Introduction
Genetic therapy is a prospective strategy for many
genetic disorders and diseases. For instance, cystic fibrosis is an
important inherited disease in which a specific membrane molecule,
the cystic fibrosis transmembrane conductance regulator (CFTR), is
absent or not functional (1). The
CFTR is a chloride channel and responsible for the chloride
concentration and viscosity of secretions (1). However, there remains to be a need for
safe and efficient gene therapy carrier systems for different cell
types. Recently, a novel carrier system was developed (2) and implemented for the delivery of drugs
or plasmids to human macrophages (3).
A corncob-like microparticle composed of amorphous silica
nanoparticles was created and loaded with a gene construct for
luciferase. Those particles could serve as a gene delivery system
to phagocytosing cells in vitro and in vivo; notably,
it was demonstrated that the particles were engulfed by
professional phagocytes, alveolar macrophages. Days after
engulfment of the microparticles, the alveolar macrophages were
confirmed to be transfected with luciferase (2). Thus macrophages, as the primary
phagocytes, may even engulf these relatively large microparticles
of 10 µm in length and 3 µm in width. However, a prevailing issue
was whether non-phagocytotic cells could also exhibit carrier
uptake. It was therefore investigated in the current study whether
the carrier system could be engulfed by epithelial cells. For the
current study the human epithelial cell line A549 was selected.
This cell line was derived from a lung tumour and reflects
properties of the alveolar epithelial type II cells, meaning that
the cells grow to a confluent layer in vitro (4). In the present study, to confirm the
epithelial phenotype of A549 cells, a freeze-fracture electron
microscopy technique was employed. Overall the aim of the present
study was to clarify whether these non-phagocytic A549 cells could
engulf relatively large microrod carrier systems comprised of
microparticles. Therefore, to observe the fate of the microrods
within the alveolar epithelial cells, different imaging methods
were applied: fluorescence microscopy and second confocal laser
scanning microscopy. In addition, the viability of the A549 cells
following microrod incubation for different times was investigated
using the MTT assay.
Materials and methods
Cells and materials
The human epithelial cell line A549 was obtained
from Merck KGaA, Darmstadt, Germany. Cells were cultivated at 37°C
in a humidified atmosphere with 5% CO2 in RPMI-1640
medium with 10% fetal bovine serum (Merck KGaA), 100 units
ml−1 penicillin and 100 µg ml−1 streptomycin.
The microparticles were produced from amorphous silica
nanoparticles (Polysciences GmbH, Eppelheim, Germany) as described
previously (2) and had a length of 10
µm and diameter of 3 µm. The microparticles were suspended in aqua
dest distilled water, and the number of particles per µl were
determined by the use of a Neubauer counting chamber. A total of
2×106 particles were incubated with 1×105
cells to establish a ratio of 20:1. The incubation times with the
microparticles were 3, 6, 8, 24 and 48 h.
Fluorescence and confocal laser
scanning microscopy
The living cells incubated with microrods were
analysed by fluorescence microscopy (Carl Zeiss AG, Oberkochen,
Germany) and confocal laser scanning microscopy (VTinfinity,
VisiTech, UK), as previously described for mouse cardiac
ventricular myocytes (5). Cell
borders were labelled using the membrane dye CellMask Deep Red
according to the instructions of the supplier (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). All microscopy examinations
were performed at room temperature (20–22°C).
Cell viability
For an MTT assay, A549 cells were cultured in
96-well plates with 200 µl RPMI-1640 medium per well. Each well
contained 1×104 cells and 0 (control), 1×104
or 1×105 microrods. Following incubation for 1, 3, 6 and
24 h the cells were washed once with sterile phosphate-buffered
saline (PBS). The cells were then incubated in a humidified
atmosphere of 5% CO2, 95% air at 37°C with 5 mg/ml MTT
(Merck KGaA) dissolved in PBS buffer at 1:10 ratio under gentle
shaking for 4 h. Removal of the MTT reagent was followed by the
addition of 100 µl dimethyl sulphoxide and incubation for 20 min in
the humidified atmosphere of 5% CO2, 95% air at 37°C to
dissolve the formazan crystals. To determine cell viability, the
absorbance at 550 nm was measured using a microplate
spectrophotometer. For each concentration and time point, triplets
were used.
Freeze-fracture and electron
microscopy
For freeze-fracture analysis, A549 cells were
cultured on poly-L-lysine coated glass coverslips. The confluent
layer was fixed with 2% paraformaldehyde at room temperature for 5
min. Incubation in 30% glycerol in Soerensen's phosphate buffer
(0.15 M, pH 7.4) as pre-vitrificational cryoprotection was
performed at 4°C for 30 min. Small pieces of the glass coverslips
were mounted headfirst onto the flat top of copper specimen
carriers (Baltic-preparation, Niesgrau, Germany) and subsequently
plunge-frozen in nitrogen-cooled liquid ethane using a
cryopreparation chamber (Leica Microsystems GmbH, Wetzlar,
Germany). Frozen samples were mounted onto a nitrogen-cooled double
replica table and inserted into a BAF060 freeze-fracture device
(Leica Microsystems GmbH). Freeze-fracturing was performed by
cracking the double carrier open at −162°C and 1×10−7
mbar pressure. Fractured samples were coated with a 1-nm pre-carbon
coat applied at a shadowing angle of 90°, a 1-nm platinum-carbon
coat applied at 60° and a second carbon coat of 20 nm applied at
90° (carbon coat: carbon rods 3×50 mm, carbon/platinum coat: carbon
rods 2×20 mm, platinum inlets 1.5×2 mm; Leica Microsystems GmbH).
The frozen replicas were stabilized on a gold index grid (Plano
GmbH, Wetzlar, Germany) using a drop of 0.5% Lexan polycarbonate
plastic dissolved in dichloroethane (DCE; Acros Organics; Thermo
Fisher Scientific, Inc.) (6). To
evaporate the DCE and consolidate the Lexan, the assembly of
carrier-sample-replica-Lexan-grid was incubated at −20°C for 16 h.
Subsequently, the samples were thawed at room temperature and the
carriers were removed. Digestion of the cell samples was performed
under agitation in SDS-digestion buffer (2.5% SDS, 10 mM Tris-HCl,
pH 8.9) at 60°C for 27 h. Prior to electron microscopy analysis,
the Lexan film was resolved and removed by dipping the replica in
DCE. Analysis was performed in a FEI Technai G2 transmission
electron microscope (Thermo Fisher Scientific, Inc.) at 100 kV.
Pictures were taken with an 8-bit camera at an image size of 1.42
megapixels (Olympus MegaView III; Olympus Soft Imaging Solutions
GmbH, Münster, Germany).
Results
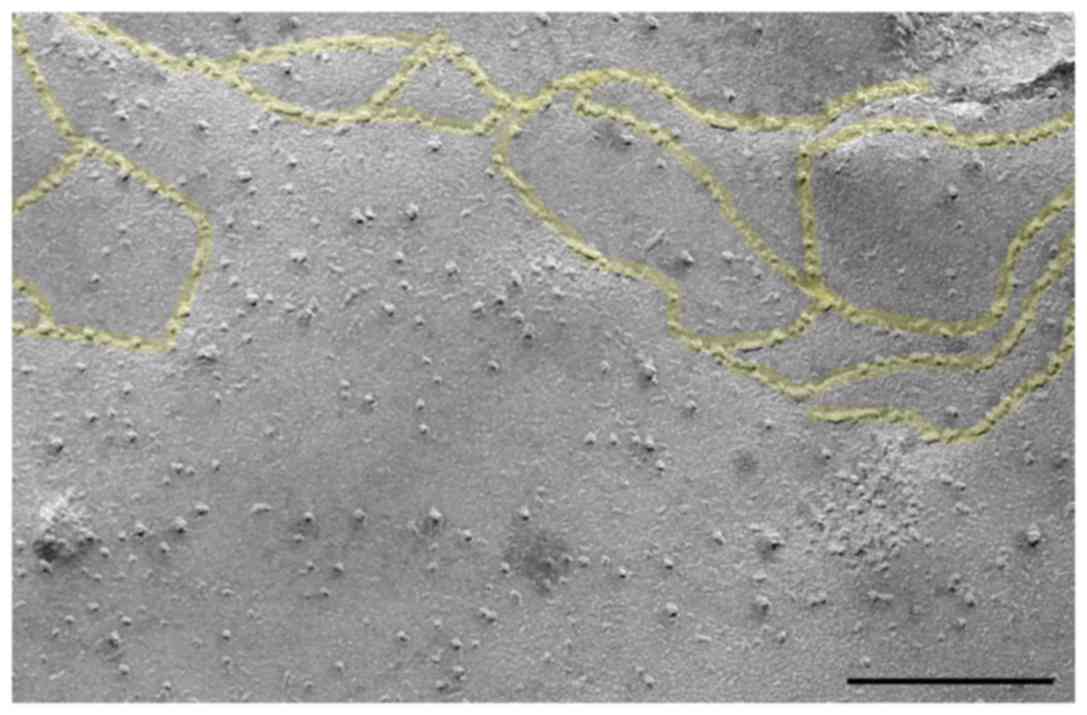
Confirmation of epithelial cell
character
It was uncertain whether the microrods are able to
enter epithelial cells. The A549 cell line used is established as
an epithelial cell line, which was supported by the freeze-fracture
analysis (Fig. 2). In this
representative image, distinct tight junctions are apparent.
Effects on viability
From the MTT assay, cell viability at a 1:1 ratio of
cells to microparticles was 101.9±0.9% after 1 h, 99.9±3.7% after 3
h, 103.6±1.5% after 6 h, 95.9±5.6% after 24 h, and at a 10:1 ratio
was 103.7±5.1% after 1 h, 94.0±3.2% after 3 h, 90.3±2.5% after 6 h
and 83.6±2.1% after 24 h (data not shown).
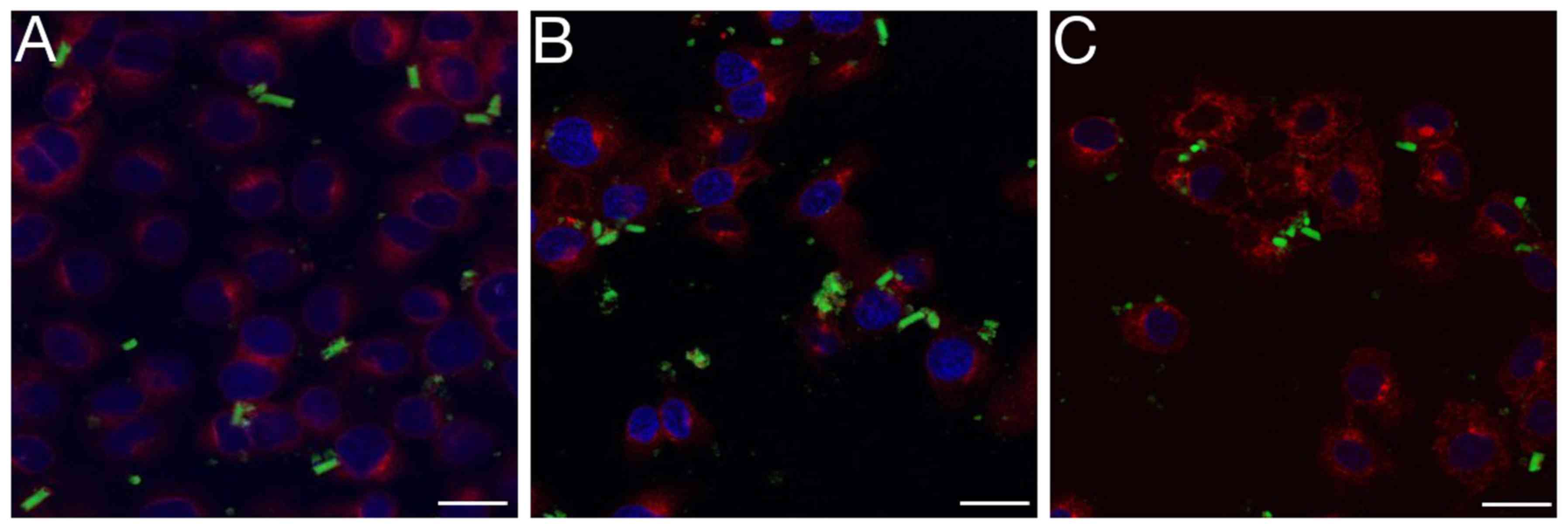
Imaging
The fluorescence microscopy provided visualisation
of the epithelial cells following co-incubation with the microrods
at different time points (Fig. 3).
After 8 h, microparticles remained extracellular (Fig. 3A). After 24 h, the majority of the
rods had started to disaggregate (Fig.
3B). After 48 h, nearly all of the rods appeared to have
disaggregated, and nanoparticles or conglomerates of nanoparticles
appeared to be intracellular (Fig.
3C). Through confocal analysis, this process could be observed
in greater detail and higher resolution (Fig. 4). After 1 h, attachments of the rods
to the cells were observed (Fig. 4A and
B). After 3 and 6 h (Fig. 4C and
D), disaggregated nanoparticles were observed within the cells.
After 48 h (Fig. 4E and F), many
epithelial cells contained nanoparticles.
Discussion
The intention of this short report was to highlight
that this novel drug delivery system may not only reach macrophages
but also enter epithelial cells. The corncob-like microrods have
previously been reported to be engulfed by macrophages and to
successfully transfect macrophages (2); however, due to their dimensions, they
were considered incapable of entering epithelial cells. The
epithelial cells used were A549 cells. These cells were originally
derived from a lung carcinoma and share many features with type II
alveolar epithelial cells (3). In
particular, the cells are characterised by fast replication and
confluential growth (4). To confirm
the epithelial character of these cells, a freeze-fracture
technique was employed presently, in order to visualize tight
junctions, the presence of which is characteristic for epithelial
cells and tissues. The toxic effects of the microrods were
generally excluded by determining the viability of the cells.
However, a notable reduction of viability (<90%) was observed
after 1 day of incubation at a particle-to-cell ratio of 10:1.
Conventional fluorescence and confocal laser scanning microscopy
were used to describe the transitional process undertaken by the
microrods and provided spatial and temporal resolution. Following
their initial adhesion to the A549 cells, the rods disaggregated to
silica nanoparticles and these appeared to penetrate the cell
membranes and enter the cytoplasm. The A549 cells are a useful cell
line since they possess some remnants of alveolar type II cells,
including the capacity for the synthesis of lamellar bodies
(4). These cells may possibly also
have certain endocytotic properties. Admittedly, inhibitors of
specific endocytosis pathways that could have detected phagocytotic
mechanisms (7) were not tested.
A potential application of this microrod-based
approach is the targeting of human bronchial and intestinal cells.
In this regard the rods could be used to carry transcripts for a
range of genetic disorders. One example would be the CFTR to rescue
hereditary mutation in this molecule. This may be a viable option
to treat patients with cystic fibrosis, as a common hereditary
disease. A long-term aim is to reach epithelial basal cells or stem
cells, in order to further target and potentially cure genetic
disorders including cystic fibrosis.
Acknowledgements
The authors thank Ms Franziska Müller (Institute of
Anatomy and Cell Biology, Saarland University, Homburg/Saar,
Germany) for creating the graphical abstract and Ms Ann Soether
(formerly Institute of Anatomy and Cell Biology, Saarland
University) for performing language editing.
Funding
The current study was supported by Saarland
University, Saarbrücken, Germany (Anschubförderung; awarded to TT)
and the Saarland government (Landesforschungsförderprogramm;
awarded to TF). The authors acknowledge financial support by the
German Research Foundation and the Saarland, who funded the
freeze-fracture unit.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TT, MS and TF planned the study. TF prepared the
microrods and performed the MTT assay. CM, AB and AG performed the
freeze-fracture, electron microscopy and interpretation of the
corresponding results. AG performed the cell culture and
fluorescence imaging. PL and AG performed the laser scanning
confocal microscopy. TT was a major contributor in the writing of
the manuscript. All authors interpreted data, and read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Armstrong DK, Cunningham S, Davies JC and
Alton EW: Gene therapy in cystic fibrosis. Arch Dis Child.
99:465–468. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Möhwald M, Pinnapireddy SR, Wonnenberg B,
Pourasghar M, Jurisic M, Jung A, Fink-Straube C, Tschernig T,
Bakowsky U and Schneider M: Aspherical, Nanostructured
Microparticles for Targeted Gene Delivery to Alveolar Macrophages.
Adv Healthc Mater. (In press).
|
|
3
|
Kohler D, Schneider M, Krüger M, Lehr C-M,
Möhwald H and Wang D: Template-assisted polyelectrolyte
encapsulation of nanoparticles into dispersible, hierarchically
nanostructured microfibers. Adv Mater. 23:1376–1379. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lieber M, Smith B, Szakal A, Nelson-Rees W
and Todaro G: A continuous tumor-cell line from a human lung
carcinoma with properties of type II alveolar epithelial cells. Int
J Cancer. 17:62–70. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Oberhofer M, Tian Q, Ruppenthal S, Wegener
S, Reil JC, Körbel C, Hammer K, Menger M, Neuberger HR, Kaestner L,
et al: Calcium dysregulation in ventricular myocytes from mice
expressing constitutively active Rac1. Cell Calcium. 54:26–36.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rash JE, Duffy HS, Dudek FE, Bilhartz BL,
Whalen LR and Yasumura T: Grid-mapped freeze-fracture analysis of
gap junctions in gray and white matter of adult rat central nervous
system, with evidence for a “panglial syncytium” that is not
coupled to neurons. J Comp Neurol. 388:265–292. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Diler E, Schwarz M, Nickels R, Menger MD,
Beisswenger C, Meier C and Tschernig T: Influence of external
calcium and thapsigargin on the uptake of polystyrene beads by the
macrophage-like cell lines U937 and MH-S. BMC Pharmacol Toxicol.
15:162014. View Article : Google Scholar : PubMed/NCBI
|