Introduction
Dental pulp cells (DPCs), the resident mesenchymal
stromal cells in dental pulp tissue, may be isolated as migrating
cells from pulp tissue explants or as colony-forming cells in
low-density cultures (1,2). These cells are able to differentiate
into dentin, bone, periodontal ligament, fat, vascular cells and
nerve-like cells in vitro and in vivo (1,3–15). DPCs and bone marrow-derived
mesenchymal stem cells (BM-MSCs) have similar differentiation
potentials, though the growth activity of DPCs may be greater than
that of BM-MSCs (1,13,16).
DPCs, as well as BM-MSCs, are promising in
cell-based therapy for various diseases including ischemia
(6) and spinal cord injury (15). Fetal bovine serum (FBS) has been used
for ex vivo expansion of DPCs; however, this carries a risk
of contamination with prions or viruses. Furthermore, the
proliferation activity and differentiation potential of DPCs
depends upon the batch of serum (17). Therefore, serum-free, chemically
defined media should be used for the expansion of DPCs destined for
clinical application (17).
A range of serum-free media have been developed for
culturing adult and embryonic stem cells (17). In the present study, a culture of DPCs
under serum-free conditions was attempted using STK2, a serum-free
medium for BM-MSCs. Previous studies have demonstrated that STK2 is
suitable for ex vivo expansion of BM-MSCs. For instance,
when compared with Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% FBS, STK2 further increased the proliferation
of BM-MSCs (18), but did not promote
the growth of immortalized human gingival fibroblasts or cancer
cell lines (19). In addition, neural
crest and endometrial carcinoma cells grown in STK2 exhibited
mesenchymal-like features (20,21).
Therefore, the present study examined whether STK2 could support
the proliferation of human DPCs. In addition, the differentiation
ability of DPCs grown in STK2 was assessed.
Materials and methods
Culture media
STK2 was purchased from DS Pharma Biomedical (Osaka
Japan). DMEM (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
supplemented with 10% FBS (HyClone; GE Healthcare Life Sciences,
Logan, UT, USA) and 1X antibiotic-antimycotic containing 100 U/ml
penicillin, 100 g/ml streptomycin and 0.25 g/ml amphotericin B
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) was
used as control medium. α-minimal essential medium (α-MEM)
supplemented with 10% FBS, 100 nM dexamethasone (Sigma-Aldrich;
Merck KGaA), 50 mg/l ascorbic acid 2-phosphate (Sigma-Aldrich;
Merck KGaA) and 10 mM β-glycerophosphate (Tokyo Chemical Industry,
Co., Ltd., Tokyo, Japan) was used for induction of
osteogenesis.
Cells
Healthy upper third molars were obtained from 4
healthy female donors (aged 23–27 years) at Hiroshima University
Hospital (Hiroshima, Japan) from April 2008 to March 2009 with
informed consent following a protocol approved by the Ethics
Committee at Hiroshima University (approval no. D88-2).
Fibroblast-like cells were grown out from tooth pulp tissue
explants individually derived from the donors and were used as DPC
lines (DPCs-2, DPCs-3, DPCs-4 and DPCs-5), as previously described
(22). BM-MSCs (lot no. OF3853) from
a 20-year-old female donor were obtained from Lonza Group, Ltd.
(Basel, Switzerland).
Cell growth
The DPCs grown out from tissue explants were
harvested with 0.2% trypsin and 0.02% EDTA in phosphate-buffered
saline (PBS). The cells were seeded onto 10 cm plastic tissue
culture dishes at a 1:5 split ratio and incubated with 10 ml DMEM
supplemented with 10% FBS (DMEM/10% FBS) at 37°C in 95% air and 5%
CO2. For experimentation, cells obtained from 3rd-6th
passage cultures were seeded at 5×103
cells/cm2 into each well of a 12-well plate (Corning
Incorporated, Corning, NY, USA) with 1.0 ml STK2 or DMEM/10% FBS.
The cultures were fed with the respective media every 2–3 days.
When cultures became 80–90% confluent, the cells were incubated
with Accutase (Innovative Cell Technologies, Inc., San Diego, CA,
USA) for 3 min at 37°C, and the number of dispersed cells was
counted. These cells were then sub-cultured at the same initial
density with the respective media, and cell counting was repeated
as above until the experiment endpoint (37 days).
For a Cell Counting Kit-8 (CCK-8) assay, DPCs
obtained from 4th-6th passage cultures were seeded on 96-well
plates (Corning Incorporated) at 5×103, 1×104
or 2×104 cells/cm2, and incubated in 0.1 ml
STK2 or DMEM/10% FBS at 37°C in 95% air and 5% CO2. The
human BM-MSCs were also used as a positive control. At 7 days after
cell seeding, 0.01 ml of a WST-8 solution (CCK-8; Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) was added to the culture
medium, and the cultures were incubated for 1 h at 37°C in 95% air
and 5% CO2. The absorbance of the medium at 450 nm was
measured with a microplate reader (VersaMax; Molecular Devices,
LLC, Sunnyvale, CA, USA).
Alizarin Red S staining
DPCs were seeded on 24-well plates at
5×103 cells/cm2 and incubated for 7 days in
0.5 ml STK2 or DMEM/10% FBS at 37°C in 95% and 5% CO2.
When the cultures became 100% confluent, cells were incubated with
osteogenesis induction medium for 14, 21 and 28 days. The cell
layers were washed twice with PBS, and fixed with 97% ethanol for
10 min at room temperature. They were then washed twice with PBS,
and incubated for 30 min with 1% Alizarin Red S solution
(Sigma-Aldrich; Merck KGaA) at room temperature. Finally, the cell
layers were washed with water, and the red staining indicating
calcium deposition was observed by naked eye.
Alkaline phosphatase (ALP)
activity
DPCs were seeded on 48-well plates at
5×103 cells/cm2 and incubated in 0.3 ml STK2
or DMEM/10% FBS at 37°C in 95% air and 5% CO2. When the
cultures became confluent, cells were incubated with osteogenesis
induction medium for 0, 4, 8, 12 and 16 days. The cell layers were
washed twice with saline, incubated for 15 min with 0.25 ml 1%
Nonidet P-40 (NP-40) solution (Sigma-Aldrich; Merck KGaA), and then
homogenized with the same solution for 1 min on ice. Using the cell
extract and LabAssay ALP (Wako Pure Chemical Industries, Ltd.,
Osaka, Japan), ALP activity was determined according to the
manufacturer's instructions. In this assay, p-nitrophenylphosphate
is hydrolyzed into p-nitrophenol and phosphoric acid by ALP. The
released p-nitrophenol exhibiting yellow color was optically
measured at 405 nm as the enzyme activity. The quantity of DNA in
the cell extract was determined using a Quant-iT PicoGreen dsDNA
Assay kit (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. Fluorescence was measured at an
excitation of 480 nm and emission of 520 nm. Enzyme activity was
expressed as nmol/min/µg DNA.
DNA microarray
DPCs-2, −3 and −4 from 5th passage cultures were
seeded on 10 cm plastic tissue culture dishes at 5×103
cells/cm2 and incubated in STK2 or DMEM/10% FBS at 37°C
in 95% air and 5% CO2. Total RNA was isolated with
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,), an
RNeasy Mini kit (Qiagen, Inc., Valencia, CA, USA) and a TURBO
DNA-Free kit (Applied Biosystems; Thermo Fisher Scientific, Inc.)
24 h after the cultures became confluent. cDNA was synthesized
using a GeneAmp RNA PCR kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.). Labeled cRNA was synthesized using a Quick Amp
Labeling kit (Agilent Technologies, Inc., Santa Clara, CA, USA),
and hybridization was performed on a DNA microarray (SurePrint G3
Human, 8×60k; Agilent Technologies, Inc.), according to the
manufacturer's protocols. Finally, the mRNA expression levels were
analyzed using Agilent GeneSpring GX version 12 software (Agilent
Technologies, Inc.). A correlation coefficient was calculated in
GeneSpring to compare the gene expression profiles in DPCs cultured
in DMEM/10% FBS with those in DPCs cultured in STK2. The resulting
raw data have been deposited in the Gene Expression Omnibus
database (GSE97199; http://www.ncbi.nlm.nih.gov/geo/) (23).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated as described above and cDNA
was synthesized using the GeneAmp RNA PCR kit as described
previously (24). Quantitative
real-time PCR analysis was performed using an ABI Prism 7900HT
Sequence Detection System with the included software (SDS version
2.4; Applied Biosystems; Thermo Fisher Scientific, Inc.,), based on
the ΔΔCq method (25). The cDNA was
amplified using a Universal PCR Master Mix (Applied Biosystems;
Thermo Fisher Scientific, Inc.) with appropriate forward and
reverse primers (Table I) with a
primer kit for eukaryotic 18S rRNA (Applied Biosystems; Thermo
Fisher Scientific, Inc.) used as an endogenous control. The PCR
cycling conditions comprised of incubation at 50°C for 2 min,
denaturation at 95°C for 10 min, followed by 40 cycles of 95°C for
15 sec and 60°C for 1 min. TaqMan probes (Table I) were purchased from Roche
Diagnostics (Basel, Switzerland). Data were normalized against 18S
ribosomal RNA levels.
 | Table I.Primer and probe sequences used for
reverse transcription-quantitative polymerase chain reaction. |
Table I.
Primer and probe sequences used for
reverse transcription-quantitative polymerase chain reaction.
| Gene | Primer (5′-3′) | Roche universal
probe no. |
|---|
| TXNIP | F:
CTTCTGGAAGACCAGCCAAC | 85 |
|
| R:
GAAGCTCAAAGCCGAACTTG |
|
| PGA3 | F:
CCCGTCTTTGACAACATCTG | 81 |
|
| R:
ATCACCACGCTGCCACTC |
|
| TNNT1 | F:
GACTACATGGGGGAGGAACA | 17 |
|
| R:
GCCATCAGGTCGAACTTCTC |
|
| SCRG1 | F:
TGTTACTGCAACTTCAGCGAAT | 44 |
|
| R:
TTGCAAGGAATCACGAAAGA |
|
| INHBE | F:
CAGGGAGTGTGGCTCCAG | 52 |
|
| R:
TGTAGGCTGAAGTGGAGTCTGT |
|
| GLI1 | F:
CAGGGAGGAAAGCAGACTGA | 76 |
|
| R:
ACTGCTGCAGGATGACTGG |
|
Statistical analysis
Experiments were performed at least in triplicate.
Results are expressed as the mean ± standard deviation. Differences
between groups were analyzed by two-way analysis of variance
(ANOVA) with Sidak's post hoc test for multiple comparisons. In all
analyses, P<0.05 indicated statistically significant differences
between values.
Results
STK2 medium enhances the proliferation
of DPCs
The effects of STK2 on the proliferation of four DPC
lines (DPCs-2, DPCs-3, DPCs-4 and DPCs-5) were examined. These
cells were seeded at low, medium or high density (5×103,
1×104 or 2×104 cells/cm2,
respectively) and incubated in STK2 or DMEM/10% FBS. Cell numbers
were determined by CCK-8 assay on day 7. The majority of the cell
lines exhibited higher growth rates in STK2 than in DMEM/10% FBS
under conditions of low and medium initial cell densities (Fig. 1A-D). However, at high initial cell
density, STK2 did not enhance the growth rates of the DPCs-3 and
DPCs-4 cell lines compared with DMEM/10% FBS (Fig. 1B and C). By contrast, STK2 enhanced
the proliferation of human BM-MSCs at the low, medium and high
initial cell densities, compared with DMEM/10% FBS (P<0.0001;
Fig. 1E). In addition, at the low
initial density, DPCs-5 cells obtained from 3rd-6th passage
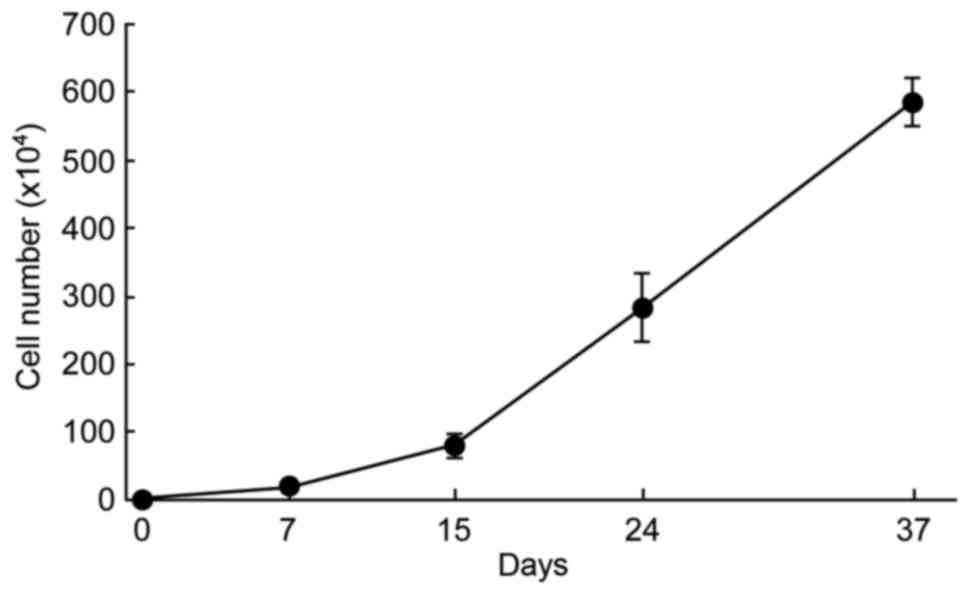
continued to proliferate actively in STK2 through successive
passages (Fig. 2).
Comparison of gene expression levels
in cells cultured in DMEM/10% FBS or STK2
The gene expression profiles of DPCs (DPCs-2, −3 and
−4) that had been grown in STK2 or DMEM/10% FBS were compared.
Overall, the gene expression profile was only marginally altered by
the medium (correlation coefficient between the two media = 0.97 to
0.99; data not shown). A total of 155, 38 and 8 genes were
upregulated by more than 2-, 5- and 10-fold, respectively, in DPCs
grown in STK2, while 254, 106 and 37 genes were downregulated by
more than 2-, 5- and 10-fold, respectively. Tables II and III list the top 20 genes upregulated and
downregulated by STK2, respectively. The upregulated genes included
those involved in proliferation [including thioredoxin interacting
protein (TXNIP), platelet factor 4 variant 1, placental
growth factor and inhibin-βE (INHBE)], metabolism [including
pepsinogen 3 (PGA3) and alcohol dehydrogenase 1A] and
differentiation [including troponin T type 1 (TNNT1),
stimulator of chondrogenesis 1 (SCRG1),
hairy/enhancer-of-split related with YRPW motif 1 and GLI family
zinc finger 1 (GLI1)]. Indeed, the mRNA levels of TXNIP,
PGA3, TNNT1, SCRG1, INHBE and GLI1 were increased in
DPCs grown in STK2, compared with their levels in DMEM/10% FBS
cultures, as confirmed by RT-qPCR (Fig.
3).
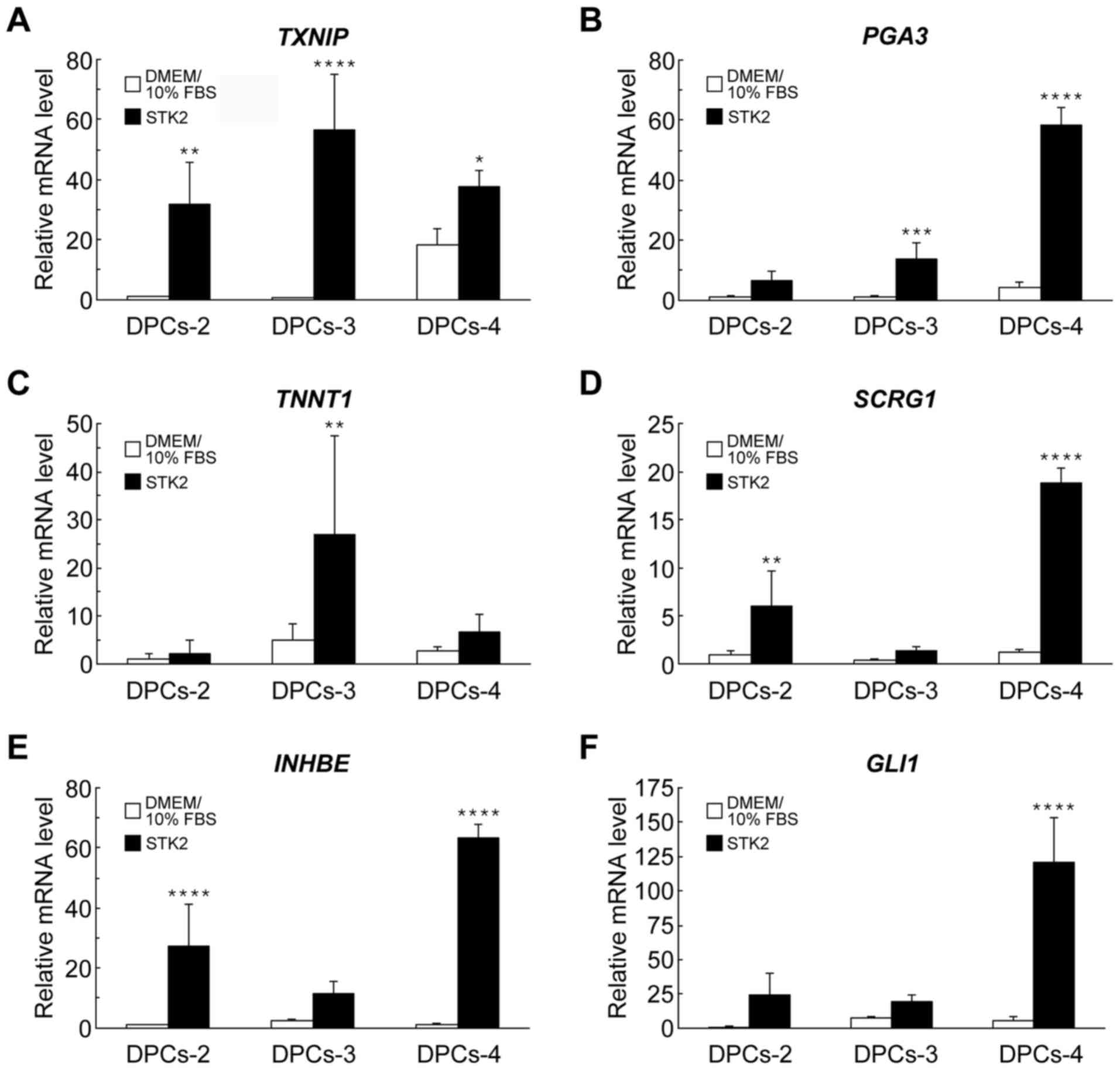 | Figure 3.RT-qPCR analyses of gene expression
in DPCs grown in DMEM/10% FBS or STK2. DPCs (DPCs-2, DPCs-3 and
DPCs-4) were cultured in DMEM/10% FBS or STK2 for 7 days. The mRNA
levels of (A) TXNIP, (B) PGA3, (C) TNNT1, (D)
SCRG1, (E) INHBF and (F) GLI1 were quantified
by RT-qPCR analysis. Values are the means ± standard deviation of
four replicate cultures. *P<0.05, **P<0.01, ***P<0.001 and
****P<0.0001 vs. DMEM/10% FBS in the same cell line. DPCs,
dental pulp cells; DMEM/10% FBS, Dulbecco's modified Eagle's medium
supplemented with 10% fetal bovine serum; TXNIP, thioredoxin
interacting protein; PGA3, pepsinogen 3; TNNT1,
troponin T type 1; SCRG1, stimulator of chondrogenesis 1;
INHBE, inhibin-βE; GLI1, GLI family zinc finger 1;
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction. |
 | Table II.Top 20 upregulated genes in dental
pulp cells grown in STK2 relative to those in Dulbecco's modified
Eagle's medium supplemented with 10% fetal bovine serum. |
Table II.
Top 20 upregulated genes in dental
pulp cells grown in STK2 relative to those in Dulbecco's modified
Eagle's medium supplemented with 10% fetal bovine serum.
| Gene symbol | Gene name | NCBI ID | Fold change |
|---|
| TXNIP | Thioredoxin
interacting protein | NM_006472 | 101.0 |
| PGA3 | Pepsinogen 3 | NM_001079807 |
23.0 |
| TNNT1 | Troponin T type
1 | NM_003283 |
19.2 |
| SCRG1 | Stimulator of
chondrogenesis 1 | NM_007281 |
17.9 |
| HEY1 |
Hairy/enhancer-of-split related with YRPW
motif 1 | NM_001040708 |
15.0 |
| ADH1A | Alcohol
dehydrogenase 1A | NM_000667 |
12.7 |
| PF4V1 | Platelet factor 4
variant 1 | NM_002620 |
11.2 |
| PGF | Placental growth
factor | NM_002632 |
10.8 |
| INHBE | Inhibin-βE | NM_031479 |
9.8 |
| GLI1 | GLI family zinc
finger 1 | NM_005269 |
9.7 |
| PLAC1 | Placenta-specific
1 | NM_021796 |
9.2 |
|
NDUFA4L2 | NADH dehydrogenase
(ubiquinone) 1 alpha subcomplex, 4-like 2 | NM_020142 |
8.8 |
| FER1L4 | Fer-1-like protein
4 | NR_024377 |
8.7 |
| LRRC15 | Leucine rich repeat
containing 15 | NM_130830 |
8.6 |
| ZP1 | Zona pellucida
glycoprotein 1 | NM_207341 |
8.4 |
| ADH1C | Alcohol
dehydrogenase 1C | NM_000669 |
8.2 |
|
MGC16121 | Hypothetical
protein MGC16121 | NR_024607 |
8.1 |
| ANXA8L2 | Annexin A8-like
2 | NM_001630 |
8.0 |
| CA9 | Carbonic anhydrase
IX | NM_001216 |
7.5 |
| BCL11A | B-cell CLL/lymphoma
11A | NM_022893 |
7.4 |
 | Table III.Top 20 downregulated genes in dental
pulp cells grown in STK2 relative to those in Dulbecco's modified
Eagle's medium supplemented with 10% fetal bovine serum. |
Table III.
Top 20 downregulated genes in dental
pulp cells grown in STK2 relative to those in Dulbecco's modified
Eagle's medium supplemented with 10% fetal bovine serum.
| Gene symbol | Gene name | NCBI ID | Fold change |
|---|
| HTR2B | 5-Ηydroxytryptamine
receptor 2B | NM_000867 | 51.0 |
| GPNMB | Glycoprotein
nmb | NM_001005340 | 45.4 |
| NPTX1 | Neuronal pentraxin
I | NM_002522 | 38.8 |
| NTN1 | Netrin 1 | NM_004822 | 36.3 |
| SLC16A6 | Solute carrier
family 16 member 6 | NM_004694 | 35.5 |
| PTGDS | Prostaglandin D2
synthase | NM_000954 | 32.8 |
| RRAGD | Ras-related GTP
binding D | NM_021244 | 30.4 |
| A2M |
α2-macroglobulin | NM_000014 | 30.1 |
| CLDN1 | Claudin 1 | NM_021101 | 24.0 |
| SPON2 | Spondin 2 | NM_012445 | 23.2 |
| NFIB | Nuclear factor
I/B | NM_005596 | 23.2 |
| BIRC3 | Baculoviral IAP
repeat-containing 3 | NM_001165 | 22.2 |
| KCNJ2 | Potassium
voltage-gated channel subfamily J member 2 | NM_000891 | 21.2 |
| MALL | Mal, T-cell
differentiation protein-like | NM_005434 | 19.5 |
| GRIA4 | Glutamate
ionotropic receptor AMPA type subunit 4 | NM_001077244 | 18.5 |
| SLC7A14 | Solute carrier
family 7 member 14 | NM_020949 | 18.1 |
| SCG2 | Secretogranin
II | NM_003469 | 17.9 |
| AGT |
Angiotensinogen | NM_000029 | 17.9 |
| GPX3 | Glutathione
peroxidase 3 | NM_002084 | 17.3 |
| ANO1 | Anoctamin 1 | NM_018043 | 15.4 |
STK2 medium enhances osteogenic
differentiation in DPCs
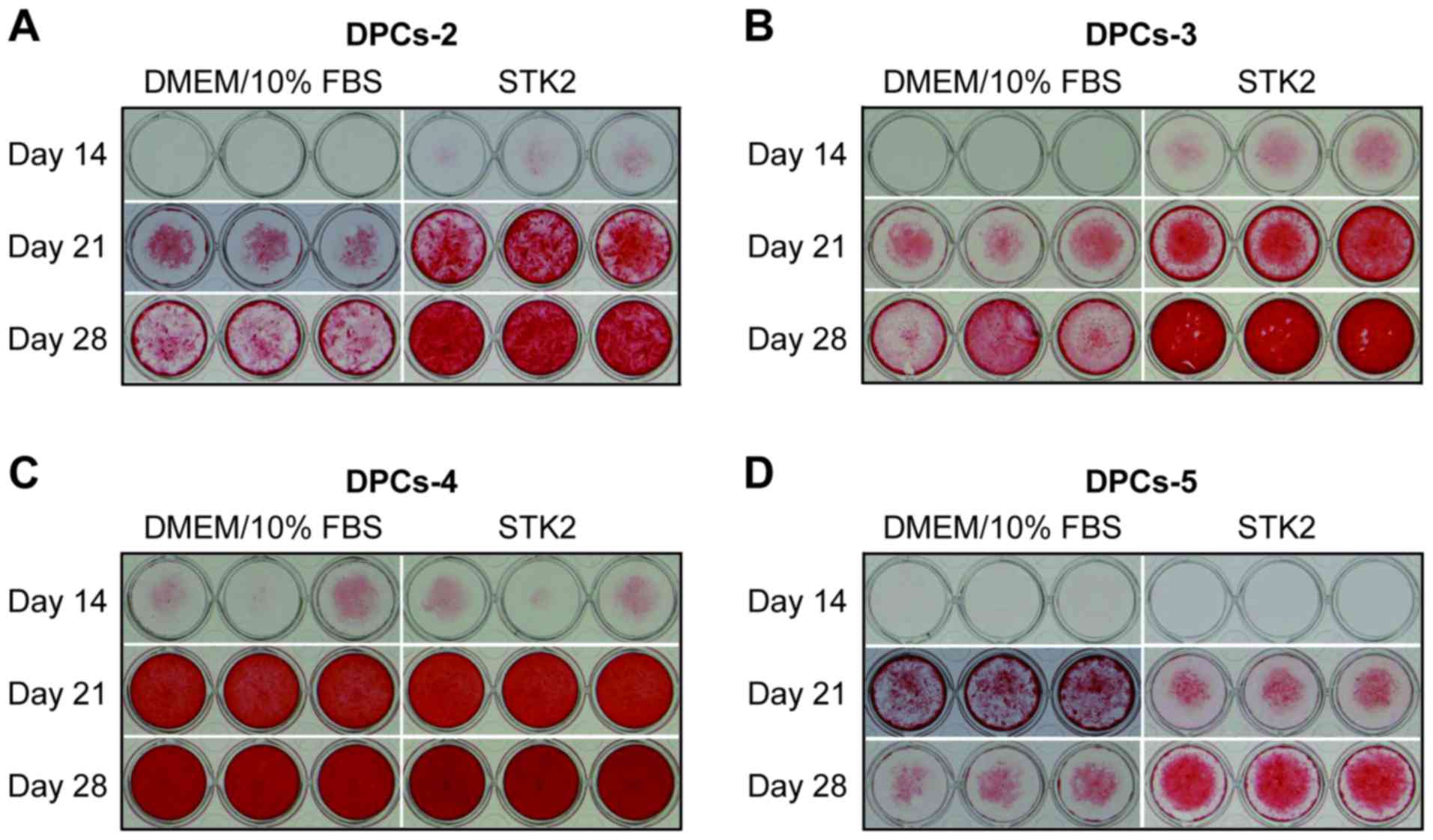
Subsequently, it was determined whether DPCs
incubated in STK2 maintained their differentiation potential via
Alizarin Red S staining (Fig. 4).
DPCs (DPCs-2, −3, −4 and −5) were seeded at a low density and
expanded for 7 days in STK2 or DMEM/10% FBS. When these cultures
became confluent, they were exposed to osteogenesis induction
medium for up to 28 days. On days 21 and 28, DPCs-2 and DPCs-3
expanded in STK2 exhibited more extensive calcification than those
expanded in DMEM/10% FBS (Fig. 4A and
B), while DPCs-4 exhibited extensive calcification on days 21
and 28 irrespective of the medium (Fig.
4C), probably due to the higher differentiation potential of
this cell line. The calcification level of DPCs-5 grown in STK2 was
higher than that of cells grown in DMEM/10 FBS on day 28 but lower
on day 21 (Fig. 4D).
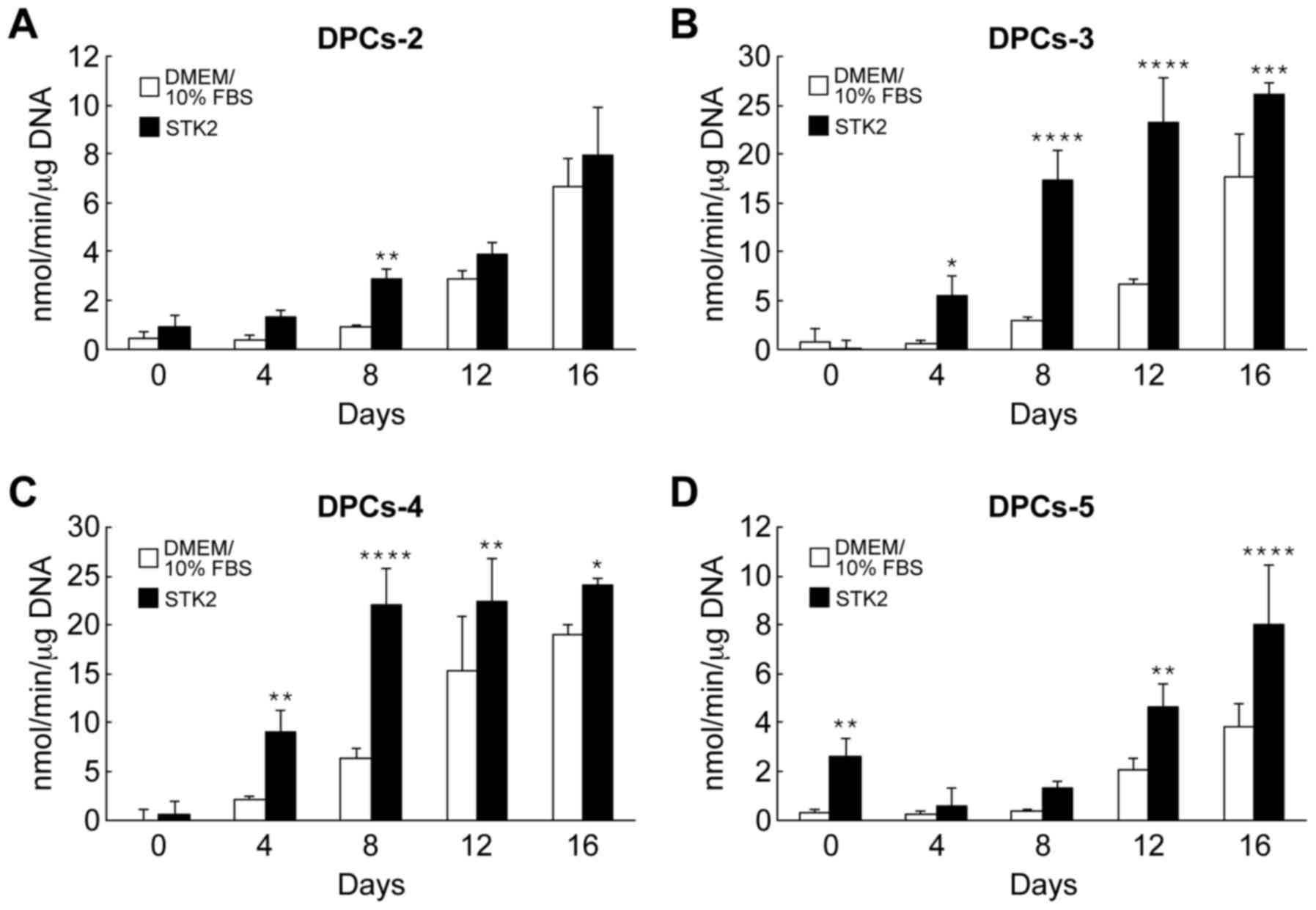
The effects of STK2 pre-culture on ALP activity were
also assessed. Following exposure of the cell cultures to
osteogenesis induction medium, ALP activity began to increase on
day 4 or 8, depending on the cell line and medium (Fig. 5). All cell lines grown in STK2
exhibited greater ALP activity than those grown in DMEM/10% FBS
(Fig. 5).
Discussion
DPCs have been reported to have stem cell-like
properties (6,7,10,15,26), and
are thereby expected to be a source of transplantable cells in
regenerative medicine for dental (7,9,27), bone (10,11),
vascular (6,12) and nerve diseases (26,28).
However, it is necessary for safe and stable cell therapy to
establish a serum-free culture system for DPCs, since the use of
serum has the potential risk of viral contamination and the
components in serum vary depending on batch. In the present study,
human DPCs were successfully cultured in a serum-free condition.
DPCs were efficiently grown in STK2 with maintained osteogenic
potential. This finding may promote clinical applications of DPCs
in regenerative medicine.
Various serum-free media have a lower
proliferation-promoting activity than serum-containing media.
However, in the current study, DPCs generally proliferated at a
higher rate in STK2 than in DMEM/10% FBS. The increased
proliferation in STK2 was consistently observed with the four DPC
lines examined, even though an optimum batch of FBS was used,
selected from more than 10 batches. It may be speculated that FBS
contains not only growth factors but also growth inhibitors and
proteases, which decrease the growth rate of DPCs.
DNA microarray analysis demonstrated that the gene
expression profiles of DPCs grown in STK2 and DMEM/10% FBS were
similar. Thus, STK2 did not induce gross changes in the phenotypic
expression of DPCs even in the absence of serum, suggesting that
STK2 may be used for routine cultures of DPCs. It was identified
that the number of downregulated genes was greater than that of
upregulated genes in STK2 cultures, relative to DMEM/10% FBS
cultures, which may be due to the absence of certain serum
compounds, including proteins and other biologically active
molecules, in the serum-free medium. While most genes exhibited no
marked changes in gene expression levels, 38 genes were upregulated
more than 5-fold in STK2-cultured cells. Some of these genes,
including placental growth factor and INHBE, may account for the
growth-promoting effect of STK2. Further experiments, including
small interfering RNA knockdowns, are required to reveal which
upregulated genes serve a role in the STK2-mediated simulation of
proliferation.
Another important finding was the maintenance of the
differentiation potential of DPCs under serum-free conditions. DPCs
expanded in STK2 generally exhibited greater increases in ALP
activity and calcified matrix following exposure to osteogenesis
induction medium than cells expanded in DMEM/10% FBS. This may be
due to the presence of certain proteins in serum, but not in STK2,
that decrease the osteogenic potential of DPCs. DPCs are able to
differentiate into adipocytes, chondrocytes and neural cells as
well as osteoblasts (1,11). In future studies, our group plans to
assess whether DPCs expanded in STK2 maintain their potency for
differentiation into these cell types.
STK2 was originally developed using human BM-MSCs in
order to create a serum-free culture medium for MSCs (17). Indeed, STK2 has been reported to exert
a significantly greater proliferation effect on BM-MSCs compared
with DMEM/10% FBS or Lonza MSCGMTM MSC growth medium (18). In addition, Sawada et al
(29) demonstrated that a number of
genes related to cell proliferation, including insulin-like growth
factor binding protein 6, NRAS proto-oncogene,
phosphoinositide-3-kinase regulatory subunit 3 and Jun
proto-oncogene, were upregulated in BM-MSCs following culture in
STK2 through DNA microarray analysis. However, these upregulated
genes were not similar to those of the present study, probably due
to differences in experimental conditions. The present study
assessed DPCs in STK2 culture for 7 days, whereas Sawada's group
used BM-MSCs cultured in STK2 for 50 days. Although it is difficult
to directly compare the effect of STK2 on human DPCs and BM-MSCs
due to inter-individual variations, STK2 appeared to be more
effective for BM-MSCs than for DPCs in the current assays, since
STK2 enhanced the proliferation of human BM-MSCs under all
conditions of initial cell density. Modifications of the
composition of STK2 may be required to optimize the expansion and
differentiation of DPCs, despite STK2 being sufficient in
supporting proliferation and maintaining the differentiation
potency of DPCs.
A recent study demonstrated that STK2 could induce
differentiation into mesenchymal-like cells; by culturing in STK2,
human pluripotent stem cells-derived neural crest cells were
induced into MSCs, which expressed specific cell surface markers
and were able to differentiate into osteogenic, chondrogenic and
adipogenic cells (20). In addition,
STK2 induced the epithelial-mesenchymal transition when endometrial
carcinoma cell lines transformed into mesenchymal-like cells
(21). In the current study, it was
demonstrated that DPCs proliferated more actively in STK2 than in
DMEM/10% FBS while maintaining their osteogenic potential.
Therefore, the serum-free culture system with STK2 may be useful
for basic experimental research and cell therapy applications with
DPCs as well as BM-MSCs.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Grant-in-Aid
for Challenging Exploratory Research (grant no. 24659876) from the
Ministry of Education, Culture, Sports, Science and Technology of
Japan.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the Gene Expression Omnibus (GEO)
repository, with accession number GSE97199 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE97199).
Authors' contributions
SF, KF, FN and YK conceived and designed the
experiments. SF, NG, AI, KK, MK, AS and KS performed the
experiments. SF, KF, YA, AI and MK analyzed the data, and SF, KF,
NG, JS, MK and YK interpreted the data. JS and KK contributed
reagents/materials/analysis tools. SF, KF and YK wrote the
paper.
Ethics approval and consent to
participate
Ethical approval for the study protocols was
provided by Hiroshima University according to the guidelines of its
Ethics Committee (approval no. D88-2). The healthy samples from
donors at Hiroshima University Hospital were obtained with informed
consent. The donor sample from Lonza Group, Ltd. (Basel,
Switzerland) was initially donated after obtaining permission for
research use by informed consent and legal authorization.
Consent for publication
Not applicable.
Competing interests
YK is a director of Two Cells Co., Ltd., and JS is
an employee of Two Cells Co., Ltd. All other authors declare no
competing interests.
Glossary
Abbreviations
Abbreviations:
|
DPCs
|
dental pulp cells
|
|
BM-MSCs
|
bone marrow-derived mesenchymal stem
cells
|
|
FBS
|
fetal bovine serum
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
α-MEM
|
α-minimal essential medium
|
|
PBS
|
phosphate-buffered saline
|
|
DMEM/10% FBS
|
DMEM supplemented with 10% FBS
|
|
CCK-8
|
Cell Counting Kit-8
|
|
ALP
|
alkaline phosphatase
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
TXNIP
|
thioredoxin interacting protein
|
|
PGA3
|
pepsinogen 3
|
|
TNNT1
|
troponin T type 1
|
|
SCRG1
|
stimulator of chondrogenesis 1
|
|
INHBE
|
inhibin-βE
|
|
GLI1
|
GLI family zinc finger 1
|
References
|
1
|
Gronthos S, Mankani M, Brahim J, Robey PG
and Shi S: Postnatal human dental pulp stem cells (DPSCs) in vitro
and in vivo. Proc Natl Acad Sci USA. 97:pp. 13625–13630. 2000;
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stanislawski L, Carreau JP, Pouchelet M,
Chen ZH and Goldberg M: In vitro culture of human dental pulp
cells: Some aspects of cells emerging early from the explant. Clin
Oral Investig. 1:131–140. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Couble ML, Farges JC, Bleicher F,
Perrat-Mabillon B, Boudeulle M and Magloire H: Odontoblast
differentiation of human dental pulp cells in explant cultures.
Calcif Tissue Int. 66:129–138. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
He H, Yu J, Liu Y, Lu S, Liu H, Shi J and
Jin Y: Effects of FGF2 and TGFbeta1 on the differentiation of human
dental pulp stem cells in vitro. Cell Biol Int. 32:827–834. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Iohara K, Zheng L, Ito M, Tomokiyo A,
Matsushita K and Nakashima M: Side population cells isolated from
porcine dental pulp tissue with self-renewal and multipotency for
dentinogenesis, chondrogenesis, adipogenesis, and neurogenesis.
Stem Cells. 24:2493–2503. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iohara K, Zheng L, Wake H, Ito M, Nabekura
J, Wakita H, Nakamura H, Into T, Matsushita K and Nakashima M: A
novel stem cell source for vasculogenesis in ischemia: Subfraction
of side population cells from dental pulp. Stem Cells.
26:2408–2418. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iohara K, Imabayashi K, Ishizaka R,
Watanabe A, Nabekura J, Ito M, Matsushita K, Nakamura H and
Nakashima M: Complete pulp regeneration after pulpectomy by
transplantation of CD105+ stem cells with stromal
cell-derived factor-1. Tissue Eng Part A. 17:1911–1920. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Min JH, Ko SY, Cho YB, Ryu CJ and Jang YJ:
Dentinogenic potential of human adult dental pulp cells during the
extended primary culture. Hum Cell. 24:43–50. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shi S, Bartold PM, Miura M, Seo BM, Robey
PG and Gronthos S: The efficacy of mesenchymal stem cells to
regenerate and repair dental structures. Orthod Craniofac Res.
8:191–199. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sloan AJ and Waddington RJ: Dental pulp
stem cells: What, where, how? Int J Paediatr Dent. 19:61–70. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Spath L, Rotilio V, Alessandrini M,
Gambara G, De Angelis L, Mancini M, Mitsiadis TA, Vivarelli E, Naro
F, Filippini A and Papaccio G: Explant-derived human dental pulp
stem cells enhance differentiation and proliferation potentials. J
Cell Mol Med. 14(6b): 1–1644. 2010.
|
|
12
|
Tran-Hung L, Mathieu S and About I: Role
of human pulp fibroblasts in angiogenesis. J Dent Res. 85:819–823.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamada Y, Fujimoto A, Ito A, Yoshimi R and
Ueda M: Cluster analysis and gene expression profiles: A cDNA
microarray system-based comparison between human dental pulp stem
cells (hDPSCs) and human mesenchymal stem cells (hMSCs) for tissue
engineering cell therapy. Biomaterials. 27:3766–3781. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu H, Gronthos S and Shi S: Dental pulp
stem cells. Methods Enzymol. 419:99–113. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sakai K, Yamamoto A, Matsubara K, Nakamura
S, Naruse M, Yamagata M, Sakamoto K, Tauchi R, Wakao N, Imagama S,
et al: Human dental pulp-derived stem cells promote locomotor
recovery after complete transection of the rat spinal cord by
multiple neuro-regenerative mechanisms. J Clin Invest. 122:80–90.
2012.PubMed/NCBI
|
|
16
|
Nakamura S, Yamada Y, Katagiri W, Sugito
T, Ito K and Ueda M: Stem cell proliferation pathways comparison
between human exfoliated deciduous teeth and dental pulp stem cells
by gene expression profile from promising dental pulp. J Endod.
35:1536–1542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gottipamula S, Muttigi MS, Kolkundkar U
and Seetharam RN: Serum-free media for the production of human
mesenchymal stromal cells: A review. Cell Prolif. 46:608–627. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ishikawa I, Sawada R, Kato Y, Tsuji K,
Shao J, Yamada T, Kato R and Tsuchiya T: Effectivity of the novel
serum-free medium STK2 for proliferating human mesenchymal stem
cells. Yakugaku Zasshi. 129:381–384. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsugeno Y, Sato F, Muragaki Y and Kato Y:
Cell culture of human gingival fibroblasts, oral cancer cells and
mesothelioma cells with serum-free media, STK1 and STK2. Biomed
Rep. 2:644–648. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fukuta M, Nakai Y, Kirino K, Nakagawa M,
Sekiguchi K, Nagata S, Matsumoto Y, Yamamoto T, Umeda K, Heike T,
et al: Derivation of mesenchymal stromal cells from pluripotent
stem cells through a neural crest lineage using small molecule
compounds with defined media. PLoS One. 9:e1122912014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Inoue H, Takahashi H, Hashimura M, Eshima
K, Akiya M, Matsumoto T and Saegusa M: Cooperation of Sox4 with
β-catenin/p300 complex in transcriptional regulation of the Slug
gene during divergent sarcomatous differentiation in uterine
carcinosarcoma. BMC Cancer. 16:532016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fujii S, Fujimoto K, Goto N, Kanawa M,
Kawamoto T, Pan H, Srivatanakul P, Rakdang W, Pornprasitwech J,
Saskianti T, et al: Characteristic expression of MSX1, MSX2, TBX2
and ENTPD1 in dental pulp cells. Biomed Rep. 3:566–572. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Edgar R, Domrachev M and Lash AE: Gene
Expression Omnibus: NCBI gene expression and hybridization array
data repository. Nucleic Acids Res. 30:207–210. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sasamoto T, Fujimoto K, Kanawa M, Kimura
J, Takeuchi J, Harada N, Goto N, Kawamoto T, Noshiro M, Suardita K,
et al: DEC2 is a negative regulator for the proliferation and
differentiation of chondrocyte lineage-committed mesenchymal stem
cells. Int J Mol Med. 38:876–884. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakashima M, Iohara K and Sugiyama M:
Human dental pulp stem cells with highly angiogenic and neurogenic
potential for possible use in pulp regeneration. Cytokine Growth
Factor Rev. 20:435–440. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakashima M, Iohara K, Murakami M,
Nakamura H, Sato Y, Ariji Y and Matsushita K: Pulp regeneration by
transplantation of dental pulp stem cells in pulpitis: A pilot
clinical study. Stem Cell Res Ther. 8:612017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Song M, Lee JH, Bae J, Bu Y and Kim EC:
Human dental pulp stem cells are more effective than human bone
marrow-derived mesenchymal stem cells in cerebral ischemic injury.
Cell Transplant. 26:1001–1016. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sawada R, Yamada T, Tsuchiya T and
Matsuoka A: Microarray analysis of the effects of serum-free medium
on gene expression changes in human mesenchymal stem cells during
the in vitro culture. Yakugaku Zasshi. 130:1387–1393. 2010.
View Article : Google Scholar : PubMed/NCBI
|