Introduction
The controlled production of nitric oxide (NO) has
an important role in mediating neurotransmission and regulating
vascular tone (1). However, early
after the finding of the signal transducing physiological functions
of the free radical NO in the vasculature and nervous system, it
became evident that NO could also participate as a cytotoxic
effector molecule and/or a pathogenic mediator when produced at
high rates by either inflammatory stimuli-induced NO synthase
(iNOS) or overstimulation of the constitutive forms (eNOS and
nNOS). Much of NO-mediated pathogenicity depends on the formation
of secondary intermediates such as peroxynitrite anion
(ONOO−) and nitrogen dioxide (NO2) that are
typically more reactive and toxic than NO (2,3).
ONOO− is a powerful oxidant and cytotoxic
agent formed by the near-diffusion limited reaction between NO and
superoxide (O2−) (4,5).
ONOO− can damage DNA, membrane lipids, and mitochondria,
and has been shown to modify proteins at intrinsic methionine,
tryptophan, and cysteine residues (6). The most important property of
ONOO− is its ability to nitrate free tyrosine and
tyrosine residues in proteins (7).
One of the molecular footprints left by the reactions of reactive
nitrogen species with biomolecules is nitration (i.e., addition of
the nitro group, NO2) of protein tyrosine residues to
3-nitrotyrosine (3-NT). It is generally suggested that increases in
tyrosine nitration, whether tyrosine is free or part of a
polypeptide chain, reflect the actions of ONOO−
(8). Nitration pathways involve free
radical biochemistry with carbonate radicals and/or oxo-metal
complexes oxidizing tyrosine to tyrosyl radical followed by the
diffusion-controlled reaction with NO2 to yield 3-NT.
Although protein tyrosine nitration is a low-yield process in
vivo, 3-NT has been revealed as having a higher yield and is a
relevant biomarker of NO-dependent oxidative stress.
Previous studies (9)
have suggested that noise exposure leads to hair cell death and
significant production of nitrotyrosine in the OHCs and stria
vascularis. The purpose of this study was to demonstrate
distribution of 3-NT, ONOO− marker, in the organ of
corti and in the lateral wall of the cochlea in the guinea pig and
to examine the influence of broad band noise exposure to 3-NT
distribution in the cochlea.
Materials and methods
Animal preparation
A total of 24 guinea pigs (albinos of both sexes,
200–250 g) with normal hearing were used in this study. The animals
were divided into the control and experimental groups. Animals in
the control group (n=12) were kept in a quiet room with food and
water (constant temperature of 21–24°C and humidity of 40–70%).
Animals in the experimental group (n=12) were placed in the noise
exposure chamber and exposed for 4 h/day to broadband noise at 122
dB SPL (A-weighted) for 2 consecutive days. Hearing of the animal
was estimated using auditory brain-stem response thresholds before
and after noise exposure. This exposure level resulted in a
permanent threshold shift (10).
Guinea pigs were processed for the detection of the distribution
and change of 3-NT in the organ of corti and cochlear lateral wall
immediately following noise exposure.
Specimen preparation
The animals were anesthetized and cardiac perfusion
was performed with saline following the second day's noise
exposure, then a further vascular perfusion with 4%
paraformaldehyde followed. One ear from each animal was used for
whole mount surface preparation, while the contralateral ear was
used for frozen section methods. The cochlea was gently isolated
after fixing. For whole mount preparation, the lateral wall tissues
and the organ of corti were harvested after removal of the bony
capsule. For the frozen section methods, cochleae were harvested
with the bony capsule. After rinsing with 0.1 M PB, the cochleae
were decalcified in 8% ethylene diamine tetraacetic acid (EDTA) in
PBS in microwave-assisted decalcification. After infiltration with
10 and 30% sucrose, the cochlea was then placed overnight into a
Tissue-Tek mold and frozen in the mold with dry ice. Eight-micron
cryostat (Bright Instrument, Huntingdon, Cambridgeshire, UK)
sections were obtained, and thaw-mounted onto slides pre-coated
with silane (Sigma Diagnostics, Inc., St. Louis, MO, USA). The
tissue was then processed for immunohistochemistry.
Immunohistochemistry
The specimens were incubated overnight in anti-3-NT
(mouse monoclonal antibody, 39B6; Alexis Biochemicals, San Diego,
CA, USA). The specimens were washed in 1% BSA-PBS for 30 min and
incubated in Alexa Fluor 488 anti-mouse IgG (catalog no. A11001)
for 3-NT and Alexa Fluor 568 phalloidin (diluted 1:50 with 1%
BSA-PBS; Molecular Probes Life Technologies, Carlsbad, CA, USA) for
1 h. After washing in 0.02 PBS for 30 min, the surface-prepared
tissues and frozen sections were mounted separately. Negative
controls were incubated tissue with 1% BSA-PBS to replace the
primary antibody. In addition to immunoactivity labeling, the
specimens were double labeled either with Alexa Fluor 568
phalloidin, a probe for F-actin, to observe the whole cell shape or
with propidium iodide, a DNA intercalator, to observe nuclear
morphology.
Fluorescence and confocal
microscope
The laser scanning confocal microscope (MRC 1024ES;
Bio-Rad, Berkeley, CA, USA) was used to observe the fluorescent
signals. Images were captured using the same settings for gain and
illumination power. Cochleae were double labeled with red
fluorescence (PI-labeled nuclei and phalloidin labeled F-actin) and
green fluorescence (Alexa Fluor 488 anti-rabbit antibody labeled
3-nitrotyrosin). A series of 40–60 laser confocal images were taken
for each section of the organ of corti beginning at the top of the
OHC stereocilia and stepping progressively through the OHCs body to
the bottom of the OHCs at depth intervals of 1.0 µm. The images in
the figures are presented as projection sets of 40–60 images.
Statistical analysis
Quantitative analysis for fluorescence intensity of
3-NT was performed using Adobe Photoshop 7.0® software
on single confocal optical sections. To quantitate 3-NT in OHCs and
marginal cells of cochlear lateral wall before and after noise
exposure, the image to be analyzed was chosen as the brightest
Z-axis confocal section at the level of the OHCs or marginal cells.
The mean fluorescent intensity was determined using the software
over a user-selected window approximately covering the organ of
corti or marginal cells in the acquired image. A mean background
fluorescence level was determined in a small window located away
from the fluorescence tissue, and was subtracted from the mean
fluorescence intensity value. The data were averaged within the 10
experimental and 10 control animals. All values presented in
Results are mean ± SD. Differences among the different groups were
evaluated using a two-tailed Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Distribution and change of
ONOO− in the normal and noise-stimulated organ of
corti
The distribution of 3-NT, ONOO− marker,
was observed in the organ of corti both in the control and
experimental groups. For the normal organ of corti, relatively weak
3-NT immunolabeling was detected in the OHCs, pillar cells (PCs),
inner hair cells (IHCs), and spiral ganglion cells (SPCs) (Fig. 1A and C). After animals were exposed
for 4 h/day to broadband noise at 122 dB SPL (A-weighted) for two
consecutive days, the activity of 3-NT immunostaining became
stronger immediately following the second day's noise exposure in
various cochlear cells. The increased labeling was especially
prominent in OHCs, PCs and SPCs (Fig. 1D
and F). The same phalloidin staining was observed in control
(panel B) and noise exposure animal (panel E): However, quite a few
OHCs were lost in the noise exposure animal (panels D-F). No hair
cell loss was observed in the control animal (panels A-C).
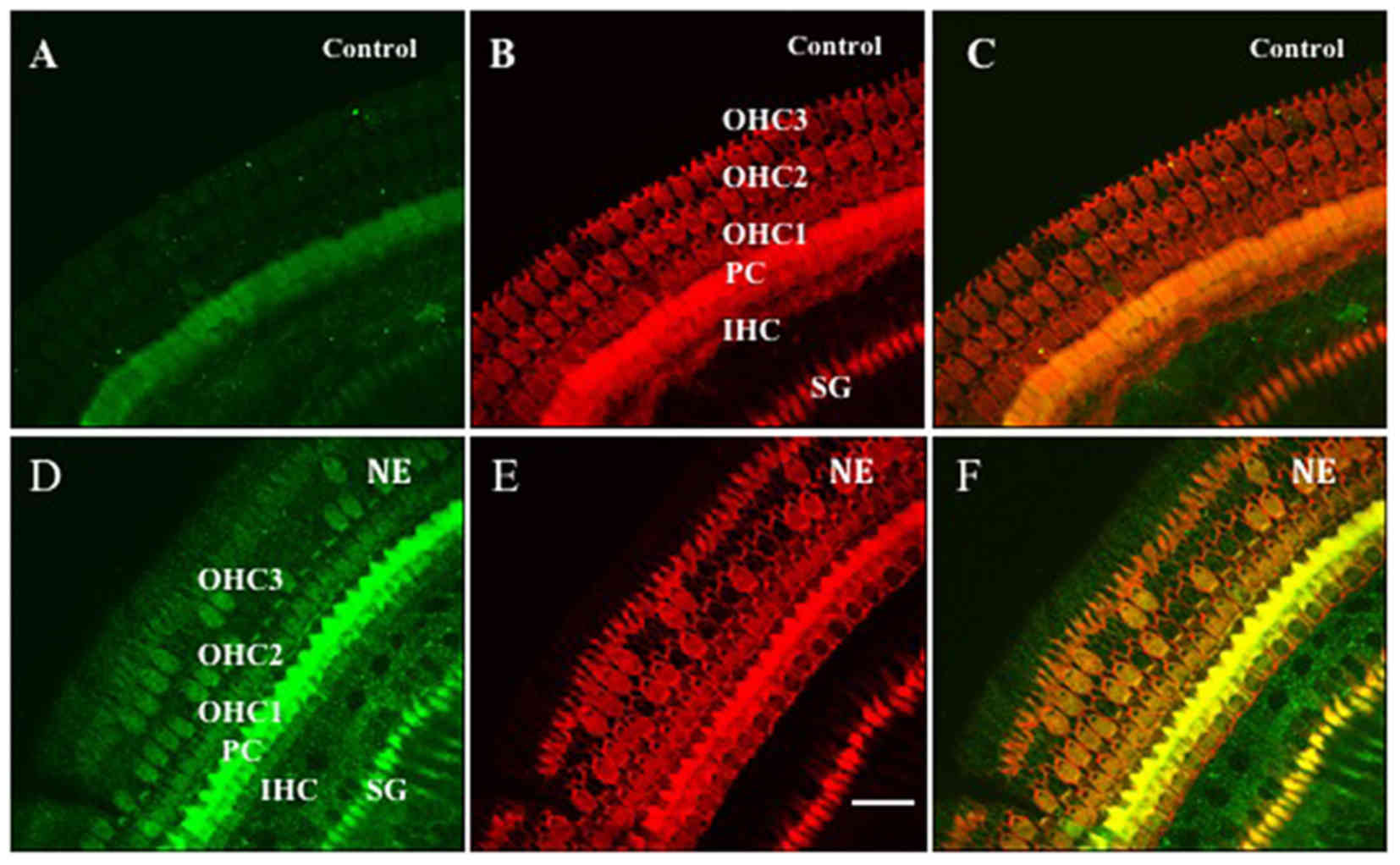 | Figure 1.Anti-3-NT and phalloidin double
staining showed the 3-NT distribution in the organ of corti before
and after NE. (A-C) Control group; (D-F) NE group. The organs of
corti were stained (red, B and E) with phalloidin showing whole
cell shape and (green, A and D) anti-3-NT antibody showing the
distribution of 3-NT. Panel C is the merged image of A and B and
panel F is that of D and E. Relatively weak staining of 3-NT was
found in the OHCs, IHCs, SPCs and PCs in the control animal (panels
A and C). Significant 3-NT immunostaining increase was observed in
the OHCs, IHCs, PCs and SPCs in the NE animal (panels D and F). The
same phalloidin staining was observed in control (panel B) and
noise exposure animals (panel E); however, quite a few OHCs were
lost in the noise exposure animal (panels D-F). No hair cell loss
was observed in the control animal (panels A-C). 3-NT, anti 3-NT;
OHC 1–3: first, second and the third row of OHCs; PC, pillar cell;
SG, spiral ganglion. Scale bar, 20 µm. 3-NT, 3-nitrotyrosine; NE,
noise exposure; OHCs, outer hair cells; IHCs, inner hair cells;
SPCs, spiral ganglion cells; PCs, pillar cells. |
Noise induced ONOO−
increase in OHCs from guinea pig cochlea
The significant change of 3-NT, ONOO−
marker, was observed in OHCs. To quantitatively determine 3-NT
increase in OHCs following noise exposure, the mean fluorescent
intensity was measured using the software in a user-selected window
approximately covering the OHCs images in the 10 control animals
and in the 10 noise exposure animals. We found weak 3-NT signals in
the OHCs of control animals (Fig.
2A). The stronger 3-NT signals were observed in OHCs
immediately following two days of noise exposure (Fig. 2B). There was no apparent
immunoreactivity in OHCs when incubated with the secondary antibody
alone (Fig. 2C). The quantitative
analysis showed 3-NT signals of OHCs significantly increased
(P<0.01, n=10) in the noise exposure group compared with that of
the control group.
ONOO− distribution outside
of nuclei in OHCs
To further determine 3-NT distribution inside of
OHCs, the organs of corti were double labeled with anti-3-NT, to
show 3-NT distribution, and propidium iodide, to show nuclei in
both the control and noise exposure groups. Relatively weak
staining of 3-NT was found in the apical end of OHCs in the control
animals (Fig. 3C and E). A
significant 3-NT immunostaining increase was observed in the apical
end of OHCs in the noise exposure animals (Fig. 3D and F). Anti-3-NT and propidium
iodide double staining showed that 3-NT is distributed mainly in
the apical end of hair cells and no distribution was evident in the
nuclear area (panels E and F).
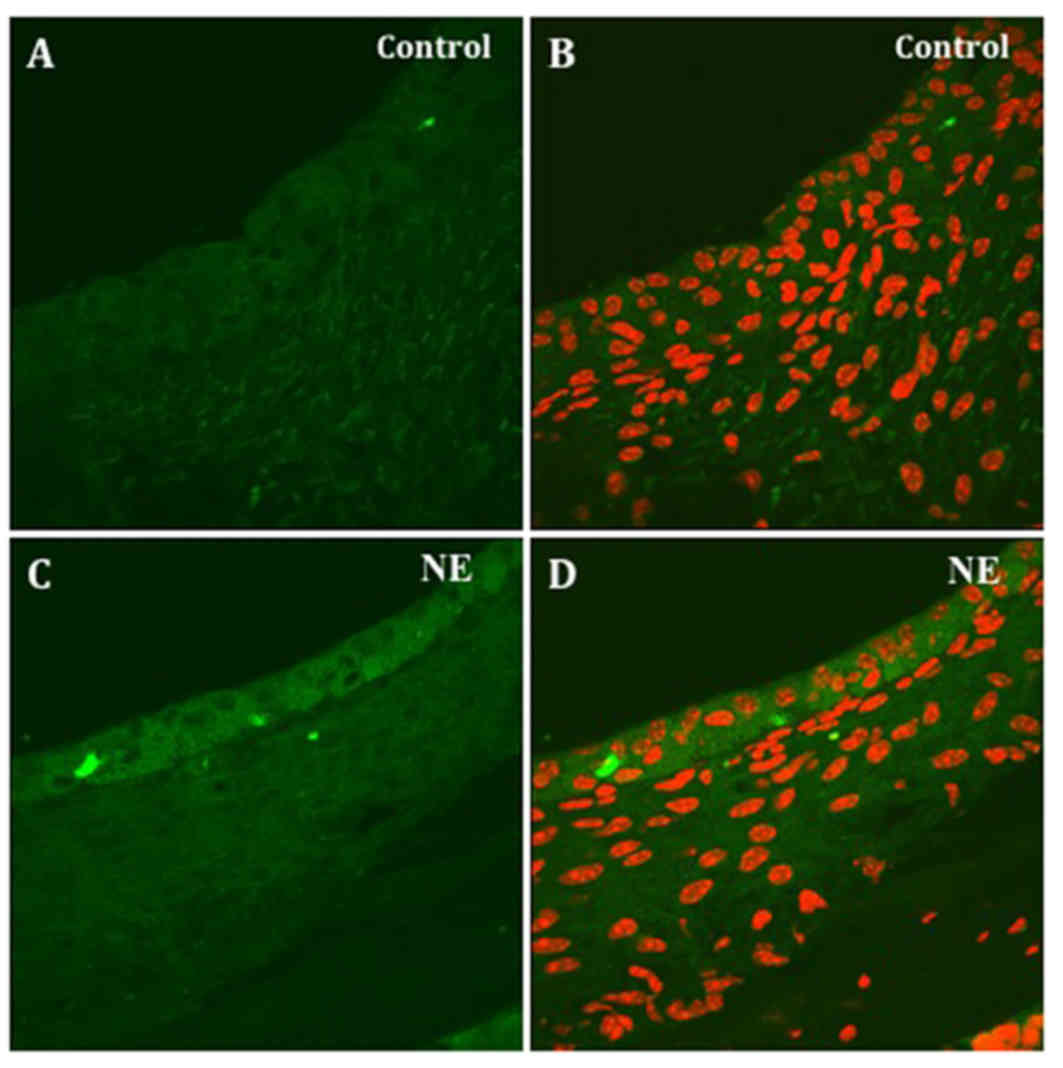
ONOO− distribution in the
cochlear lateral wall in the normal and noise exposure guinea pig
cochleae
The distributions of 3-NT, ONOO− marker,
were observed in cryostat sections of the cochlear lateral wall.
The frozen sections were double labeled with anti-3-NT and
propidium iodide. In the unstimulated guinea pig lateral wall,
relatively weak 3-NT immunolabeling was observed in stria
vascularis (Fig. 4A and B). The 3-NT
immunoactivity became stronger in stria vascularis in the tissue
specimens from noise-exposed animals (Fig. 4C and D). There was no apparent
immunoreactivity in the spiral ligament area of the cochlear
lateral wall. Anti-3-NT and propidium iodide double staining showed
that 3-NT distributes mainly in the outside of the nucleus of the
marginal cells.
Noise exposure-induced
ONOO− increase in the marginal cells from the lateral
wall of guinea pig cochleae
ONOO− distributions in cochlear lateral
wall were also observed in surface-prepared specimens from the
control and noise-exposed animals. 3-NT immunoactivity was
quantitatively analyzed in the marginal cells of lateral wall.
Relatively weak staining of 3-NT was found in marginal cells in the
control animal (Fig. 5A). Significant
3-NT immunostaining increase was observed in the marginal cells in
the noise-exposed animal (Fig. 5D and
G). The same phalloidin staining was observed in the control
(Fig. 5D) and noise-exposed animals
(Fig. 5E). Anti-3-NT and propidium
iodide double staining showed that 3-NT distributes mainly outside
of nuclei in the marginal cells and no distribution is seen in the
nuclear area (Fig. 5H and I).
Quantitative analysis comparing the intensity of 3-NT fluorescence
in the control and noise-exposed groups showed a significant
(P<0.01, n=10) 3-NT increase in the marginal cells (Fig. 5J) following noise exposure.
Discussion
Previous findings have shown that NO may play an
important role in the regulation of cochlear blood flow and
auditory neurotransmission (11,12). Much
of NO-mediated pathogenicity would depend on the formation of
secondary intermediates. NO readily diffuses through the cytosol
and cell membrane, allowing it to react with
O2− to form ONOO−. Thus, the
increase in tyrosine nitration reflects the actions of
ONOO−. 3-NT can be a marker for ONOO−-induced
neurotoxicity (13). ONOO−
that are typically more reactive and toxic than NO.
In the present study, we have examined the
distribution of NO derivatives, 3-NT, in the cochlear lateral wall
tissue and the organ of corti from the guinea pig using
fluorescence immunohistochemistry. We found that in the normal (no
noise exposure) animals, 3-NT was observed in the OHCs, IHCs, PCs,
SPCs and the marginal cells of stria vascularis in the lateral
wall. This result is consistent with a previous report which
identified the presence of NO in the IHCs, OHCs, and the stria
vascularis (14). Although the
complete actions of ONOO− remain to be determined, we
clearly found 3-NT signals in OHCs and IHCs as well as PCs and
SPCs. 3-NT distributions reflect the diffusion of NO, products of
NADPH oxidase in mitochondria and the location of tyrosine. Since
NO is a small molecule and a gas that freely diffuses (but is
short-lived), it is difficult to determine the precise location of
its production. Thus, our data of 3-NT distributions mainly reflect
the location of O2− producing and tyrosine
nitration. Mitochondria have been recognized as critical sources
and targets of nitrating species (15). Although the formation of NO mainly
occurs outside the mitochondria, the diffusion of NO into
mitochondria and its combination with mitochondrial-derived
O2− results in the formation of
ONOO−, which accounts for much of the disruption of
mitochondrial metabolism initially attributed to direct actions of
NO and to the nitration of mitochondrial proteins. In the present
study, anti-3-NT and propidium iodide double staining showed that
3-NT distributes mainly in the apical end of OHCs, and 3-NT
distributes outside of the nucleus of the OHCs. This result is
consistent with mitochondria as sources and targets of nitrating
species.
Noise-induced hearing loss reflects a direct
mechanical trauma occurring during noise exposure and oxidative
stress-induced formation of reactive oxygen species (ROS). ROS are
free radicals, reactive molecules containing oxygen, or molecules
containing oxygen that generate free radicals. ROS include NO,
O2−, ONOO− and hydrocynical
radical. These reactive species directly destroy DNA and cell
membranes, and act as signaling molecules for the upregulation of
apoptotic cell death genes. Thus, hydroxyl radicals significantly
increase in the cochlea with noise, nitrotyrosine and
4-hydroxy-2-nonel increase within 10 days following noise exposure
(16), isoprostanes, directly
reflecting ROS formation after intense noise, form in the organ of
corti and lateral wall, and oxidative-induced DNA damage follows
intense noise exposure (9). In the
present study, we quantitatively analyzed the change of 3-NT in
OHCs before and after noise exposure. We found a significant
increase of 3-NT in OHCs (P<0.01, n=10) in the noise exposure
group compared with that of the control group. The increase of 3-NT
means large quantities of ONOO− production under loud
sound stimulation. This excess ONOO− is the principal
pathogenic pathway resulting from the reaction of NO with oxygen
and oxygen radicals. The increased 3-NT means the formation of
reactive NO species, which are thought to play a crucial role in
cell injury and death by a complex process, including damage to DNA
and mitochondria, resulting in the disruption of energy metabolism
(17). Using phalloidin labeling, we
observed quite a few hair cell losses following loud sound
stimulation. In addition, apoptosis and necrosis were observed in
OHCs after noise exposure (17). Some
studies have suggested that mitochondria contain NO synthase and
are capable of producing biologically significant quantities of NO
to regulate energy metabolism and perform other physiologic
functions or to become involved in pathologic processes. NO-related
free radicals can damage the organelles leading to cell death
through irreversible inhibition of mitochondrial respiration or
other damage to a variety of mitochondrial components via oxidizing
reactions involving ONOO− (18,19).
In the present study, both whole mount and cryostat
sections were performed to observe the distribution of 3-NT in the
marginal cells of stria vascularis in the lateral wall. We observed
relatively weak 3-NT distributions in marginal cells in
unstimulated animals (Figs. 4A and
5A). By contrast, upregulated 3-NT
immunoactivity was detected in the marginal cells following
exposure to the broadband noise. The quantitative analysis revealed
3-NT in marginal cells of lateral wall increased significantly
(P<0.01, n=10) in the noise exposure group compared with that of
the control group. This is consistent with previous report that
increased NO signal was observed following exposure to noise
(14). A high level of NO can react
rapidly with O2− to produce ONOO−,
which are strong oxidants that could produce marginal cell damage.
An early study showed that O2− anion radicals
emerged along the luminal membrane of the marginal cells of the SV
following acoustic trauma in the guinea pig (20). We have observed increased 3-NT
activity in marginal cells, which may result in oxidative injury to
those cells due to ONOO− related free radical damage,
and may cause loss of homeostasis of the endolymph.
Taken together, this study demonstrates the presence
of 3-NT, an ONOO− marker, in the IHCs, OHCs, PCs, SPCs
and the marginal cells of stria vascularis in the normal guinea pig
cochlea. The 3-NT distributes mainly in the apical end of hair
cells and no distribution is seen in the nuclear area. After the
noise exposure, the increased 3-NT signals were observed in the
IHCs, OHCs, PCs, SPCs and marginal cells of the stria vascularis.
Furthermore, to increase nitrotyrosine and 3-NT, hair cell loss was
observed for the OHCs following noise exposure.
Acknowledgements
The present study was supported by grants from
National Natural Science Foundation of China (nos. 81470683 and
81770992).
References
|
1
|
Michel O, Hess A, Bloch W, Stennert E, Su
J and Addicks K: Localization of the NO/cGMP-pathway in the cochlea
of guinea pigs. Hear Res. 133:1–9. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Radi R: Nitric oxide, oxidants, and
protein tyrosine nitration. Proc Natl Acad Sci USA. 101:4003–4008.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gheddouchi S, Mokhtari-Soulimane N,
Merzouk H, Bekhti F, Soulimane F, Guermouche B, Meziane Tani A and
Narce M: Low SOD activity is associated with overproduction of
peroxynitrite and nitric oxide in patients with acute coronary
syndrome. Nitric Oxide. 49:40–46. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Koppenol WH, Moreno JJ, Pryor WA,
Ischiropoulos H and Beckman JS: Peroxynitrite, a cloaked oxidant
formed by nitric oxide and superoxide. Chem Res Toxicol. 5:834–842.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huie RE and Padmaja S: The reaction of no
with superoxide. Free Radic Res Commun. 18:195–199. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Beckman JS and Koppenol WH: Nitric oxide,
superoxide, and peroxynitrite: The good, the bad, and ugly. Am J
Physiol. 271:C1424–C1437. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ischiropoulos H: Biological selectivity
and functional aspects of protein tyrosine nitration. Biochem
Biophys Res Commun. 305:776–783. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Crow JP and Ischiropoulos H: Detection and
quantitation of nitrotyrosine residues in proteins: In vivo marker
of peroxynitrite. Methods Enzymol. 269:185–194. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han WJ, Shi XR and Nuttall A:
Noise-induced nitrotyrosine increase and outer hair cell death in
guinea pig cochlea. Chin Med J (Engl). 126:2923–2927.
2013.PubMed/NCBI
|
|
10
|
Zhang J, Chen X, Wu J and Han W: Detection
of guinea pigs' hearing functions before and after white noise
exposure. Chin J Otol. 31:141–144. 2015.
|
|
11
|
Konishi K, Yamane H, Iguchi H, Takayama M,
Nakagawa T, Sunami K and Nakai Y: Local substances regulating
cochlear blood flow. Acta Otolaryngol Suppl. 538:40–46.
1998.PubMed/NCBI
|
|
12
|
Hess A, Bloch W, Huverstuhl J, Su J,
Stennert E, Addicks K and Michel O: Expression of inducible nitric
oxide synthase (iNOS/NOS II) in the cochlea of guinea pigs after
intratympanical endotoxin-treatment. Brain Res. 830:113–122. 1999a.
View Article : Google Scholar
|
|
13
|
Kuhn DM, Sakowski SA, Sadidi M and Geddes
TJ: Nitrotyrosine as a marker for peroxynitrite-induced
neurotoxicity: The beginning or the end of the end of dopamine
neurons? J Neurochem. 89:529–536. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shi X, Ren T and Nuttall AL: Nitric oxide
distribution and production in the guinea pig cochlea. Hear Res.
153:23–31. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Radi R, Denicola A, Alvarez B,
Ferrer-Sueta G and Rubbo H: Nitric Oxide. Ignarro L: Academic
Press; San Diego; pp. 57–82. 2000, View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamashita D, Jiang HY, Schacht J and
Miller JM: Delayed production of free radicals following noise
exposure. Brain Res. 1019:201–209. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
van Campen LE, Murphy WJ, Franks JR,
Mathias PI and Toraason MA: Oxidative DNA damage is associated with
intense noise exposure in the rat. Hear Res. 164:29–38. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brown GC: Nitric oxide as a competitive
inhibitor of oxygen consumption in the mitochondrial respiratory
chain. Acta Physiol Scand. 168:667–674. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pisoschi AM and Pop A: The role of
antioxidants in the chemistry of oxidative stress: A review. Eur J
Med Chem. 97:55–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamane H, Nakai Y, Takayama M, Konishi K,
Iguchi H, Nakagawa T, Shibata S, Kato A, Sunami K and Kawakatsu C:
The emergence of free radicals after acoustic trauma and strial
blood flow. Acta Otolaryngol Suppl. 519:87–92. 1995. View Article : Google Scholar : PubMed/NCBI
|