciences).
Statistical analysis
Statistical analysis was carried out using GraphPad
Prism software (version 6, GraphPad Software, Inc., La Jolla, CA,
USA) by performing one way analysis of variance followed by Tukey's
multiple comparison post hoc test. Data is presented as mean values
± standard error of the mean. P<0.05 was considered to indicate
a statistically significant difference.
Results
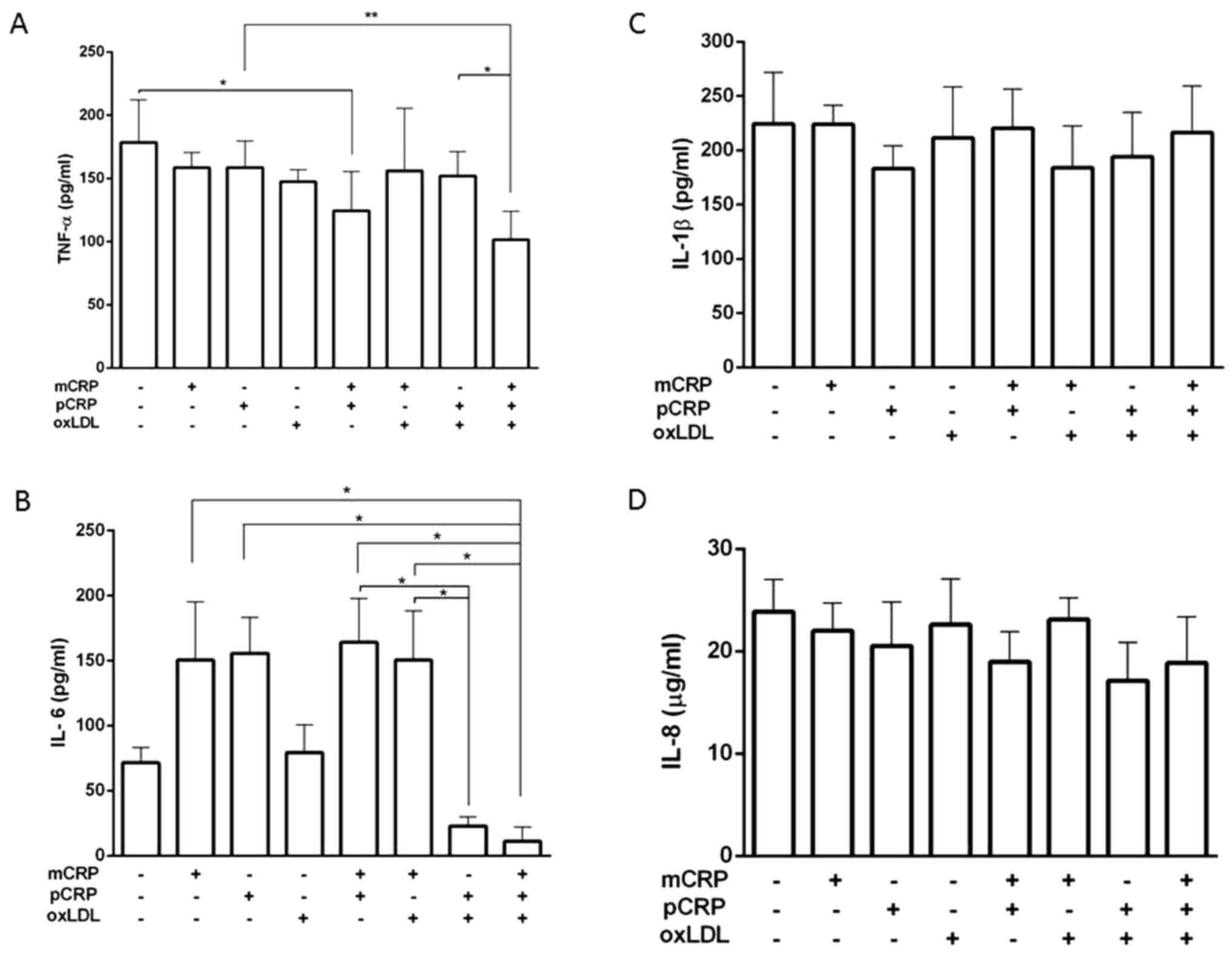
Cytokine release
TNF-α, IL-1β, IL-8 and IL-6 release by U937-derived
macrophages treated with mCRP, pCRP and/or oxLDL for 24 h was
assessed via ELISA. TNF-α levels, produced by U937-derived
macrophages, were similar to the control for all samples treated
with one of the CRP isoforms alone or in combination with oxLDL.
However, the combination of both CRP isoforms significantly reduced
TNF-α release by ~1.5-fold. Similarly, the triple combination
demonstrated a similar decrease when compared to the samples
treated with pCRP alone or combined with oxLDL (Fig. 1A).
The presence of pCRP and oxLDL in the treated sample
lowered the release of IL-6 by 7-fold when compared to the samples
treated with both CRP isoforms or in the combination of mCRP and
oxLDL. A further decrease (13-fold) was observed in the triple
combination, which was also significant as compared to the samples
treated with either isoforms (P<0.05; Fig. 1B).
In contrast, no significant variations were observed
neither for IL-1β nor IL-8 release by U937-derived macrophages
under different treatment combinations (Fig. 1C and D).
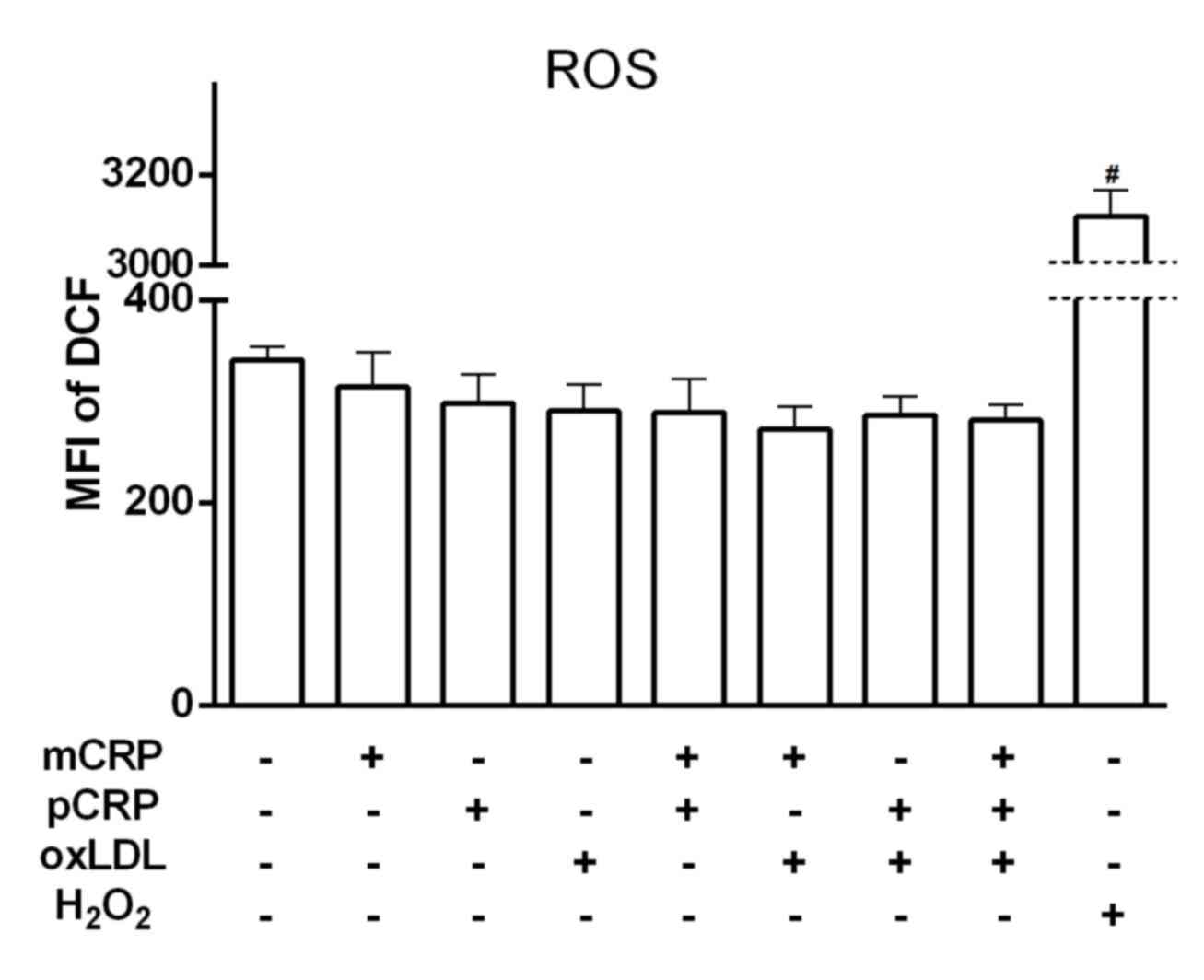
ROS detection
Although the intracellular ROS level induced by
stimulating U937-derived macrophages with
H2O2 (positive control) was significantly
high, no significant differences were observed following 24 h of
exposure to different combinations of CRP and oxLDL (P>0.05;
Fig. 2).
Discussion
The role of CRP isoforms has been controversial in
the development of atherosclerosis. Although mCRP, pCRP and oxLDL
are present at the plaque level, the combined effect of these three
factors on the cytokine release by macrophages has not been
established yet in vitro. In the present study, we adopted
the U937-derived macrophages model to investigate the effect of
these factors, as single or in different combinations, on the
release of selected inflammatory markers known to be correlated
with the atherosclerotic process.
In the current experiment, secreted TNF-α levels
were similar in samples treated with one of the parameters added
(mCRP, pCRP and oxLDL) and in those containing either one of the
CRP isoforms with oxLDL. However, the combination of the isoforms
(mCRP with pCRP) with or without oxLDL lowered TNF-α release as
compared to untreated cells, cells treated with pCRP alone or with
oxLDL.
As expected, IL-6 levels increased in the presence
of pCRP or oxLDL; however, this failed to reach statistical
significance. Interestingly, IL-6 released in presence of the
double combination lacking mCRP was less than the other treatments,
except the one with oxLDL alone. This decrease was more pronounced
in the triple combination sample. This lowering of IL-6 for
combinations containing pCRP suggested that pCRP may have the major
role in the downregulation of IL-6 release by U937-derived
macrophages stimulated with mCRP and oxLDL.
These results suggested a possible interaction
between CRP isoforms lowering TNF-α and IL-6 release by
U937-derived macrophages in presence and absence of oxLDL.
Therefore, it may be suggested that the presence of mCRP and pCRP
decreases TNF-α and IL-6 secretion by macrophages that may
decelerate the process of inflammation.
Previous studies have demonstrated that high level
of TNF-α is associated with an increased inflammatory activity in
the blood as well as increased levels of IL-6 (25). OxLDL and CRP stimulate activated
macrophages to release TNF-α, IL-6 and other cytokines that induce
vascular and macrophage activation, thus leading to inflammation
(26,27). According to the present study, the
triple combination reduces the release of these cytokines, which
may retard the inflammatory process associated with
atherosclerosis. These two pro-inflammatory cytokines levels were
increased in elderly and people prone to atherosclerosis (28). Moreover, an increased level of IL-6
exacerbates the atherosclerotic lesions (19,29).
Therefore, lowering these pro-inflammatory cytokines release may
reduce atherosclerosis progression (30).
Unlike other studies, these results are not
influenced by the presence of azide used for CRP preservation that
induces TNF-α release (3,4).
The current results support previous studies
attributing the pro-inflammatory effects to mCRP rather than pCRP.
However, we further propose a possible interaction between the
three molecules leading to the observed anti-inflammatory effects
on the model cells tested.
Neither IL-1β nor IL-8 levels were affected by any
of the treatment combinations. IL-1β, mainly secreted by monocytes
and activated macrophages, has an important role in the progression
of atherosclerosis (19,31). However, IL-8 serves an important
chemotactic role in inflammation and in recruiting neutrophils and
other cells to adhere to endothelial cells at the site of
inflammation. It is also released by various types of cells
including key cells involved in atherosclerosis, such as monocytes,
macrophages and T lymphocytes (32).
The levels of released ROS by U937-derived
macrophages were similar among all the different treatment
conditions. A previous study has reported that CRP induced ROS
production by THP-1 macrophages in a time dependent manner
(33). However, it was not indicated
which CRP isoform was responsible for the increased ROS induction
by THP-1 macrophages. Therefore, future experiments will aim to
investigate ROS production by U937-derived macrophages following
shorter treatment periods with CRP isoforms.
The present study demonstrated that a combination of
mCRP and pCRP in the presence of oxLDL would decrease TNF-α and
IL-6 production by U937-derived macrophages. However, no
significant effects were observed for IL-1β and IL-8, nor for ROS
levels in any of the treatments tested. Therefore, the combination
of CRP isoforms may stabilize and decelerate the atherosclerotic
process. However, additional in vivo and in vitro
studies are required to investigate the effects of this combination
either on the arteries or on other cell types that have crucial
roles in plaque development. Recent studies have been targeting
pCRP dissociation as a novel therapy for atherosclerosis (34).
Acknowledgements
The present study was supported by BIRG (Balamand
Internal Research Grant; grant no. 10/2013). The authors would like
to thank Dr Zeina Nasr (University of Balamand) and Dr Jad Abdallah
(Lebanese American University) as well as Dr Takla El Khoury, Mr.
Michel El Zakhem and Mr. Salah El Khatib (University of Balamand)
for their assistance and the valuable discussions that enriched
this study.
References
|
1
|
Barquera S, Pedroza-Tobías A, Medina C,
Hernández-Barrera L, Bibbins-Domingo K, Lozano R and Moran AE:
Global overview of the epidemiology of atherosclerotic
cardiovascular disease. Arch Med Res. 46:328–338. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hattori Y, Matsumura M and Kasai K:
Vascular smooth muscle cell activation by C-reactive protein.
Cardiovasc Res. 58:186–195. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eisenhardt SU, Thiele JR, Bannasch H,
Stark GB and Peter K: C-reactive protein: How conformational
changes influence inflammatory properties. Cell Cycle. 8:3885–3892.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eisenhardt SU, Habersberger J, Murphy A,
Chen YC, Woollard KJ, Bassler N, Qian H, von Zur Muhlen C,
Hagemeyer CE, Ahrens I, et al: Dissociation of pentameric to
monomeric C-reactive protein on activated platelets localizes
inflammation to atherosclerotic plaques. Circ Res. 105:128–137.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Khreiss T, József L, Potempa LA and Filep
JG: Conformational rearrangement in C-reactive protein is required
for proinflammatory actions on human endothelial cells.
Circulation. 109:2016–2022. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schwedler SB, Amann K, Wernicke K, Krebs
A, Nauck M, Wanner C, Potempa LA and Galle J: Native C-reactive
protein increases whereas modified C-reactive protein reduces
atherosclerosis in apolipoprotein E-knockout mice. Circulation.
112:1016–1023. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eisenhardt SU, Habersberger J and Peter K:
Monomeric C-reactive protein generation on activated platelets: The
missing link between inflammation and atherothrombotic risk. Trends
Cardiovasc Med. 19:232–237. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Filep JG: Platelets affect the structure
and function of C-reactive protein. Circ Res. 105:109–111. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Khreiss T, József L, Potempa LA and Filep
JG: Loss of pentameric symmetry in C-reactive protein induces
interleukin-8 secretion through peroxynitrite signaling in human
neutrophils. Circ Res. 97:690–697. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Libby P, Ridker PM and Maseri A:
Inflammation and atherosclerosis. Circulation. 105:1135–1143. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tabuchi M, Inoue K, Usui-Kataoka H,
Kobayashi K, Teramoto M, Takasugi K, Shikata K, Yamamura M, Ando K,
Nishida K, et al: The association of C-reactive protein with an
oxidative metabolite of LDL and its implication in atherosclerosis.
J Lipid Res. 48:768–781. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chang MK, Hartvigsen K, Ryu J, Kim Y and
Han KH: The pro-atherogenic effects of macrophages are reduced upon
formation of a complex between C-reactive protein and
lysophosphatidylcholine. J Inflamm (Lond). 9:422012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Frostegard J, Ulfgren AK, Nyberg P, Hedin
U, Swedenborg J, Andersson U and Hansson GK: Cytokine expression in
advanced human atherosclerotic plaques: Dominance of
pro-inflammatory (Th1) and macrophage-stimulating cytokines.
Atherosclerosis. 145:33–43. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qiu G, Ho AC, Yu W and Hill JS:
Suppression of endothelial or lipoprotein lipase in THP-1
macrophages attenuates proinflammatory cytokine secretion. J Lipid
Res. 48:385–394. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jovinge S, Ares MP, Kallin B and Nilsson
J: Human monocytes/macrophages release TNF-alpha in response to
Ox-LDL. Arterioscler Thromb Vasc Biol. 16:1573–1579. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eruslanov E and Kusmartsev S:
Identification of ROS using oxidized DCFDA and flow-cytometry.
Methods Mol Biol. 594:57–72. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Uzui H, Harpf A, Liu M, Doherty TM, Shukla
A, Chai NN, Tripathi PV, Jovinge S, Wilkin DJ, Asotra K, et al:
Increased expression of membrane type 3-matrix metalloproteinase in
human atherosclerotic plaque: Role of activated macrophages and
inflammatory cytokines. Circulation. 106:3024–3030. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaneto H, Katakami N, Matsuhisa M and
Matsuoka TA: Role of reactive oxygen species in the progression of
type 2 diabetes and atherosclerosis. Mediators Inflamm.
2010:4538922010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chávez-Sánchez L, Chávez-Rueda K,
Legorreta-Haquet MV, Zenteno E, Ledesma-Soto Y, Montoya-Díaz E,
Tesoro-Cruz E, Madrid-Miller A and Blanco-Favela F: The activation
of CD14, TLR4, and TLR2 by mmLDL induces IL-1beta, IL-6, and IL-10
secretion in human monocytes and macrophages. Lipids Health Dis.
9:1172010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Galve-de Rochemonteix B, Wiktorowicz K,
Kushner I and Dayer JM: C-reactive protein increases production of
IL-1 alpha, IL-1 beta, and TNF-alpha and expression of mRNA by
human alveolar macrophages. J Leukoc Biol. 53:439–445.
1993.PubMed/NCBI
|
|
21
|
Pue CA, Mortensen RF, Marsh CB, Pope HA
and Wewers MD: Acute phase levels of C-reactive protein enhance
IL-1 beta and IL-1ra production by human blood monocytes but
inhibit IL-1 beta and IL-1ra production by alveolar macrophages. J
Immunol. 156:1594–1600. 1996.PubMed/NCBI
|
|
22
|
Gershov D, Kim S, Brot N and Elkon KB:
C-Reactive protein binds to apoptotic cells, protects the cells
from assembly of the terminal complement components, and sustains
an antiinflammatory innate immune response: Implications for
systemic autoimmunity. J Exp Med. 192:1353–1364. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Taylor KE and van den Berg CW: Structural
and functional comparison of native pentameric, denatured monomeric
and biotinylated C-reactive protein. Immunology. 120:404–411. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Adan A, Kiraz Y and Baran Y: Cell
proliferation and cytotoxicity assays. Curr Pharm Biotechnol.
17:1213–1221. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bruunsgaard H, Andersen-Ranberg K, Jeune
B, Pedersen AN, Skinhoj P and Pedersen BK: A high plasma
concentration of TNF-αlpha is associated with dementia in
centenarians. J Gerontol A Biol Sci Med Sci. 54:M357–M364. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hansson GK and Hermansson A: The immune
system in atherosclerosis. Nat Immunol. 12:204–212. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Devaraj S, Davis B, Simon SI and Jialal I:
CRP promotes monocyte-endothelial cell adhesion via Fcgamma
receptors in human aortic endothelial cells under static and shear
flow conditions. Am J Physiol Heart Circ Physiol. 291:H1170–H1176.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bruunsgaard H, Skinhøj P, Pedersen AN,
Schroll M and Pedersen BK: Ageing, tumour necrosis factor-alpha
(TNF-alpha) and atherosclerosis. Clin Exp Immunol. 121:255–260.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huber SA, Sakkinen P, Conze D, Hardin N
and Tracy R: Interleukin-6 exacerbates early atherosclerosis in
mice. Arterioscler Thromb Vasc Biol. 19:2364–2367. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Branen L, Hovgaard L, Nitulescu M,
Bengtsson E, Nilsson J and Jovinge S: Inhibition of tumor necrosis
factor-alpha reduces atherosclerosis in apolipoprotein E knockout
mice. Arterioscler Thromb Vasc Biol. 24:2137–2142. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hansson GK and Libby P: The immune
response in atherosclerosis: A double-edged sword. Nat Rev Immunol.
6:508–519. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Harada A, Sekido N, Akahoshi T, Wada T,
Mukaida N and Matsushima K: Essential involvement of interleukin-8
(IL-8) in acute inflammation. J Leukoc Biol. 56:559–564.
1994.PubMed/NCBI
|
|
33
|
Zhao XQ, Zhang MW, Wang F, Zhao YX, Li JJ,
Wang XP, Bu PL, Yang JM, Liu XL, Zhang MX, et al: CRP enhances
soluble LOX-1 release from macrophages by activating TNF-α
converting enzyme. J Lipid Res. 52:923–933. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Thiele JR, Zeller J, Bannasch H, Stark GB,
Peter K and Eisenhardt SU: Targeting C-reactive protein in
inflammatory disease by preventing conformational changes.
Mediators Inflamm. 2015:3724322015. View Article : Google Scholar : PubMed/NCBI
|