|
1
|
Yu-Wai-Man P, Griffiths PG, Burke A,
Sellar PW, Clarke MP, Gnanaraj L, Ah-Kine D, Hudson G, Czermin B,
Taylor RW, et al: The prevalence and natural history of dominant
optic atrophy due to OPA1 mutations. Ophthalmology.
117:1538–1546.e1. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lenaers G, Hamel C, Delettre C,
Amati-Bonneau P, Procaccio V, Bonneau D, Reynier P and Milea D:
Dominant optic atrophy. Orphanet J Rare Dis. 7:462012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thiselton DL, Alexander C, Morris A,
Brooks S, Rosenberg T, Eiberg H, Kjer B, Kjer P, Bhattacharya SS
and Votruba M: A frameshift mutation in exon 28 of the OPA1 gene
explains the high prevalence of dominant optic atrophy in the
Danish population: Evidence for a founder effect. Hum Genet.
109:498–502. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferré M, Caignard A, Milea D, Leruez S,
Cassereau J, Chevrollier A, Amati-Bonneau P, Verny C, Bonneau D,
Procaccio V and Reynier P: Improved locus-specific database for
OPA1 mutations allows inclusion of advanced clinical data. Hum
Mutat. 36:20–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reynier P, Amati-Bonneau P, Verny C,
Olichon A, Simard G, Guichet A, Bonnemains C, Malecaze F, Malinge
MC, Pelletier JB, et al: OPA3 gene mutations responsible for
autosomal dominant optic atrophy and cataract. J Med Genet.
41:e110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Metodiev MD, Gerber S, Hubert L, Delahodde
A, Chretien D, Gérard X, Amati-Bonneau P, Giacomotto MC, Boddaert
N, Kaminska A, et al: Mutations in the tricarboxylic acid cycle
enzyme, aconitase 2, cause either isolated or syndromic optic
neuropathy with encephalopathy and cerebellar atrophy. J Med Genet.
51:834–838. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hanein S, Perrault I, Roche O, Gerber S,
Khadom N, Rio M, Boddaert N, Jean-Pierre M, Brahimi N, Serre V, et
al: TMEM126A, encoding a mitochondrial protein, is mutated in
autosomal-recessive nonsyndromic optic atrophy. Am J Hum Genet.
84:493–498. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maresca A, la Morgia C, Caporali L,
Valentino ML and Carelli V: The optic nerve: A ‘mito-window’ on
mitochondrial neurodegeneration. Mol Cell Neurosci. 55:62–76. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Optic atrophy. http://compbio.charite.de/hpoweb/showterm?id=HP:0000648July
18–2016
|
|
10
|
Grenier J, Meunier I, Daien V, Baudoin C,
Halloy F, Bocquet B, Blanchet C, Delettre C, Esmenjaud E, Roubertie
A, et al: WFS1 in optic neuropathies: Mutation findings in
nonsyndromic optic atrophy and assessment of clinical severity.
Ophthalmology. 123:1989–1998. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Charif M, Roubertie A, Salime S, Mamouni
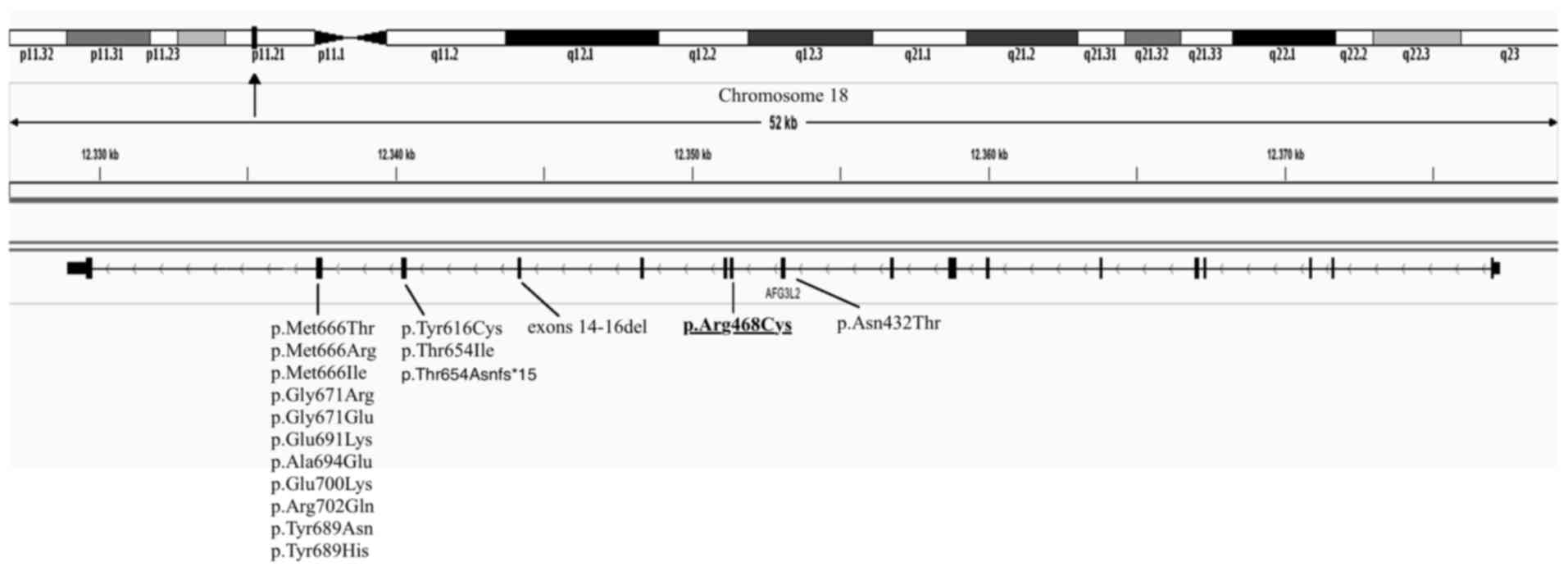
S, Goizet C, Hamel CP and Lenaers G: A novel mutation of AFG3L2
might cause dominant optic atrophy in patients with mild
intellectual disability. Front Genet. 6:3112015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li H and Durbin R: Fast and accurate short
read alignment with Burrows-Wheeler transform. Bioinformatics.
25:1754–1760. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Van der Auwera GA, Carneiro MO, Hartl C,
Poplin R, Del Angel G, Levy-Moonshine A, Jordan T, Shakir K, Roazen
D, Thibault J, et al: From FastQ data to high confidence variant
calls: The Genome Analysis Toolkit best practices pipeline. Curr
Protoc Bioinformatics. 11:11.10.1–11.10.33. 2013.
|
|
14
|
Richards S, Aziz N, Bale S, Bick D, Das S,
Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, et al ACMG
Laboratory Quality Assurance Committee, : Standards and guidelines
for the interpretation of sequence variants: A joint consensus
recommendation of the American College of Medical Genetics and
Genomics and the Association for Molecular Pathology. Genet Med.
17:405–424. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zemojtel T, Köhler S, Mackenroth L, Jäger
M, Hecht J, Krawitz P, Graul-Neumann L, Doelken S, Ehmke N,
Spielmann M, et al: Effective diagnosis of genetic disease by
computational phenotype analysis of the disease-associated genome.
Sci Transl Med. 6:252ra1232014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smedley D and Robinson PN:
Phenotype-driven strategies for exome prioritization of human
Mendelian disease genes. Genome Med. 7:812015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Di Bella D, Lazzaro F, Brusco A, Plumari
M, Battaglia G, Pastore A, Finardi A, Cagnoli C, Tempia F, Frontali
M, et al: Mutations in the mitochondrial protease gene AFG3L2 cause
dominant hereditary ataxia SCA28. Nat Genet. 42:313–321. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zühlke C, Mikat B, Timmann D, Wieczorek D,
Gillessen-Kaesbach G and Bürk K: Spinocerebellar ataxia 28: A novel
AFG3L2 mutation in a German family with young onset, slow
progression and saccadic slowing. Cerebellum Ataxias. 2:192015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maltecca F, Aghaie A, Schroeder DG,
Cassina L, Taylor BA, Phillips SJ, Malaguti M, Previtali S, Guénet
JL, Quattrini A, et al: The mitochondrial protease AFG3L2 is
essential for axonal development. J Neurosci. 28:2827–2836. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cagnoli C, Stevanin G, Brussino A,
Barberis M, Mancini C, Margolis RL, Holmes SE, Nobili M, Forlani S,
Padovan S, et al: Missense mutations in the AFG3L2 proteolytic
domain account for ~1.5% of European autosomal dominant cerebellar
ataxias. Hum Mutat. 31:1117–1124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pierson TM, Adams D, Bonn F, Martinelli P,
Cherukuri PF, Teer JK, Hansen NF and Cruz P: Mullikin For The Nisc
Comparative Sequencing Program JC Blakesley RW et al. Whole-exome
sequencing identifies homozygous AFG3L2 mutations in a spastic
ataxia-neuropathy syndrome linked to mitochondrial m-AAA proteases.
PLoS Genet. 7:e10023252011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Muona M, Berkovic SF, Dibbens LM, Oliver
KL, Maljevic S, Bayly MA, Joensuu T, Canafoglia L, Franceschetti S,
Michelucci R, et al: A recurrent de novo mutation in KCNC1 causes
progressive myoclonus epilepsy. Nat Genet. 47:39–46. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Löbbe AM, Kang JS, Hilker R, Hackstein H,
Müller U and Nolte D: A novel missense mutation in AFG3L2
associated with late onset and slow progression of spinocerebellar
ataxia type 28. J Mol Neurosci. 52:493–496. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Musova Z, Kaiserova M, Kriegova E,
Fillerova R, Vasovcak P, Santava A, Mensikova K, Zumrova A,
Krepelova A, Sedlacek Z and Kanovsky P: A novel frameshift mutation
in the AFG3L2 gene in a patient with spinocerebellar ataxia.
Cerebellum. 13:331–337. 2014. View Article : Google Scholar : PubMed/NCBI
|