Introduction
The clonal hematopoietic stem cell is associated
with chronic myeloid leukemia (CML), which results due to the
balance translocation among the long arms of chromosomes
(9;22)(q34;q11) commonly known as Philadelphia (Ph) chromosome
(1,2).
CML consists of monocytic, megakaryocytic, myeloid, erythroid,
B-lymphoid and T-lymphoid linkages (3). The nonfunctional crossbreed (PBCR-ABL)
protein produced by the chimeric BCR-ABL gene with tyrosine kinase
activity independently leads to myeloid proliferation and leukemic
makeover (1,4). The translocation of chromosomes
(9;22)(q34;p15) is perceived in almost 90–95% of patients with CML,
and only 5–8% CML patients have established variant complex
translocation, which is due to the participation of one or more
chromosomes other than 9 and 22 chromosome (5–7).
BCR-ABL protein tyrosine kinase is inhibited by the
imatinib mesylate (IM), a conventional oral therapy for patients
suffering from CML, irrespective of phases, with a response rate of
65–90% in CML cases. IM acts by blocking the production and
inducing apoptosis of BCR-ABL gene expression in CML cells. It
plays a vital role regarding continued survival and better quality
of life (8–11).
In the present study, we report a rare three-way
Ph-positive complex variant translocation
46,XX,t(6;9;22)(p21:q34;q11) in a CML patient.
Patient and methods
Case report
We report here a 47-year-old female patient who had
established CML in Sundayman Civil Hospital (Quetta) on the 8th
August, 2014. She was referred to the hospital because of symptoms
including fever, anemia, weight loss, sweating, depression,
swelling on the body and high blood pressure. The laboratory
characteristics of the patient were WBC (414000/mm3),
hemoglobin (8.9 g/dl), platelets (619000/mm3), MCV (56.2
FL), MCH (22.5/pg), lymphocytes (7%), neutrophils (20%), HCT
(30.2%), monocytes (0.3%), MCHC (39.9 g/dl), meta-myelocytes (33%),
blast (2%), myelocytes (10%) and normoblasts (04/100 WBCs). The
result of ultrasound report of the CML patient showed mild
hepatomegaly and massive splenomegaly. The patient was treated with
Glivec (imatinib) 600 mg/day. Approval for the study was obtained
from the Ethics Committee of the Institute/University (BUITEMS,
Quetta, Pakistan).
Methods
CBC laboratory test
Hematological markers such as hemoglobin, platelets,
red blood cells and white blood cells were determined within a day
of sample collection by Nihon Codon Hematological analyzer (Tokyo,
Japan).
Cytogenetic test
Bone marrow culture was used for cytogenetic
analysis as reported in an earlier study (12). Twenty GTG-banded unstimulated bone
marrow specimens were examined. Karyotypes were performed as per
the International System for Human Cytogenetic Nomenclature
(13).
FISH test
Fluorescence in situ hybridisation (FISH)
analysis was performed by directly labeled dual color LSI/CEP probe
for the recognition of BCR/ABL gene. We counted 500 metaphase or
interphase cells to obtain the BCR-ABL percentage.
Results
Hematological results showed an increase in WBC
414000/mm3 and a decrease in hemoglobin (8.9 mg/dl),
indicating the anemic condition in the CML patient. Furthermore,
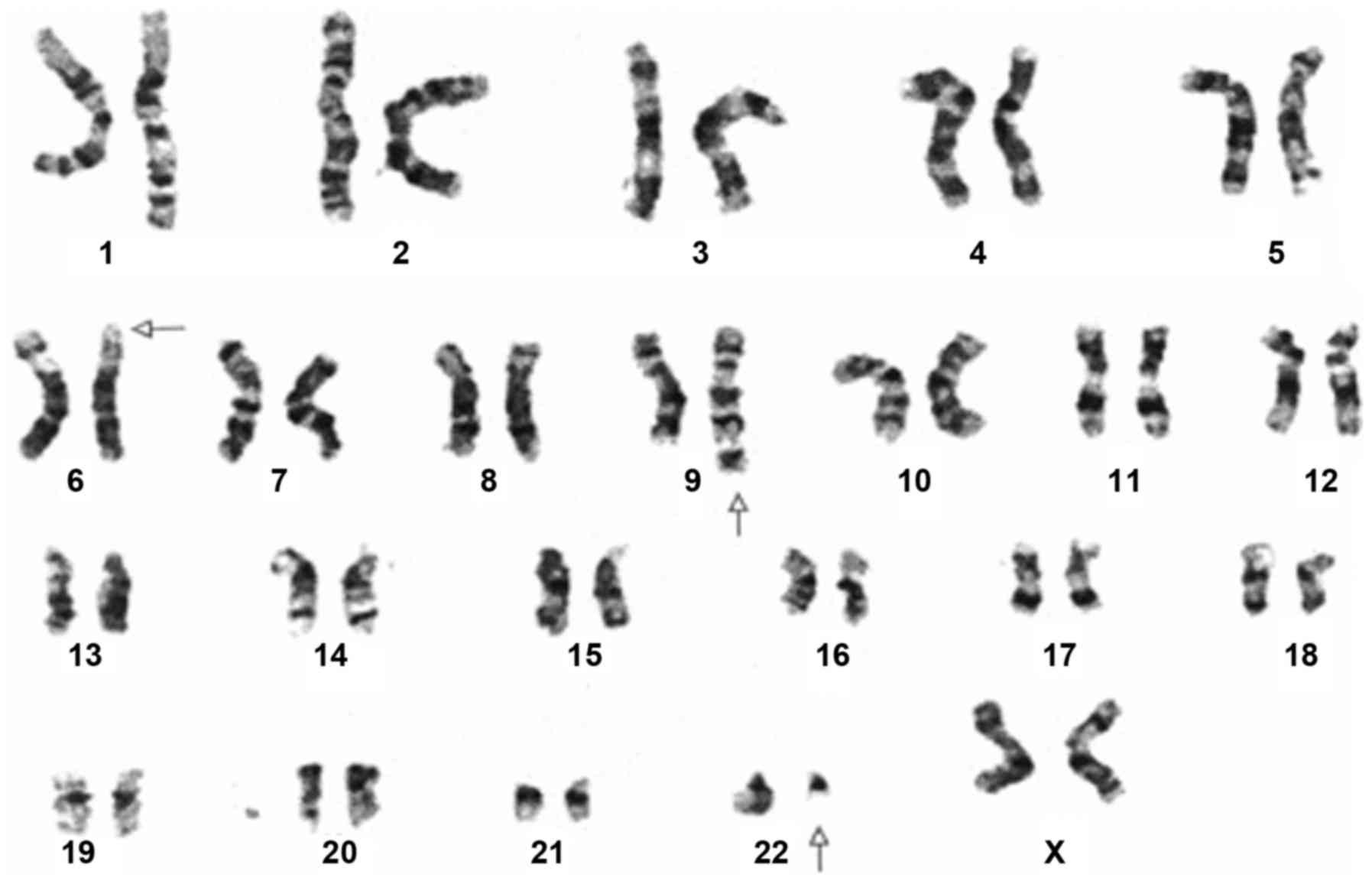
cytogenetic karyotyping results showed 46,XX,t(6;9;22)(p21;q34;q11)
in this CML patient. All 20 cells were positive for Ph chromosome
(Fig. 1).
BCR-ABL fusion genes were detected in 91% of the 500
nuclei counted in the FISH analysis. The fluorescent red dots
corresponded for the (9q34) ABL and green dots represents (22q11)
the BCR gene. However, a cell showing two isolated green and
red dots counted as a normal cell, indicating no translocation.
Conversely, a cell displaying one red, one green with fused yellow
signal was considered for irregular translocation.
Discussion
CML is associated with clonal stem cell syndrome due
to balanced translocation of long arms of (9;22) chromosomes in
most cases (90–95%) of CML. However, 5–8% of CML patients present
variant and complex translocation, referred to as Ph chromosome,
later responsible for the production of a protein BCR-ABL gene
fusion, which contains a protein kinase activity. The
BCR-ABL genes interfere with WBC, making the immune system
weak (25). This condition is observed
in 90% of CML individuals (7).
Sessarego et al (5) and Aliano et al identified that
complex variant translocation is present in almost 5–8% of CML
patients, by the participation of one extra chromosome or more than
one chromosome in addition to chromosome numbers 9 and 22 (5,26,27). In the present study, we also
investigated a complex variant case of chorionic myeloid leukemia
patient with Ph chromosome 46,XX,t(6;9;22)(p21;q34;q11) in chronic
phase. To the best of our knowledge, we are the thirteenth study to
report these cases; previously only 12 investigators reported such
type of cases.
The mechanism of variant complex Ph translocation is
an unknown phenomenon. Morel et al suggested a two-step
mechanism. The first one involves the formation of (9;22)(34q;11q)
translocation and the second is the additional translocation
involving one derivative chromosome from Ph translocation and a
third chromosome (28). Reddy and
Sulcova reported that the complex variant translocation formed by
multiple simultaneous breakages of several chromosomes were
followed by mismatch joining (29).
Imatinib (Glivec) is a potent inhibitor of the
Bcr-Abl protein tyrosine kinase. The optimal doses of Glivec
primarily attenuate the tyrosine kinase activity of the
platelet-derived growth factor (PDGF) receptor β and c-Kit, without
disturbing other associates of the type III receptor kinase family,
such as Flt-3 and Fms (29,30).
In conclusion, we reported a rare case of the
variant translocation involving Ph-positive chromosome
46,XX,t(6;9;22)(p21;q34;q11) in a CML patient in the chronic
phase.
Acknowledgements
The present study was partially supported by Office
of Research Innovational and commercialization, BUITEMS, Quetta,
Pakistan. (Registration ID no. 27934).
References
|
1
|
Aguayo A, Garcia-Alvarez E,
Cazares-Ordonez Y, Crespo-Solis E, Martinez-Baños D,
Guadarrama-Beltran E, Cervera-Ceballos EE and Lopez-Karpovitch X:
Chronic myeloid leukemia: A clinicoepidemiologic and therapeutic
description of a single institution in Mexico city. Clin Leuk.
2:261–266. 2008. View Article : Google Scholar
|
|
2
|
Au WY, Caguioa PB, Chuah C, Hsu SC, Jootar
S, Kim DW, Kweon IY, O'Neil WM, Saikia TK and Wang J: Chronic
myeloid leukemia in Asia. Int J Hematol. 89:14–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Faderl S, Talpaz M, Estrov Z, O'Brien S,
Kurzrock R and Kantarjian HM: The biology of chronic myeloid
leukemia. N Engl J Med. 341:164–172. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cowan-Jacob SW, Fendrich G, Floersheimer
A, Furet P, Liebetanz J, Rummel G, Rheinberger P, Centeleghe M,
Fabbro D and Manley PW: Structural biology contributions to the
discovery of drugs to treat chronic myelogenous leukaemia. Acta
Crystallogr D Biol Crystallogr. 63:80–93. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sessarego M, Fugazza G, Bruzzone R,
Ballestrero A, Miglino M and Bacigalupo A: Complex chromosome
rearrangements may locate the Bcr/abl fusion gene sites other than
22q11. Haematologica. 85:35–39. 2000.PubMed/NCBI
|
|
6
|
La Starza R, Testoni N, Lafage-Pochitaloff
M, Ruggeri D, Ottaviani E, Perla G, Martelli MF, Marynen P and
Mecucci C: Complex variant Philadelphia translocations involving
the short arm of chromosome 6 in chronic myeloid leukemia.
Haematologica. 87:143–147. 2002.PubMed/NCBI
|
|
7
|
Achkar WA, Wafa A, Ali BY, Manvelyan M and
Liehr T: A rare chronic myeloid leukemia case with Philadelphia
chromosome, BCR-ABL e13a3 transcript and complex translocation
involving four different chromosomes. Oncol Lett. 1:797–800. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Al-Achkar W, Wafa A and Liehr T: A new
t(9;11;20;22)(q34;p11.2;q11.21;q11) in a Philadelphia-positive
chronic myeloid leukemia case. Oncol Lett. 5:605–608.
2013.PubMed/NCBI
|
|
9
|
Cohen MH, Williams G, Johnson JR, Duan J,
Gobburu J, Rahman A, Benson K, Leighton J, Kim SK, Wood R, et al:
Approval summary for imatinib mesylate capsules in the treatment of
chronic myelogenous leukemia. Clin Cancer Res. 8:935–942.
2002.PubMed/NCBI
|
|
10
|
Koshiyama DB, Capra ME, Paskulin GA, Rosa
RF, Oliveira CA, Vanelli T, Fogliatto LM and Zen PR: Cytogenetic
response to imatinib treatment in Southern Brazilian patients with
chronic myelogenous leukemia and variant Philadelphia chromosome.
Ann Hematol. 92:185–189. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cortes J, Giles F, O'Brien S, Thomas D,
Garcia-Manero G, Rios MB, Faderl S, Verstovsek S, Ferrajoli A,
Freireich EJ, et al: Result of high-dose imatinib mesylate in
patients with Philadelphia chromosome-positive chronic myeloid
leukemia after failure of interferon-α. Blood. 102:83–86. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Claussen U, Michel S, Mühlig P, Westermann
M, Grummt UW, Kromeyer-Hauschild K and Liehr T: Demystifying
chromosome preparation and the implications for the concept of
chromosome condensation during mitosis. Cytogenet Genome Res.
98:136–146. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Simons A, Shaffer LG and Hastings RJ:
Cytogenetic nomenclature: Changes in the ISCN 2013 compared to the
2009 edition. Cytogenet Genome Res. 141:1–6. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bennour A, Sennana H, Laatiri MA, Elloumi
M, Khelif A and Saad A: Molecular cytogenetic characterization of
variant Philadelphia translocations in chronic myeloid leukemia:
Genesis and deletion of derivative chromosome 9. Cancer Genet
Cytogenet. 194:30–37. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dubé I, Dixon J, Beckett T, Grossman A,
Weinstein M, Benn P, McKeithan T, Norman C and Pinkerton P:
Location of breakpoints within the major breakpoint cluster region
(bcr) in 33 patients with bcr rearrangement-positive chronic
myeloid leukemia (CML) with complex or absent Philadelphia
chromosomes. Genes Chromosomes Cancer. 1:106–111. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hagemeijer A: The Philadelphia chromosome,
25 years later. 1960–1985. Nouv Rev Fr Hematol. 27:153–155.
1985.(In French). PubMed/NCBI
|
|
17
|
Potter AM, Sharp JC, Brown MJ and Sokol
RJ: Structural rearrangements associated with the Ph1 chromosome in
chronic granulocytic leukaemia. Humangenetik. 29:223–228.
1975.PubMed/NCBI
|
|
18
|
La Starza R, Vitale A, Serra A, Saglio G,
Fioritoni G, Falzetti D, Martelli MF, Foà R and Mecucci C:
Philadelphia-positive acute lymphoblastic leukemia with multiple
subclones including duplication of the Philadelphia chromosome and
Abelson oncogene. Cancer Genet Cytogenet. 132:46–50. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sessarego M, Ajmar F, Scarrà Bianchi GL,
Ravazzolo R, Garré C and Boccaccio P: Unusual Ph translocations in
CML: Four new cases. Cancer Genet Cytogenet. 15:199–207. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sessarego M, Defferrari R, Panarello C,
Frassoni F, Mandich P and Ajmar F: Variant Philadelphia
translocations in CML: Correlation with fragile sites. Cancer Genet
Cytogenet. 31:105–112. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Orciuolo E, Buda G, Galimberti S, Cervetti
G, Cecconi N, Papineschi F and Petrini M: Complex translocation
t(6;9;22)(p21.1;q34;q11) at diagnosis is a therapy resistance index
in chronic myeloid leukaemia. Leuk Res. 32:190–191. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reid R, de Silva MVC and Paterson L:
Poorly differentiated extraskeletal myxoid chondrosarcoma with
t(9;22)(q22;q11) translocation presenting initially as a solid
variant devoid of myxoid areas. Int J Surg Pathol. 11:137–141.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Legues ME, Encina A, Valenzuela M, Palma T
and Undurraga MS: Cytogenetic and molecular characteristics of 25
Chilean patients with a variant Ph translocation. Cancer Genet.
204:410–412. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barbouti A, Johansson B, Höglund M,
Mauritzson N, Strömbeck B, Nilsson PG, Tanke HJ, Hagemeijer A,
Mitelman F and Fioretos T: Multicolor COBRA-FISH analysis of
chronic myeloid leukemia reveals novel cryptic balanced
translocations during disease progression. Genes Chromosomes
Cancer. 35:127–137. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zangari M, Anaissie E, Stopeck A, Morimoto
A, Tan N, Lancet J, Cooper M, Hannah A, Garcia-Manero G, Faderl S,
et al: Phase II study of SU5416, a small molecule vascular
endothelial growth factor tyrosine kinase receptor inhibitor, in
patients with refractory multiple myeloma. Clin Cancer Res.
10:88–95. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Quintás-Cardama A and Cortes JE: Chronic
myeloid leukemia: Diagnosis and treatment. Mayo Clin Proc.
81:973–988. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aliano S, Cirmena G, Fugazza G, Bruzzone
R, Palermo C and Sessarego M: Standard and variant Philadelphia
translocation in a CML patient with different sensitivity to
imatinib therapy. Leuk Res Rep. 2:75–78. 2013.PubMed/NCBI
|
|
28
|
Morel F, Herry A, Le Bris MJ, Morice P,
Bouquard P, Abgrall JF, Berthou C and De Braekeleer M: Contribution
of fluorescence in situ hybridization analyses to the
characterization of masked and complex Philadelphia chromosome
translocations in chronic myelocytic leukemia. Cancer Genet
Cytogenet. 147:115–120. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Reddy KS and Sulcova V: A FISH study of
variant Philadelphia rearrangements. Cancer Genet Cytogenet.
118:121–131. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cohen MS, Hussain HB and Moley JF:
Inhibition of medullary thyroid carcinoma cell proliferation and
RET phosphorylation by tyrosine kinase inhibitors. Surgery.
132:960–967. 2002. View Article : Google Scholar : PubMed/NCBI
|