Introduction
Kidney transplantation is the optimal treatment for
patients with end-stage kidney disease (1). Although progress in immunosuppressive
regimens improved kidney allograft survival mainly by affecting
cellular alloimmunity, long-term outcomes remain relatively poor
partially due to chronic alloantibody-mediated graft rejection
(2). Thus, investigation for new
immunosuppressive medications able to suppress both cellular and
humoral alloimmunity is of great importance.
Immunometabolism is a field of research with
promising pharmacological applications. Concerning adaptive
immunity, T-cell activation results in an increase in aerobic
glycolysis and glutaminolysis providing the required building
blocks for rapid T-cell proliferation and differentiation into
effector T cells (3–5). Although most of the cell energy is
acquired by the enhanced aerobic glycolysis, a moderate Krebs cycle
and oxidative phosphorylation activity is required for effective
T-cell activation, possibly as an extra source of ATP and/or due to
the role that mitochondrial ROS play in T-cell activation (6–9). Apart from
glucose, upon T-cell activation, glutamine serves as another source
of Krebs cycle (10). Glutaminase
(GLS) converts glutamine to glutamate. Glutamate is converted to
α-ketoglutarate, which may enter the Krebs cycle (11). Additionally, in the case of
differentiation in regulatory T cells, fatty acids entering the
Krebs cycle is pivotal (3).
B cell metabolism is less well studied (5). There is evidence that upon activation, B
cells enhance both aerobic glycolysis and the Krebs cycle in a
balanced manner (12–14). Glutamine again serves as a major Krebs
cycle source (15). However,
differentiated plasma cells are mainly dependent on the Krebs cycle
and oxidative phosphorylation for covering the extremely high
demands of protein synthesis for ATP. During plasma cell
differentiation the transcription factor XBP1 increases
mitochondrial mass and function (16).
Pyruvate entry into the Krebs cycle is required for the long-term
survival of antibody-secreted plasma cells (17). Glutamine again serves as a major source
of the Krebs cycle. It is well known that high glutamine
concentration is required for antibody production by cultured
lymphocytes (18). Analysis of the
exometabolome with advanced techniques has shown that massive
antibody production is paralleled by glutamine use as a +carbon and
energy source (12).
Dichloroacetate (DCA) is an inhibitor of pyruvate
dehydrogenase (PDH) kinase (PDK) and by decreasing the
phosphorylation of PDH, the pyruvate entry into the Krebs cycle is
increased instead of its being converted to lactate, thereby
decreasing aerobic glycolysis (19). A
previous study has shown that in mixed lymphocyte reactions (MLRs),
DCA at a concentration of 1 mM decreases aerobic glycolysis
assessed by lactate production (20).
At this concentration, DCA does not affect significantly cell
proliferation but favors CD4+ T-cell apoptosis and differentiation
towards a regulatory instead of an effector phenotype (20).
Malate dehydrogenase-2 (MDH2) catalyzes the
conversion of malate to oxaloacetate in the Krebs cycle, and the
3-(aryloxyacetylamino)-4-hydroxybenzoic acid derivative LW6 by
inhibiting MDH2, thereby suppressing the Krebs cycle (21). A previous study has shown that LW6 at a
concentration of 30 µM decreases the proliferation of activated
T-cells without inducing apoptosis (22). The Krebs cycle is only partially
inhibited (22), possibly due to the
pyruvate-malate cycle. In this cycle, malate, which is expected to
be accumulated in case of MDH2 inhibition, is transferred from the
mitochondria to the cytosol where it is converted by the malic
enzyme to pyruvate. The formed pyruvate can be converted by PDH to
acetyl-CoA and re-enters the Krebs cycle (23). Notably, the malic enzyme is upregulated
following T-cell activation (4).
Additionally, the pyruvate-malate cycle occurs in activated B cells
(15). LW6 increased the expression
GLS1 in activated T cells (22), which
may also contribute to the only partial inhibition of the Krebs
cycle by enhancing the use of glutamine.
Considering the significant role of cell metabolism
shifts in the immune response and the possible clinical
applications of pharmacological intervention on cell metabolism
pathways, in this study, we compared the effect of the aerobic
glycolysis inhibitor DCA and Krebs cycle inhibitor LW6 on cellular
and humoral alloimmunity. For this purpose, human peripheral blood
mononuclear cells (PBMCs) and MLRs were used; and an assay for
assessing alloantibody production was developed.
Materials and methods
Subjects
Blood samples were collected from 4 healthy
volunteers (aged 33.5±6.45 years). All the subjects were male and
without a history of blood transfusion in order to exclude any
pre-sensitization event. Informed consent was obtained from each
individual enrolled in the study. The Ethics Committee of the
Faculty of Medicine, University of Thessaly, approved the study
protocol.
Cell isolation and culture
conditions
PBMCs were isolated from blood with Ficoll-Hypaque
density gradient centrifugation using Histopaque-1077
(Sigma-Aldrich; Merck Millipore, Darmstadt, Germany). Isolated
PBMCs were counted on a Neubauer chamber (Paul Marienfeld GmbH
& Co. KG, Lauda-Königshofen, Germany) with an optical
microscope. Cell viability was assessed using the trypan blue
exclusion assay (Sigma-Aldrich; Merck Millipore). Cell cultures
were performed in RPMI-1640 medium, supplemented with L-glutamine,
10 mM [4-(2-hydroxyethyl) piperazineethanesulfonic acid] (HEPES)
(Sigma-Aldrich; Merck Millipore), 10% fetal bovine serum
(Sigma-Aldrich; Merck Millipore) and antibiotic antimycotic
solution (Sigma-Aldrich; Merck Millipore). Cultures were incubated
at 37°C in an atmosphere of 95% relative humidity and 5%
CO2.
Lactate dehydrogenase (LDH) release
cytotoxicity assay
PBMCs (1×105) were cultured in 96-well
plates for a 7-day period in the presence or not of DCA
(Sigma-Aldrich; Merck Millipore) at a concentration of 1 mM or of
LW6 (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at a
concentration of 30 µM. The cytotoxicity of the above compounds was
assessed by LDH release assay using the Cytotox Non-Radioactive
Cytotoxic Assay kit (Promega Corporation, Madison, WI, USA)
according to the manufacturers protocol. Cytotoxicity was
calculated by the equation: Cytotoxicity (%) = (LDH in the
supernatant ÷ total LDH) × 100. The experiments were performed in
triplicate and the results refer to the mean of the three
measurements.
Two-way MLRs and assessment of T-cell
proliferation
T-cell proliferation was assessed by assessing cell
proliferation in two-way MLRs. Two-way MLRs were performed in
96-well plates in the presence or not of DCA at a concentration of
1 mM or of LW6 at a concentration of 30 µM and lasted 7 days. Each
member of the couple of individuals contributed to the respective
MLR with 5×104 PBMCs, bringing the total cell number to
1×105 cells/MLR. In parallel, 1×105 resting
PBMCs from each member were cultured in the same 96-well plate and
served as controls.
Cell proliferation was evaluated with Cell
Proliferation ELISA (Roche Diagnostics, Indianapolis, IN, USA)
using bromodeoxyuridine labeling and immunoenzymatic detection
according to the protocol provided by the manufacturer. The
proliferation index was calculated using the equation:
Proliferation index (%) = [optical density (OD) derived from each
MLR ÷ mean of the ODs derived from the control resting PBMCs
cultures of the two subjects that constituted the specific MLR] ×
100. Six such MLRs were performed, each in triplicate and the
results refer to the mean of the three measurements.
One-way MLRs and assessment of
alloantibody production
One-way MLRs were performed in 24-well plates.
Mitomycin C-treated PBMCs (0.5×106 cells) were used as
stimulator cells. For the mitomycin C treatment, PBMCs were
incubated for 30 min with 50 µg/ml mitomycin C (Sigma-Aldrich;
Merck Millipore) at 37°C and then washed 3 times with complete
RPMI-1640. As responder cells, 0.5×106 PBMCs from
another individual were used. MLRs lasted for 7 days in the
presence or not of 1 mM DCA or 30 µM LW6. Then, supernatants from
each one-way MLR were harvested to obtain antibodies produced
against the stimulator PBMCs, since a 7-day period is enough for
the production of IgM and IgG alloantibodies in MLRs (24).
A modification of an antibody-mediated
complement-dependent cytotoxicity (CDC) assay protocol developed
for assessing antigen-specific antibodies in serum was used in
order to assess alloantibody production (25,26). In
parallel with the above mentioned one-way MLRs, resting untreated
PBMCs were cultured in 6-well plates. At the end of the 7-day
period of one-way MLRs, resting PBMCs similar to those used as
stimulator cells in MLRs, albeit untreated, were counted and seeded
in 96-well plates at a number of 0.5×105 and in a volume
of 50 µl. To assess antibody-mediated CDC, 50 µl of supernatant
collected from each respective one-way MLR or 1:2 dilution with
complete RPMI-1640 were added into the 96-well plates already
seeded with the resting PBMCs serving as target cells. The plates
were incubated for 30 min on ice. Subsequently, 11 µl of rabbit
complement (Low-Tox®-H rabbit complement, Cedarlane
Corporation, Burlington, Ontario, Canada) were added to each well
at a final concentration of 10%. The 96-well plates were incubated
for another 2 h at 37°C. As a control, 50 µl of complete RMPI-1640
were added instead of the one-way MLR supernatant, along with 11 µl
of rabbit complement.
Since cell-mediated cytotoxicity occurs in the
course of an MLR (27), and inevitably
LDH is released in the supernatants and the used compounds affect
the intensity of the MLRs, we avoided assessing antibody-mediated
CDC with LDH release assay as it would provide us with erroneous
data. Instead, we assessed cell survival by measuring the reduction
of sodium
2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)-carbonyl]-2H-tetrazolium
(XTT), a yellow tetrazolium salt, to the orange formazan by
metabolically viable active target cells. Target cells were
incubated with the XTT reagent for 1 h. For this purpose, the TACS
XTT assay kit (Trevigen, Gaithersburg, MD, USA) was used according
to the manufacturers protocol provided and cell survival was
calculated as cell survival (%) = (XTT assay OD of the control ÷
XTT assay OD of the evaluated condition) × 100. Twelve such
experiments were performed, each in triplicates and the results
refer to the mean of the three measurements.
Statistical analysis
The normality of the evaluated variables was
confirmed by the one-sample Kolmogorov-Smirnov test. For comparison
of means, paired t-test or one-way repeated measures analysis of
variance (ANOVA) followed by Bonferronis correction test were used.
Results were expressed as mean ± standard deviation (SD). P<0.05
was considered statistically significant. The SPSS 13.0 software
for Windows (SPSS Inc., Chicago, IL, USA) was used for the
statistical analysis.
Results
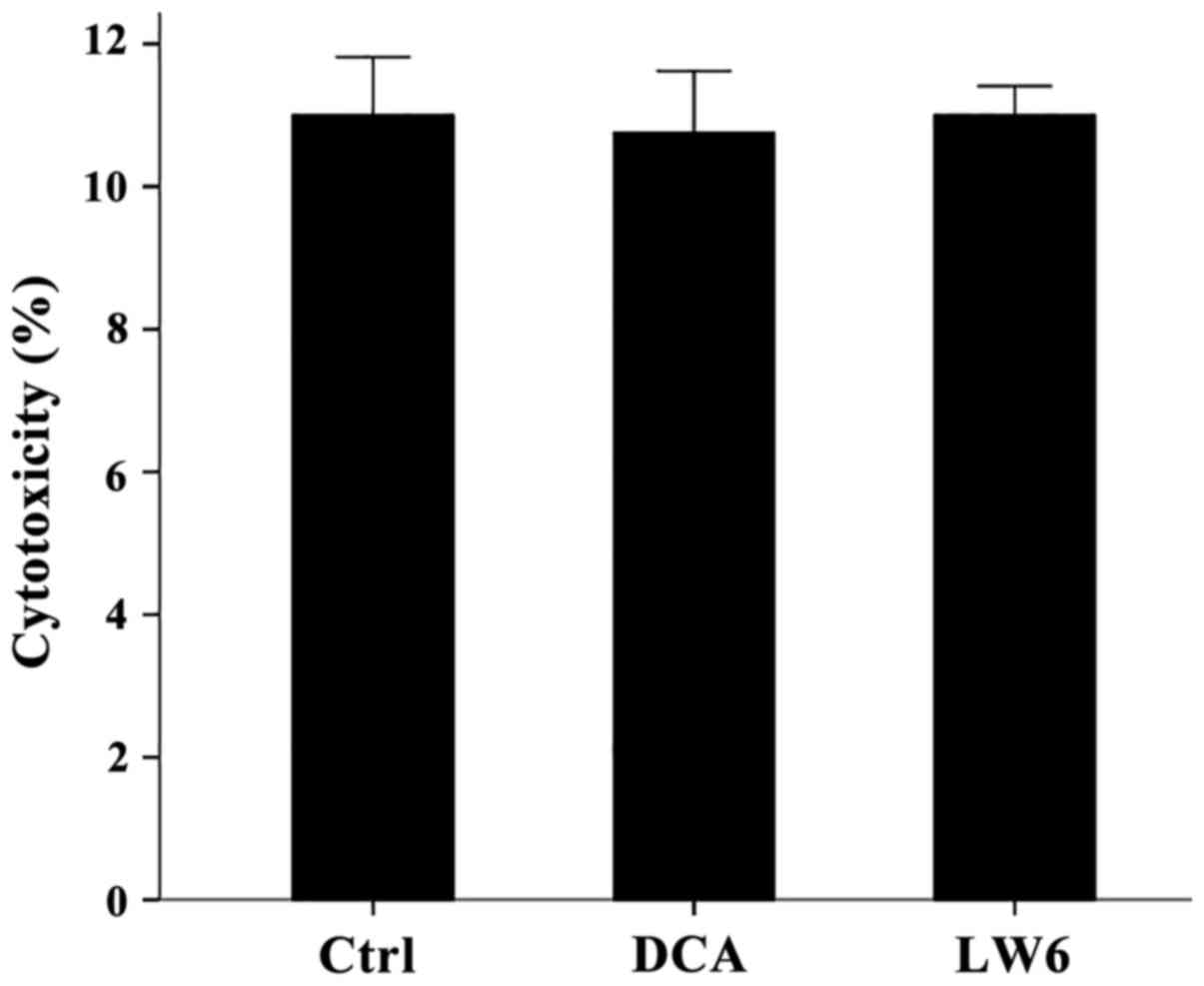
At the used concentrations neither DCA
nor LW6 was cytotoxic for resting PBMCs
Compared to the untreated PBMCs, in which LDH
release assay detected a cytotoxicity of 11±0.82%, neither DCA at a
concentration of 1 mM nor LW6 at a concentration of 30 µM was
cytotoxic. Cytotoxicity was 10.75±0.87% in case of DCA treatment
(p>0.05) and 11.00±0.41% in case of LW6 treatment (p>0.05)
(Fig. 1).
In two-way MLRs DCA had minor effect
on T-cell proliferation, whereas LW6 inhibited T-cell proliferation
significantly
In two-way MLRs DCA increased cell proliferation
(p<0.05), but only slightly. The proliferation index was
160.41±5.66% in untreated MLRs and 187.99±13.20% in DCA-treated
MLRs, which corresponds to an alteration rate of 17.5% (Fig. 2).
LW6 decreased T-cell clonal expansion in two-way
MLRs almost to the control values (p<0.001). In this case, the
proliferation index was 116.95±3.78% (Fig.
2).
DCA increases alloantibody production
in one-way MLRs, whereas LW6 decreases alloantibody production
Antibody-mediated CDC assay revealed that in one-way
MLRs alloantibodies were formed. Undiluted or 1:2 diluted
supernatants from these MLRs decreased target cell survival
significantly to the 64.38±4.42% (p<0.001) and 80.92±3.63%
(p<0.001) compared to the control. Notably, compared to
undiluted supernatants, 1:2 diluted supernatants decreased cell
survival less (p<0.001) (Fig. 3A and
B).
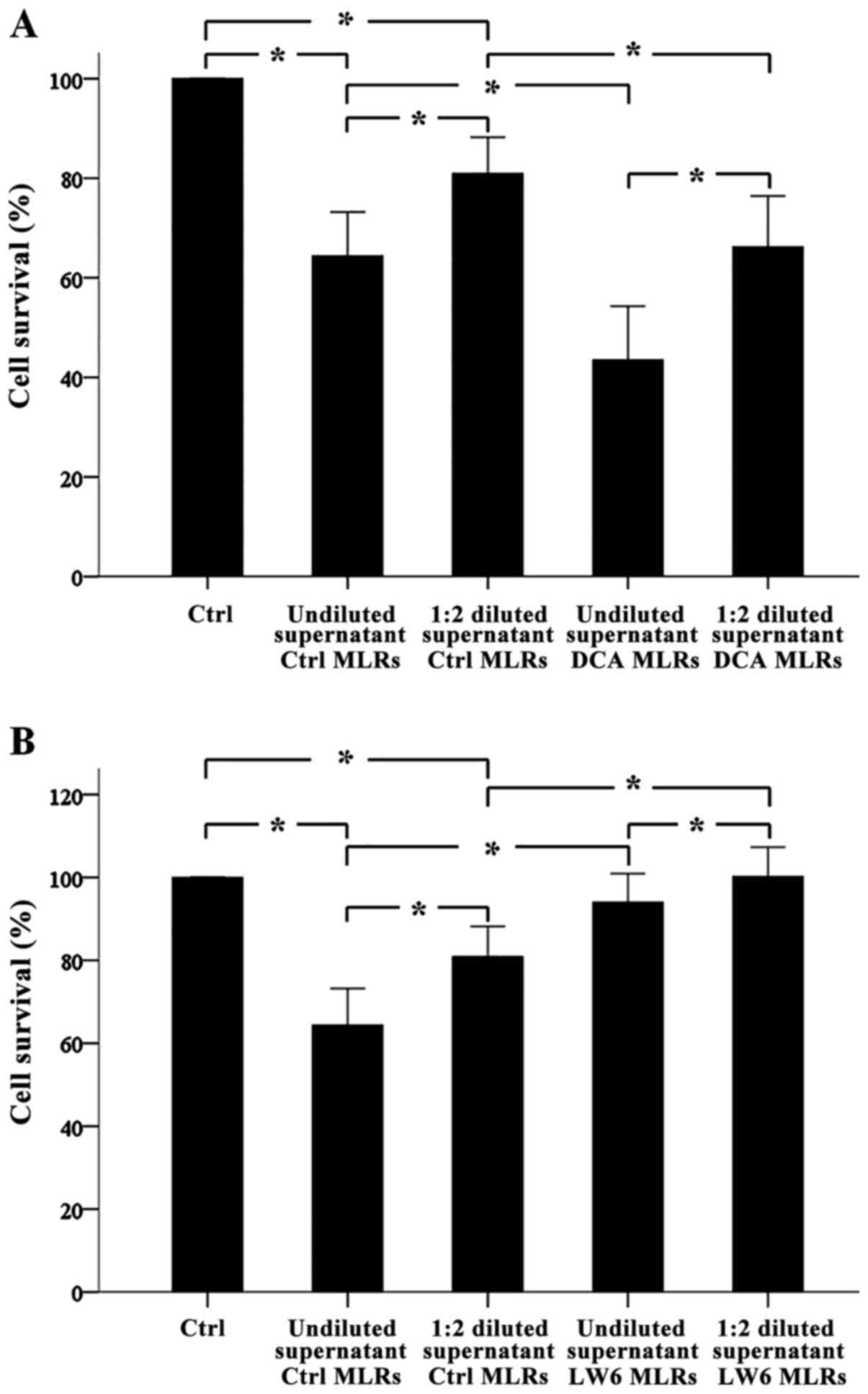 | Figure 3.In one-way MLRs, DCA increased
alloantibody production, whereas LW6 decreased alloantibody
production. One-way MLRs lasted 7 days. Then the supernatants were
collected and the production of the antibodies in them was tested
with an antibody-mediated CDC assay against PBMCs target cells
derived from the subjects who confer the stimulator PMBCs of the
respective one-way MLRs. Twelve different one-way MLRs were
performed. CDC assay revealed that alloantibodies were present in
the supernatants. Of note, when supernatants were used at a 1:2
dilution, less CDC was detected (A and B). CDC assay showed that
DCA increased alloantibody production (A), whereas LW6 decreased
alloantibody production. Error bars correspond to 2 SD. Statistical
significant differences (p<0.05) of interesting comparisons are
denoted with an asterisk. MLRs, mixed lymphocyte reactions; DCA,
dichloroacetate; CDC, complement-dependent cytotoxicity; PBMCs,
peripheral blood mononuclear cells; SD, standard deviation. |
Undiluted supernatants from DCA-treated one way MLRs
decreased target cell survival significantly from 64.38±4.42% to
43.49±5.40% (p<0.001) and from 80.92±3.63% to 66.22±5.10%
(p<0.001), respectively. Diluted supernatants affected cell
survival less (p<0.001). Thus, DCA induced alloantibody
production in one-way MLRs (Fig.
3A).
Undiluted or 1:2 diluted supernatants from
LW6-treated one way MLRs increased target cell survival
significantly from 64.38±4.42% to 94.01±3.47% (p<0.001) and from
80.92±3.63% to 101.17±3.57% (p<0.001), respectively. Diluted
supernatants affected cell survival less (p<0.001). Thus, LW6
inhibited alloantibody production in one-way MLRs (Fig. 3B).
Discussion
In the present study, the effect of the aerobic
glycolysis inhibitor DCA and Krebs cycle enzyme MDH2 inhibitor LW6
on humoral alloimmunity was assessed. For this purpose, non-toxic
concentrations of DCA and LW6 were used, which, however, are
effective in inhibiting the aerobic glycolysis and Krebs cycle,
respectively (20,22). Their effect on T-cell clonal expansion
was also assessed.
In consensus with a previous study (20), DCA increased T-cell clonal expansion
but only slightly. This is in contrast with another study, which
has shown that DCA inhibits T-cell clonal expansion (28). However, in that study T cells were
murine, activated with anti-CD3/CD28 antibodies and the
supra-pharmacological concentration of 20 mM DCA was used (28). We used MLRs, which is a model of
alloimmunity (27), and DCA at a
concentration of 1 mM, which is close to the blood concentrations
of this drug when used for the treatment of hereditary lactic
acidosis (19). Nevertheless, besides
its minor effect on T-cell proliferation found in this study, DCA
may exert an immunosuppressive effect, since it favors CD4+ T-cell
differentiation toward a regulatory instead of an effector
phenotype (20).
As identified in a previous study, in which isolated
T cells were activated with anti-CD3/CD28 covered beads (22), in this study LW6 also inhibited T-cell
clonal expansion in two-way MLRs. However, compared to the role of
aerobic glycolysis, the role of Krebs cycle in T-cell activation
has not been emphasized as Krebs cycle activity is required for an
effective T-cell response (6–10). Thus, regarding the T-cell clonal
expansion part of cellular alloimmunity, the inhibitor of aerobic
glycolysis DCA had a minor effect, whereas the inhibitor of the
Krebs cycle LW6 decreased it significantly.
A number of studies using different murine
experimental models have shown varying results regarding the effect
of DCA on antibody production. In a previous study it was shown
that DCA administration in lupus prone or normal mice enhances
serum immunoglobulin levels (29),
whereas another study has shown that in lupus prone mice DCA had no
effect on the levels of anti-dsDNA IgG (9). On the other hand, in a murine model of
collagen type II-induced arthritis, DCA alleviated arthritis only
in female mice, in which it decreased anti-collagen type II
antibodies without affecting the T cell response (30). In addition, although upon activation
murine B-cells exhibit a balanced increase in lactate production
and oxygen consumption, the induction of glycolysis is critical for
B-cell proliferation and antibody production since DCA abrogated
both of these processes (14).
However, in that study, the human B cells, when used, were isolated
and stimulated with a TLR9 activator (14).
In our model of alloimmunity, DCA increased
alloantibody production in one-way MLRs. This may be attributed to
the direct effect of DCA on B cells. Since plasma cells are mainly
dependent on the Krebs cycle for covering their high energy
requirements (12,16–18),
DCA-induced increased pyruvate entry into the mitochondria may
enhance their ability to produce antibodies. However, an indirect
effect cannot be excluded. During T-cell proliferation, enhanced
aerobic glycolysis produced large amounts of lactate. Lactate
enhances arginase-1 expression on myeloid-derived suppressor cells
(31), which then can suppress
antibody production (32,33). By decreasing lactate production, DCA
may decrease arginase-1 expression and consequently enhance
antibody production. Regardless of the exact molecular mechanism,
our results indicate that although DCA has been already used for
the treatment of hereditary lactic acidosis and is well-tolerated
(19), it is not a good candidate as
an immunosuppressant in the field of transplantation, where chronic
antibody-mediated rejection plays a significant role in late graft
loss.
The inhibitor of the Krebs cycle LW6 inhibited
alloantibody production significantly. This may be the indirect
result of the inhibition of T-cell clonal expansion by LW6, but it
may also be the direct effect of the Krebs cycle inhibition on
plasma cells. As already noted, plasma cells are dependent on the
Krebs cycle for covering the high energy demands for the high
protein synthesis (12,16–18). The
Krebs cycle and the consequent oxidative phosphorylation are more
effective than aerobic glycolysis in ATP production. Every 12 h, a
blood-derived plasma cell can produce up to 1.7 ng of antibodies
(34), whereas it has been calculated
that for each peptide bond formation 7.52 molecules of ATP are
required (35). It is of note that at
least in T cells, the Krebs cycle is not totally inhibited by LW6
(22), possibly due to the
pyruvate-malate cycle and increased GLS1 expression (11,23). This
prevents energy collapse of the T cells and may explain the lack of
apoptosis when they are treated with LW6 (22). It remains to be elucidated if the same
occurs in B cells and plasma cells. Reducing T-cell proliferation
and antibody production without affecting cell survival is a
feature that may mean rapid reversibility of the LW6
immunosuppressive effect when needed, for instance in the case of a
life-threatening infection. Regardless of the exact molecular
mechanism, the ability of LW6 to inhibit both cellular and humoral
alloimmunity renders MDH2 a possible target for the development of
new immunosuppressive medications.
In conclusion, the inhibitor of aerobic glycolysis
DCA exerts a minor effect on alloimmune T-cell clonal expansion,
while it enhances alloantibody production. The inhibitor of the
Krebs cycle LW6 suppresses both alloimmune T-cell clonal expansion
and alloantibody production. These results indicate that MDH2 may
be an optimal target for the development of new immunosuppressive
medications, especially when inhibition of both cellular and
humoral alloimmunity is required.
References
|
1
|
Wolfe RA, Ashby VB, Milford EL, Ojo AO,
Ettenger RE, Agodoa LY, Held PJ and Port FK: Comparison of
mortality in all patients on dialysis, patients on dialysis
awaiting transplantation, and recipients of a first cadaveric
transplant. N Engl J Med. 341:1725–1730. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hricik DE: Transplant immunology and
immunosuppression: Core curriculum 2015. Am J Kidney Dis.
65:956–966. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Michalek RD, Gerriets VA, Jacobs SR,
Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG and
Rathmell JC: Cutting edge: Distinct glycolytic and lipid oxidative
metabolic programs are essential for effector and regulatory
CD4+ T cell subsets. J Immunol. 186:3299–3303. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang R, Dillon CP, Shi LZ, Milasta S,
Carter R, Finkelstein D, McCormick LL, Fitzgerald P, Chi H, Munger
J, et al: The transcription factor Myc controls metabolic
reprogramming upon T lymphocyte activation. Immunity. 35:871–882.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Caro-Maldonado A, Gerriets VA and Rathmell
JC: Matched and mismatched metabolic fuels in lymphocyte function.
Semin Immunol. 24:405–413. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roos D and Loos JA: Changes in the
carbohydrate metabolism of mitogenically stimulated human
peripheral lymphocytes. II. Relative importance of glycolysis and
oxidative phosphorylation on phytohaemagglutinin stimulation. Exp
Cell Res. 77:127–135. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chang CH, Curtis JD, Maggi LB Jr, Faubert
B, Villarino AV, OSullivan D, Huang SC, van der Windt GJ, Blagih J,
Qiu J, et al: Posttranscriptional control of T cell effector
function by aerobic glycolysis. Cell. 153:1239–1251. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sena LA, Li S, Jairaman A, Prakriya M,
Ezponda T, Hildeman DA, Wang CR, Schumacker PT, Licht JD, Perlman
H, et al: Mitochondria are required for antigen-specific T cell
activation through reactive oxygen species signaling. Immunity.
38:225–236. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yin Y, Choi SC, Xu Z, Zeumer L, Kanda N,
Croker BP and Morel L: Glucose oxidation is critical for
CD4+ T cell activation in a mouse model of systemic
lupus erythematosus. J Immunol. 196:80–90. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bental M and Deutsch C: Metabolic changes
in activated T cells: An NMR study of human peripheral blood
lymphocytes. Magn Reson Med. 29:317–326. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matés JM, Segura JA, Martín-Rufián M,
Campos-Sandoval JA, Alonso FJ and Márquez J: Glutaminase isoenzymes
as key regulators in metabolic and oxidative stress against cancer.
Curr Mol Med. 13:514–534. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Garcia-Manteiga JM, Mari S, Godejohann M,
Spraul M, Napoli C, Cenci S, Musco G and Sitia R: Metabolomics of B
to plasma cell differentiation. J Proteome Res. 10:4165–4176. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dufort FJ, Gumina MR, Ta NL, Tao Y, Heyse
SA, Scott DA, Richardson AD, Seyfried TN and Chiles TC:
Glucose-dependent de novo lipogenesis in B lymphocytes: A
requirement for atp-citrate lyase in lipopolysaccharide-induced
differentiation. J Biol Chem. 289:7011–7024. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Caro-Maldonado A, Wang R, Nichols AG,
Kuraoka M, Milasta S, Sun LD, Gavin AL, Abel ED, Kelsoe G, Green
DR, et al: Metabolic reprogramming is required for antibody
production that is suppressed in anergic but exaggerated in
chronically BAFF-exposed B cells. J Immunol. 192:3626–3636. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Le A, Lane AN, Hamaker M, Bose S, Gouw A,
Barbi J, Tsukamoto T, Rojas CJ, Slusher BS, Zhang H, et al:
Glucose-independent glutamine metabolism via TCA cycling for
proliferation and survival in B cells. Cell Metab. 15:110–121.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shaffer AL, Shapiro-Shelef M, Iwakoshi NN,
Lee AH, Qian SB, Zhao H, Yu X, Yang L, Tan BK, Rosenwald A, et al:
XBP1, downstream of Blimp-1, expands the secretory apparatus and
other organelles, and increases protein synthesis in plasma cell
differentiation. Immunity. 21:81–93. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lam WY, Becker AM, Kennerly KM, Wong R,
Curtis JD, Llufrio EM, McCommis KS, Fahrmann J, Pizzato HA, Nunley
RM, et al: Mitochondrial pyruvate import promotes long-term
survival of antibody-secreting plasma cells. Immunity. 45:60–73.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Crawford J and Cohen HJ: The essential
role of L-glutamine in lymphocyte differentiation in vitro. J Cell
Physiol. 124:275–282. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stacpoole PW: The pharmacology of
dichloroacetate. Metabolism. 38:1124–1144. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eleftheriadis T, Sounidaki M, Pissas G,
Antoniadi G, Liakopoulos V and Stefanidis I: In human alloreactive
CD4+ T-cells, dichloroacetate inhibits aerobic glycolysis, induces
apoptosis and favors differentiation towards the regulatory T-cell
subset instead of effector T-cell subsets. Mol Med Rep.
13:3370–3376. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee K, Ban HS, Naik R, Hong YS, Son S, Kim
BK, Xia Y, Song KB, Lee HS and Won M: Identification of malate
dehydrogenase 2 as a target protein of the HIF-1 inhibitor LW6
using chemical probes. Angew Chem Int Ed Engl. 52:10286–10289.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eleftheriadis T, Pissas G, Antoniadi G,
Liakopoulos V and Stefanidis I: Malate dehydrogenase-2 inhibitor
LW6 promotes metabolic adaptations and reduces proliferation and
apoptosis in activated human T-cells. Exp Ther Med. 10:1959–1966.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Friedman B, Goodman EH Jr, Saunders HL,
Kostos V and Weinhouse S: Estimation of pyruvate recycling during
gluconeogenesis in perfused rat liver. Metabolism. 20:2–12. 1971.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rümke HC, Terpstra FG, Huis B, Out TA and
Zeijlemaker WP: Immunoglobulin production in human mixed lymphocyte
cultures: Implications for co-cultures of cells from patients and
healthy donors. J Immunol. 128:696–701. 1982.PubMed/NCBI
|
|
25
|
Konishi E, Kitai Y and Kondo T:
Utilization of complement-dependent cytotoxicity to measure low
levels of antibodies: Application to nonstructural protein 1 in a
model of Japanese encephalitis virus. Clin Vaccine Immunol.
15:88–94. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eleftheriadis T, Pissas G, Sounidaki M,
Antoniadi G, Antoniadis N, Liakopoulos V and Stefanidis I: In human
cell cultures, everolimus is inferior to tacrolimus in inhibiting
cellular alloimmunity, but equally effective as regards humoral
alloimmunity. Int Urol Nephrol. May 15–2017.(Epub ahead of print).
View Article : Google Scholar
|
|
27
|
Sato T, Deiwick A, Raddatz G, Koyama K and
Schlitt HJ: Interactions of allogeneic human mononuclear cells in
the two-way mixed leucocyte culture (MLC): Influence of cell
numbers, subpopulations and cyclosporin. Clin Exp Immunol.
115:301–308. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ostroukhova M, Goplen N, Karim MZ,
Michalec L, Guo L, Liang Q and Alam R: The role of low-level
lactate production in airway inflammation in asthma. Am J Physiol
Lung Cell Mol Physiol. 302:L300–L307. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cai P, Boor PJ, Khan MF, Kaphalia BS,
Ansari GA and Konig R: Immuno- and hepato-toxicity of
dichloroacetic acid in MRL+/+ and
B6C3F1 mice. J Immunotoxicol.
4:107–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bian L, Josefsson E, Jonsson IM, Verdrengh
M, Ohlsson C, Bokarewa M, Tarkowski A and Magnusson M:
Dichloroacetate alleviates development of collagen II-induced
arthritis in female DBA/1 mice. Arthritis Res Ther. 11:R1322009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ohashi T, Akazawa T, Aoki M, Kuze B,
Mizuta K, Ito Y and Inoue N: Dichloroacetate improves immune
dysfunction caused by tumor-secreted lactic acid and increases
antitumor immunoreactivity. Int J Cancer. 133:1107–1118. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lelis FJN, Jaufmann J, Singh A, Fromm K,
Teschner AC, Pöschel S, Schäfer I, Beer-Hammer S, Rieber N and
Hartl D: Myeloid-derived suppressor cells modulate B-cell
responses. Immunol Lett. 188:108–115. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Simioni PU, Fernandes LG and Tamashiro WM:
Downregulation of L-arginine metabolism in dendritic cells induces
tolerance to exogenous antigen. Int J Immunopathol Pharmacol.
30:44–57. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bromage E, Stephens R and Hassoun L: The
third dimension of ELISPOTs: Quantifying antibody secretion from
individual plasma cells. J Immunol Methods. 346:75–79. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Aoyagi Y, Tasaki I, Okumura J and
Muramatsu T: Energy cost of whole-body protein synthesis measured
in vivo in chicks. Comp Biochem Physiol A Comp Physiol.
91:765–768. 1988. View Article : Google Scholar : PubMed/NCBI
|