Introduction
Parkinson's disease (PD) is regarded as the most
common neurodegenerative movement disorder, affecting ~1% of the
population aged 65 or older worldwide (1). The key pathological characteristic of PD
is the selective loss of dopaminergic neurons in the
substantianigra in the midbrain. The current clinical diagnosis of
PD is based on the presence of parkinsonian motor features, such as
slowness in movement (bradykinesia) and variable expressions of
tremor, rigidity and postural instability. With the aging
population and increasing life expectancy in the world, the number
of people with PD is expected to increase by >50% by 2030
(2). However, to date, dopamine (DA)
replacement therapeutic strategies, such as the DA precursor
levodopa, only improve the symptoms. However, such therapeutic
strategies cannot slow or stop the neurodegenerative process in PD
and its therapeutic effect is reduced after 3–5 years of use, which
then requires an increased dose and may lead to exacerbated side
effects (3). Furthermore, surgical or
medical therapeutic strategies that provide superior
anti-parkinsonian benefits than levodopa have not been fully
investigated. Cell therapy has been reported as a promising
strategy for potential neuromodulation in animal models of PD.
In recent years, animal models have been developed,
with the most promising research demonstrated in cell therapy that
evaluates pathogenesis and treatment effect in PD (4). Neurotoxins or pesticides have been used
in animals to kill DA neurons and produce parkinsonian symptoms
(4). Fetal ventral midbrain cells
(5,6)
or embryonic stem cells (ESCs) (7–9), as well
as mesenchymal stem cells (MSCs) (4,10–12) have been transplanted into these animal
models after in vitro differentiation into neural precursors
or DA neurons, and functional recovery has been observed in these
studies. However, there are various obstacles in developing these
stem cells for treatment: i) Ethical concerns of ESCs; ii) limited
tissue supply; iii) the need for immunosuppressive treatment in
order to prevent graft rejection and graft versus host disease; iv)
graft-induced dyskinesia (13,14); and
v) poor improvements for behavioral deficits (15). To avoid these adverse outcomes, it is
critical to eliminate the unwanted cells from the donor cell
population (16). In addition to
fetal ventral midbrain cells, ESCs and MSCs, induced pluripotent
stem cells (iPSCs) are another type of cell-based therapeutic
strategy. Human adult somatic cells may be reprogrammed to iPSCs in
culture, thus providing allogenic dopaminergic neurons and an
almost unlimited cell supply for replacement therapy (17). Human iPSCs offer the opportunity to
bypass the immune rejection issue associated with allogenic cell
transplants and may also diminish the bioethical questions
surrounding human embryonic stem cell (hESC) therapeutic strategies
(16).
In order to evaluate the efficiency of iPSCs, PD rat
models were lesioned using 6-hydroxydopamine (6-OHDA). Various
previous studies have reported that positive results have been
observed upon immunohistochemical and behavioral testing. To the
best of our knowledge, a systematic review of all these studies in
an objective and quantitative manner has never been conducted.
Therefore, it is necessary to obtain conclusive evidence to
identify whether iPSC treatment is effective in PD models.
Therefore, a systematic review and meta-analysis were performed in
the present study to evaluate the treatment effect of iPSCs in
experimental rat models of PD.
Materials and methods
Search strategy
All studies reporting the use of iPSCs in PD rat
models were identified. The studies were searched based upon the
keywords or Medical Subjective Heading (MeSH) terms of ‘Parkinson
disease, induced pluripotent stem cell(s)’. To ensure a
comprehensive systematic search, four databases, PubMed, EMBASE
(Ovid, 1974 to 2017 March 31), MEDLINE (Ovid, 1946 to Present), and
Cochrane Library (Ovid, Cochrane Central Register of Controlled
Trials March 2017) were searched up to March 2017 for all English
language publications.
Inclusion and exclusion criteria and
data extraction
The inclusion criteria for animal models of PD
studies were as follows: i) Object of study: Parkinson's animal
model (rat or mice); ii) intervention: Test the effects of unsorted
iPSCs on PD in at least one experimental group; iii) comparisons:
Sham-controlled group or condition; and iv) outcome: Adequate data
on behavioral testing to measure response to treatment. Trials were
excluded if any of the following factors were identified: i) Case
report, conference abstract, comment, editorial and review; ii) not
available in English; and iii) duplicate publications.
Two participants extracted data independently using
a predefined data extraction form, with disagreements resolved by
careful discussion. The information from each article was collected
as follows: First author name and publication year, the source of
iPSCs, dose of iPSCs, experimental animal models, including
species, number, sex and weight; route of iPSC administration;
duration of follow-up period; outcome measures and outcome data
from behavioral tests were also included. When reported data for
meta-analysis were insufficient or only expressed graphically, data
were measured using digital ruler software [engauge digitizer,
version 4.1; http://markummitchell.github.io/engauge-digitizer
(18)]. In addition, attempts were
made to contact the study author to inquire about further
information. Data presented as means, standard deviation (SD) or
standard error of the mean (SEM) were extracted. When only SEM was
reported, it was converted to SD for the current meta-analysis.
Quality assessment
The methodological quality of the included studies
was evaluated by modifying a previously published 11-item quality
scale (4,19). This modified scale consisted of the
following six items: i) Random allocation to group; ii)
pretreatment behavioral assessment; iii) blinded assessment of
outcome; iv) assessment of ≥2 outcomes; v) compliance with animal
welfare regulations; vi) statement of a potential conflict of
interest. For the calculation of an aggregate study quality score,
one point was attributed for each checklist item reported. Studies
were classified into three levels as follows: high risk of bias,
0–2 points; unclear risk of bias, 3–4 points; low risk of bias, 5–6
points.
Statistical analysis
Outcomes consisted of a rotation behavior test
induced by amphetamine or apomorphine and limb function tests,
namely cylinder and adjustment stepping tests (4,16). The
outcomes were considered as continuous data. Continuous outcomes
measured on the same scale were expressed as a mean value and SD,
and were analyzed using standard mean differences (SMDs). The
I2 test was performed to assess the impact of
study heterogeneity on the results of the meta-analysis. An
I2 value <25% was considered to below risk of
heterogeneity and a fixed-effect model was used for meta-analysis.
A value between 25% and 50% was regarded as indicative of a
moderate level of heterogeneity and >50% was considered
statistically significant between included studies. For the two, a
random-effect model was used to estimate the combined effect sizes.
Forest plot was generated to depict the SMD along with its 95%
confidence interval (95% CI) for each study, as well as the pooled
mean difference by combining all studies. Visual inspection of the
funnel plot was performed to assess publication bias. Furthermore,
sensitivity analysis was conducted by deleting each study
individually to evaluate the quality and consistency of the
results. All analyses were performed with Review Manager (RevMan;
version 5.3, Copenhagen: The Nordic Cochrane Centre, The Cochrane
Collaboration, 2014) and P<0.05 was considered to indicate a
statistically significant difference.
Results
Study inclusion
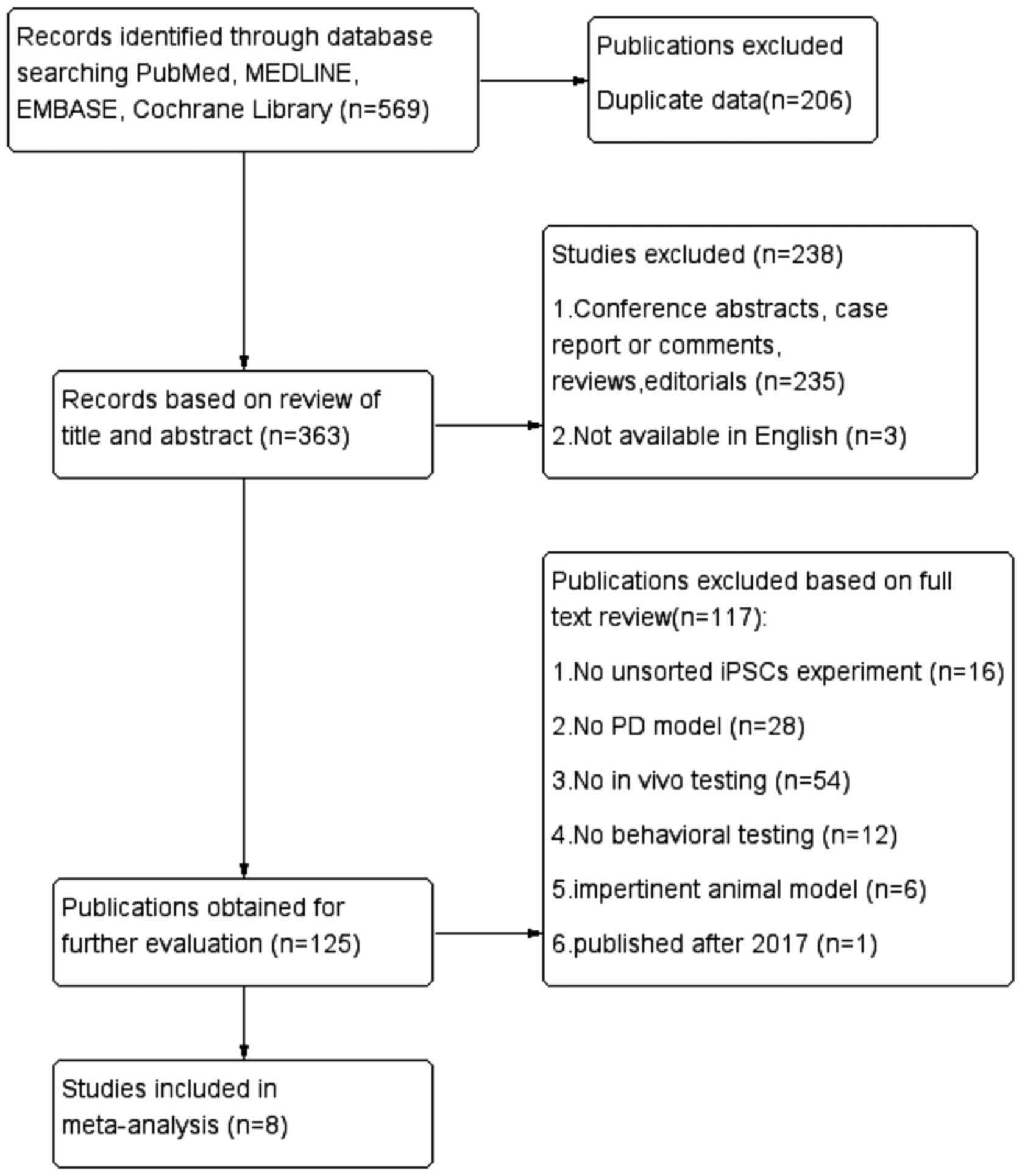
In total, 569 potentially relevant articles were
identified from four databases and 363 records remained after
removal of duplicates. After screening the titles and abstracts,
238 papers were excluded for the following reasons: i) Case
reports, conference abstracts, comments, reviews and editorials;
ii) not available in English. By reading the full text of the
remaining 125 articles carefully, 16 studies were excluded due to
not having unsorted iPSC experiments; 28 studies were excluded
because there was no PD model; 54 were excluded because in
vivo testing had not been performed; 12 studies were removed
because the animal model was impertinent; and one was published
after March 2017. Therefore, eight studies (16,17,20–25)
met the eligibility criteria and were included in the
meta-analyses. The systematic search to identify all articles for
the meta-analysis is presented in Fig.
1.
Study characteristics
A total of eight studies (Table I) described the effects of iPSCs on
rat models of PD. The studies that were published between 2008 and
2016, and five out of eight (62.5%) studies utilized Sprague-Dawley
rats; the other studies did not describe the type of rat used. All
the studies used 6-OHDA models and the delivery routes were
intrastriatal. The duration of the follow-up period ranged from 4
to 24 weeks. When a study used multiple outcomes or tests and used
a separate control group, these separate outcomes or tests were
treated as individual experiments. There were eight experiments
conducted to assess amphetamine-induced rotational behavior, five
experiments conducted to evaluate apomorphine-induced rotational
behavior and only one study evaluated limb function. In addition,
there was one study using the rotation-rod test. Certain studies
did not include full data in this area, therefore the limb function
and rotation-rod test were not included in the meta-analysis.
 | Table I.Basic characteristics of the included
studies. |
Table I.
Basic characteristics of the included
studies.
| First author,
year | iPSC source | iPSC dose | Animal models (sex,
weight) | Experiment
(no.) | Control (no.) | Lesion model | Route of iPSC
administration | Behavioral
analysis | Duration of
follow-up period (weeks) | P-value | Refs. |
|---|
| Wernig, 2008 | Mouse
fibroblasts |
1–3×105 | Rats | 5 | 10 | 6-OHDA | Intrastriatal | Rotational test:
Amphetamine-induced (4 mg/kg; 90 min) | 4 | P=0.0185 | (20) |
| Swistoaski,
2010 |
Fibroblast/mesenchymal stem cell |
| Rats (F) | 7 | 7 | 6-OHDA | Intrastriatal | Rotational test:
Amphetamine-induced (2.5 mg/kg; 90 min) | 12 | P<0.05 | (17) |
| Deleidi, 2011 | Primary skin
cells/dermal (CM) fibroblasts |
4×105 | SD rats (F, 200–250
g) | 11/9 | 6/4 | 6-OHDA | Intrastriatal | Rotational test:
Amphetamine-induced (4 mg/kg; 90 min); apomorphine-induced (0.1
mg/kg; 40 min) | 16/24 | P<0.05 | (21) |
|
|
|
|
|
|
|
|
|
|
| P<0.01 |
|
| Rhee, 2011 | Newborn fibroblast
(human) |
7.5×105/3×105 | Rats | 9/6 | 6 | 6-OHDA | Intrastriatal | Rotational test:
Amphetamine induced | 8 | P<0.01 | (22) |
| Sundberg, 2013 | Skin fibroblasts
(human, CM) |
1–2×105 | SD rats (F, 200–250
g) | 5 | 4 | 6-OHDA | Intrastriatal | Rotational test:
Amphetamine-induced (4 mg/kg; 90 min); apomorphine-induced (0.1
mg/kg; 40 min). Cylinder test. Adjustment stepping test | 16 | P<0.05 | (16) |
|
|
|
|
|
|
|
|
|
|
| P<0.05 |
|
| Doi, 2014 | MEF |
4×105 | SD rats (F, 200–250
g) | 11 | 6 | 6-OHDA | Intrastriatal | Rotational test:
Methamphetamine-induced (2.5 mg/kg; 90 min) | 16 | P=0.0017 | (23) |
| Han, 2015 | Skin
fibroblasts |
5×105 | SD rats (M, 200–250
g) | 10 | 9 | 6-OHDA | Intrastriatal | Rotational test:
Apomorphine-induced (30 min). Rotation-rod test | 16 | P=0.041 | (24) |
| Samata, 2016 | Embryo fibroblast
(mouse) |
2.6×105 | SD rats (F) | 7/7 | 8/7 | 6-OHDA | Intrastriatal | Rotational test:
Methamphetamine-induced (2.5 mg/kg; 90 min); apomorphine-induced
(0.1 mg/kg; 60 min) | 16 | P<0.05 | (25) |
Quality assessment
The quality assessment of each study is described in
Table II. The study quality
checklist items that were scored ranged from 2–5 out of a total of
6 points. Two studies got 2 points (25%); two studies got 3 points
(25%); and three studies got 4 points (37.5%); and one study got 5
points (12.5%). Only one study utilized random allocation of
groups. Pretreatment behavioral assessments and blinded assessments
of outcomes were described in four and three studies, respectively.
Four studies assessed ≥2 outcomes. All studies reported compliance
with animal welfare regulations and only one study did not mention
the statement of potential conflicts of interest.
 | Table II.Risk of bias of included studies. |
Table II.
Risk of bias of included studies.
| Author, year | A | B | C | D | E | F | Total | Refs. |
|---|
| Wernig, 2008 |
|
| 1 |
| 1 | 1 | 3 | (20) |
| Swistoaski,
2010 |
|
| 1 |
| 1 | 1 | 3 | (17) |
| Deleidi, 2011 |
| 1 |
| 1 | 1 | 1 | 4 | (21) |
| Rhee, 2011 |
|
|
|
| 1 | 1 | 2 | (22) |
| Sundberg, 2013 | 1 | 1 |
| 1 | 1 | 1 | 5 | (16) |
| Doi, 2014 |
| 1 |
|
|
| 1 | 2 | (23) |
| Han, 2015 |
| 1 |
| 1 | 1 | 1 | 4 | (24) |
| Samata, 2016 |
|
| 1 | 1 | 1 | 1 | 4 | (25) |
Efficacy of iPSCs
All the data for meta-analysis were expressed by
diagram, and engauge digitizer 4.1 was used to calculate the means
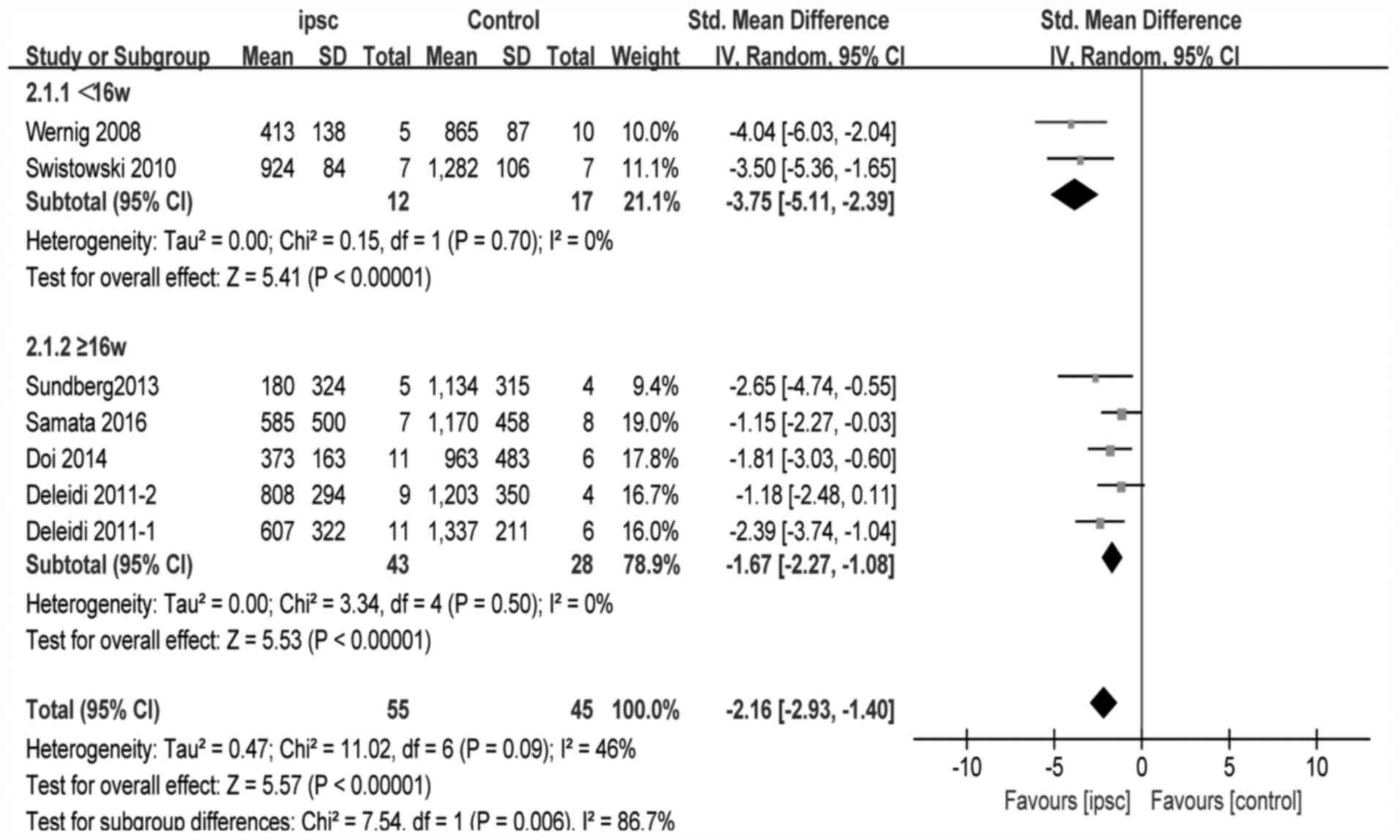
and standard error. For amphetamine-induced rotational tests, data
from six studies (16,17,20,21,23,25)
were reported. One study (21) used
two different iPSC lines, and had separate control group and
outcomes. One study (22) reported
ratios as outcomes, therefore the required data could not be
extracted. The pooled effect size of iPSC therapy was estimated
based upon the random-effects model. These experiments reported
substantial and significant effects of iPSCs for improving the
amphetamine-induced rotational behavior compared with the control
group (SMD, −2.16; 95% CI −2.93 to −1.40; P<0.00001;
heterogeneity: χ2=11.02, P=0.09,
I2=46%; Fig. 2).
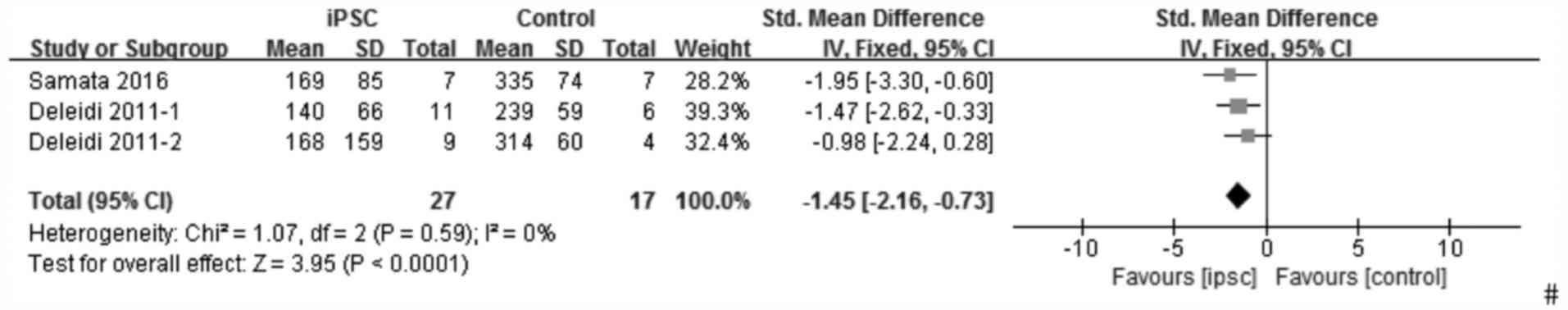
For apomorphine-induced rotation tests, similarly, one study
(24) was excluded from the pooled
analysis due to the data being represented in the form of a ratio
and one study (16) did not describe
the outcome in detail; therefore, it was not possible to calculate
the mean and SD. Thus only three experiments (21,25) were
reported for evaluating the effect of iPSCs on apomorphine-induced
rotation tests. The outcome used the fixed-effects model and
indicated that iPSCs also improved the apomorphine-induced rotation
tests (SMD, −1.45; 95% CI, −2.16 to −0.73; P<0.00001;
heterogeneity: χ2=1.07, P=0.59, I2=0%;
Fig. 3). The forest plot, stratified
by different durations of follow-up period, for the
amphetamine-induced rotational test is presented in Fig. 4. The pooled effect size in studies
following ≥16 weeks and <16 weeks with 95% CI was −1.67 (−2.27,
−1.08) with P<0.00001 and −3.75 (−5.11, −2.39) with
P<0.00001, respectively. The heterogeneity of each was 0%, but
was 46% when viewed as a whole.
Sensitivity analysis
The pooled effect size of each study was evaluated
for rotation behavior by excluding individual's studies
sequentially. The results demonstrated that the stability of
results significantly changed when the study by Wernig et al
(20) was removed from the
meta-analysis; the pooled effect size of iPSC therapy with 95%CI
and I2 changed to −1.90 (−2.57,-1.23) and 26%,
respectively (data not shown), which are narrower and lower than
the original.
Publication bias
The funnel plot of the studies included in the
present meta-analysis for the outcome of amphetamine-induced
rotation test is presented in Fig. 5.
No evident publication bias was observed through the visual
distribution of the funnel plot. The use of a funnel plot was
limited for the outcomes of the apomorphine-induced rotation test
due to the small number of studies that was evaluated.
Discussion
In the current study, the efficacy of iPSCs on
behavior tests was analyzed in animal models of PD using a
meta-analysis to obtain a powerful conclusion. The results
demonstrated that iPSCs exert a significant treatment effect on
rotation behavior. Behavior tests were regarded as outcomes of this
analysis as it is a common parameter to measure functional
impairments and recovery in animal models (26) and rotational behavior has also been
frequently examined as the measure of functional condition in
hemiparkinsonian rodent models (27).
In addition, certain studies indicate that limb function is a good
indicator of nigrostriatal DA consumption (28). To the best of our knowledge, this is
the first meta-analysis providing comprehensive insights into the
effect size of unsorted iPSCs in animal models of PD.
In addition to common clinical treatments, such as
pharmaceutical drugs and deep brain stimulation, cell replacement
therapies have offered a solid foundation for developing an
effective therapeutic strategy for PD. The epoch of cell therapy
for PD began when Brundin et al (29) transplanted human ventral midbrain
tissue into the striatum of PD patients in 1987. Various source
tissues have been examined for therapeutic replacement of DA
neurons subsequently, such as hESCs, MSCs and DA grafts directly
converted from somatic cells. Although numerous studies have
demonstrated that those tissues certainly have a significant effect
on PD, unfortunately, there were various issues with the studies,
such as ethical, technical and practical limitations. Thus, a
standardized and limitless cell source for PD is required. Many
studies have turned to other appropriate sources, such as iPSCs,
which have the potential capacity for self-renewal and are able to
differentiate into any somatic cells, including DA neurons
(30); therefore, they are ethically
more acceptable than certain other stem cell sources. Compared with
other stem cells, iPSCs are notable for their powerful
pluripotency, infrequent immune rejection and few ethical issues.
Furthermore, iPSC-derived neurons capture whole disease genomes
without age- and damage-associated epigenetic alterations, which
maybe used to model the earliest stages of disease pathogenesis
(31). However, there are certain
limitations that must be resolved before transplantation of iPSCs,
including safety issues associated with tumorigenicity and
identifying the mechanisms that enable iPSCs to restore brain
function (32).
The results of the present analysis indicate that
iPSCs may be a promising candidate for preclinical and clinical
trials. All of the included studies used 6-OHDA to induce
parkinsonian symptoms rather than other mechanisms, such as
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and rotenone, as these
promote dopaminergic neuron death with associated motor impairment.
Their side effects and lack of specificity are major drawbacks
(33). Lesions in the nigrostriatal
DA neurons of rats are generated with 6-OHDA, which must be
injected into the medial forebrain bundle, substantianigra pars
compacta or striatum, rather than systemic administration, to
produce the PD model, as it cannot cross the blood-brain barrier
(34). The pathological and
behavioral phenotypes of this model may differ from the human
condition due to differences between species, therefore it is
difficult to extrapolate the results obtained from animal models to
humans (35). However, the results
contribute to understanding the underlying genetic forms of PD and
facilitate with establishing disease modifiers and novel targets
for possible therapeutic intervention (36).
The evidence that iPSCs may be an effective
treatment for PD is encouraging. However, there were certain
limitations in the current meta-analysis. First, potential
publication bias is likely to exist, despite no evidence of this in
the statistical tests, as negative or neutral studies are less
likely to be published compared with positive studies. Furthermore,
certain outcomes were reported in the form of ratios, therefore
could not be included in the current meta-analysis. In addition,
behavior tests in animal models of PD cannot fully represent all
the components of neurological impairments of PD in humans. The
neurological symptoms of PD models consist of motor tests, as well
as non-motor deficits. Additionally, there were different sources
for each experiment, such as mouse fibroblasts and cynomolgus
macaque dermal fibroblasts, and the duration of the follow-up
period for rotational tests ranged from 4 to 24 weeks, which may
influence the total effect size. Finally, almost all of the
included studies had a small sample size (the average number of
animals per iPSC and control group was eight and six,
respectively). Trials with inadequate sample sizes often run the
risk of overestimating intervention effects (37), thus, the current results require
careful interpretation.
In conclusion, iPSCs may improve behavioral outcomes
in animal models of PD. The current analysis demonstrated that
iPSCs provide a potential approach for developing novel treatment
strategies for PD, and designing future preclinical and clinical
studies.
Acknowledgements
The authors thank the staff of the Department of
Geriatrics Medicine, West China Hospital (Sichuan University,
Chengdu, China) for their guidance and support. We also thank Dr
Joseph Flaherty, a visiting professor in geriatrics, from Saint
Louis University (Saint Louis, MO, USA), for helping with language
editing.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the study are
included in this published article.
Authors' contributions
YZ was responsible for formulating the research
question, designing the study, collecting the data, screening the
papers and quality assessment, and producing the initial draft of
the manuscript. MG was responsible for designing the study,
screening the studies, collecting the data and statistical
analysis. QH was responsible for quality assessment and statistical
analysis. BD was responsible for revision of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kalia LV and Lang AE: Parkinson's disease.
Lancet. 386:896–912. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dorsey ER, Constantinescu R, Thompson JP,
Biglan KM, Holloway RG, Kieburtz K, Marshall FJ, Ravina BM,
Schifitto G, Siderowf A, et al: Projected number of people with
Parkinson disease in the most populous nations, 2005 through 2030.
Neurology. 68:384–386. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lane EL, Handley OJ, Rosser AE and Dunnett
SB: Potential cellular and regenerative approaches for the
treatment of Parkinson's disease. Neuropsychiatr Dis Treat.
4:835–845. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Riecke J, Johns KM, Cai C, Vahidy FS,
Parsha K, Furr-Stimming E, Schiess M and Savitz SI: A Meta-Analysis
of Mesenchymal Stem Cells in Animal Models of Parkinson's Disease.
Stem Cells Dev. 24:2082–2090. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rath A, Klein A, Papazoglou A, Pruszak J,
Garcia J, Krause M, Maciaczyk J, Dunnett SB and Nikkhah G: Survival
and functional restoration of human fetal ventral mesencephalon
following transplantation in a rat model of Parkinson's disease.
Cell Transplant. 22:1281–1293. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Freed CR, Greene PE, Breeze RE, Tsai WY,
DuMouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ,
et al: Transplantation of embryonic dopamine neurons for severe
Parkinson's disease. N Engl J Med. 344:710–719. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brederlau A, Correia AS, Anisimov SV, Elmi
M, Paul G, Roybon L, Morizane A, Bergquist F, Riebe I, Nannmark U,
et al: Transplantation of human embryonic stem cell-derived cells
to a rat model of Parkinson's disease: Effect of in vitro
differentiation on graft survival and teratoma formation. Stem
Cells. 24:1433–1440. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ambasudhan R, Dolatabadi N, Nutter A,
Masliah E, Mckercher SR and Lipton SA: Potential for cell therapy
in Parkinson's disease using genetically programmed human embryonic
stem cell-derived neural progenitor cells. J Comp Neurol.
522:2845–2856. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grealish S, Diguet E, Kirkeby A, Mattsson
B, Heuer A, Bramoulle Y, Van Camp N, Perrier AL, Hantraye P,
Björklund A, et al: Human ESC-derived dopamine neurons show similar
preclinical efficacy and potency to fetal neurons when grafted in a
rat model of Parkinson's disease. Cell Stem Cell. 15:653–665. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Y, Chen J, Wang L, Zhang L, Lu M and
Chopp M: Intracerebral transplantation of bone marrow stromal cells
in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of
Parkinson's disease. Neurosci Lett. 316:67–70. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Camp DM, Loeffler DA, Farrah DM, Borneman
JN and LeWitt PA: Cellular immune response to intrastriatally
implanted allogeneic bone marrow stromal cells in a rat model of
Parkinson's disease. J Neuroinflammation. 6:172009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Inden M, Takata K, Nishimura K, Kitamura
Y, Ashihara E, Yoshimoto K, Ariga H, Honmou O and Shimohama S:
Therapeutic effects of human mesenchymal and hematopoietic stem
cells on rotenone-treated parkinsonian mice. J Neurosci Res.
91:62–72. 2013.PubMed/NCBI
|
|
13
|
Carlsson T, Carta M, Winkler C, Björklund
A and Kirik D: Serotonin neuron transplants exacerbate
L-DOPA-induced dyskinesias in a rat model of Parkinson's disease. J
Neurosci. 27:8011–8022. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Politis M, Wu K, Loane C, Quinn NP, Brooks
DJ, Rehncrona S, Bjorklund A, Lindvall O and Piccini P:
Serotonergic neurons mediate dyskinesia side effects in Parkinson's
patients with neural transplants. Sci Transl Med. 2:38ra462010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Olanow CW, Goetz CG, Kordower JH, Stoessl
AJ, Sossi V, Brin MF, Shannon KM, Nauert GM, Perl DP, Godbold J, et
al: A double-blind controlled trial of bilateral fetal nigral
transplantation in Parkinson's disease. Ann Neurol. 54:403–414.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sundberg M, Bogetofte H, Lawson T, Jansson
J, Smith G, Astradsson A, Moore M, Osborn T, Cooper O, Spealman R,
et al: Improved cell therapy protocols for Parkinson's disease
based on differentiation efficiency and safety of hESC-, hiPSC-,
and non-human primate iPSC-derived dopaminergic neurons. Stem
Cells. 31:1548–1562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Swistowski A, Peng J, Liu Q, Mali P, Rao
MS, Cheng L and Zeng X: Efficient generation of functional
dopaminergic neurons from human induced pluripotent stem cells
under defined conditions. Stem Cells. 28:1893–1904. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mitchell M, Muftakhidinov B, Winchen T, et
al: Engauge Digitizer Software. http://markummitchell.github.io/engauge-digitizerMay
11–2017
|
|
19
|
Schmidt A, Wellmann J, Schilling M,
Strecker JK, Sommer C, Schäbitz WR, Diederich K and Minnerup J:
Meta-analysis of the efficacy of different training strategies in
animal models of ischemic stroke. Stroke. 45:239–247. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wernig M, Zhao JP, Pruszak J, Hedlund E,
Fu D, Soldner F, Broccoli V, Constantine-Paton M, Isacson O and
Jaenisch R: Neurons derived from reprogrammed fibroblasts
functionally integrate into the fetal brain and improve symptoms of
rats with Parkinson's disease. Proc Natl Acad Sci USA. 105:pp.
5856–5861. 2008; View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deleidi M, Hargus G, Hallett P, Osborn T
and Isacson O: Development of histocompatible primate-induced
pluripotent stem cells for neural transplantation. Stem Cells.
29:1052–1063. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rhee YH, Ko JY, Chang MY, Yi SH, Kim D,
Kim CH, Shim JW, Jo AY, Kim BW, Lee H, et al: Protein-based human
iPS cells efficiently generate functional dopamine neurons and can
treat a rat model of Parkinson disease. J Clin Invest.
121:2326–2335. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Doi D, Samata B, Katsukawa M, Kikuchi T,
Morizane A, Ono Y, Sekiguchi K, Nakagawa M, Parmar M and Takahashi
J: Isolation of human induced pluripotent stem cell-derived
dopaminergic progenitors by cell sorting for successful
transplantation. Stem Cell Reports. 2:337–350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Han F, Wang W, Chen B, Chen C, Li S, Lu X,
Duan J, Zhang Y, Zhang YA, Guo W, et al: Human induced pluripotent
stem cell-derived neurons improve motor asymmetry in a
6-hydroxydopamine-induced rat model of Parkinson's disease.
Cytotherapy. 17:665–679. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Samata B, Doi D, Nishimura K, Kikuchi T,
Watanabe A, Sakamoto Y, Kakuta J, Ono Y and Takahashi J:
Purification of functional human ES and iPSC-derived midbrain
dopaminergic progenitors using LRTM1. Nat Commun. 7:130972016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kirik D, Rosenblad C and Björklund A:
Characterization of behavioral and neurodegenerative changes
following partial lesions of the nigrostriatal dopamine system
induced by intrastriatal 6-hydroxydopamine in the rat. Exp Neurol.
152:259–277. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cadet JL, Zhu SM and Shu Ming Zhu: The
intrastriatal 6-hydroxydopamine model of hemiparkinsonism:
Quantitative receptor autoradiographic evidence of correlation
between circling behavior and presynaptic as well as postsynaptic
nigrostriatal markers in the rat. Brain Res. 595:316–326. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Emborg ME: Evaluation of animal models of
Parkinson's disease for neuroprotective strategies. J Neurosci
Methods. 139:121–143. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brundin P, Strecker RE, Lindvall O,
Isacson O, Nilsson OG, Barbin G, Prochiantz A, Forni C, Nieoullon
A, Widner H, et al: Intracerebral grafting of dopamine neurons.
Experimental basis for clinical trials in patients with Parkinson's
disease. Ann N Y Acad Sci. 495:473–496. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brundin P, Barker RA and Parmar M: Neural
grafting in Parkinson's disease Problems and possibilities. Prog
Brain Res. 184:265–294. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jacobs BM: Stemming the hype: What can we
learn from iPSC models of Parkinson's disease and how can we learn
it? J Parkinsons Dis. 4:15–27. 2014.PubMed/NCBI
|
|
32
|
Xu X, Huang J, Li J, Liu L, Han C, Shen Y,
Zhang G, Jiang H, Lin Z, Xiong N, et al: Induced pluripotent stem
cells and Parkinson's disease: Modelling and treatment. Cell
Prolif. 49:14–26. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Valadas JS, Vos M and Verstreken P:
Therapeutic strategies in Parkinson's disease: What we have learned
from animal models. Ann N Y Acad Sci. 1338:16–37. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Blandini F, Armentero MT and Martignoni E:
The 6-hydroxydopamine model: News from the past. Parkinsonism Relat
Disord. 14 Suppl 2:S124–S129. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Seibler P, Graziotto J, Jeong H, Simunovic
F, Klein C and Krainc D: Mitochondrial Parkin recruitment is
impaired in neurons derived from mutant PINK1 induced pluripotent
stem cells. J Neurosci. 31:5970–5976. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li W, Chen S and Li JY: Human induced
pluripotent stem cells in Parkinson's disease: A novel cell source
of cell therapy and disease modeling. Prog Neurobiol. 134:161–177.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kjaergard LL, Villumsen J and Gluud C:
Reported methodologic quality and discrepancies between large and
small randomized trials in meta-analyses. Ann Intern Med.
135:982–989. 2001. View Article : Google Scholar : PubMed/NCBI
|