Introduction
Rheumatoid arthritis (RA) is an autoimmune disease
that causes chronic inflammation in synovial tissues. Hyperplasia
of synovial tissue leads to the formation of pannus, which invades
joint cartilage and bone, resulting in joint destruction. Previous
reports have indicated that a number of features of transformed
long-lived cells are observed in the hyperplastic synovial tissues
of patients with RA, including oncogene expression, resistance to
apoptosis and the presence of somatic mutations (1-3).
Several explanations for the resistance of RA-FLS to apoptosis have
been suggested, including deregulation of the Bcl-2 family of
proteins critical to intrinsic pathway regulation, deregulation of
the nuclear factor (NF)-κB signaling pathway, p53 mutations and a
low expression of PUMA, found in the RA synovium and FLS, which
provides an explanation for the lack of p53-induced FLS apoptosis
(4).
Tumor necrosis factor (TNF)-like ligand 1A
(TL1A)/TNFSF15, a member of the TNF superfamily, is expressed by
endothelial cells (5), macrophages
(6,7),
T cells (8,9), monocytes (10,11),
dendritic cells (11), chondrocytes
(12) and synovial fibroblasts
(12), and contributes to the
pathogenesis of cancer and autoimmune diseases via the apoptotic,
stress, mitogenic and inflammation pathways by binding to death
receptor 3 (DR3) and decoy receptor 3 (DcR3) (5,13).
Previous studies have reported that the expression of TL1A is
increased in the synovial fluid and serum from patients with RA
(12,14), and that TL1A increases the production
of interleukin (IL)-6 on rheumatoid fibroblast-like synoviocytes
(RA-FLS) (15). In a previous in
vivo study, it was demonstrated that TL1A treatment increased
the severity of arthritis and destruction of bone in a
collagen-induced arthritis mouse model of RA (12).
DcR3/TR6/M68/TNFRSF6b, a member of the TNF receptor
superfamily, binds to three ligands, Fas ligand (FasL), LIGHT and
TL1A, which are members of the TNF superfamily (16). The overexpression of DcR3 may benefit
tumors by enabling them to avoid the cytotoxic and regulatory
effects of FasL (17,18), LIGHT (19) and TL1A (5). In our previous studies, it was
demonstrated that DcR3 is expressed in RA-FLS (20), and that DcR3 binds to TL1A expressed
on RA-FLS, resulting in the negative regulation of cell
proliferation induced by inflammatory cytokines (21). The expression profiles of genes
regulated by DcR3 in RA-FLS were further revealed, which were
obtained through the use of a cDNA microarray (22). Based on these profiles, it was
suggested that DcR3-TL1A signaling is involved in the pathogenesis
of RA (23-25).
Although the gene expression profiles regulated by
DcR3 were revealed in our previous study, how TL1A, one of the
ligands of DcR3, contributes to the pathogenesis of RA remains to
be fully elucidated. As the functions of TL1A are diverse, it was
hypothesized that TL1A controls the expression of genes potentially
involved in the pathogenesis of RA.
In the present study, a search was performed to
identify those genes whose expression in RA-FLS is regulated by
TL1A through use of a cDNA microarray. The gene expression profiles
revealed a series of genes that may serve a significant role in the
pathogenesis of RA in the TL1A-DcR3/DR3 signaling pathway.
Materials and methods
Isolation and culture of synovial
fibroblasts
RA-FLS were obtained from four patients (samples
1-4) with RA who fulfilled the 1987 criteria of the American
College of Rheumatology (formerly, the American Rheumatism
Association) (26) during total knee
replacement surgery. The patients were four women aged 73.0±11.2
years old. Their C-reactive protein levels and erythrocyte
sedimentation rates were 2.04±2.16 mg/dl and 60.0±22.1 mm/h,
respectively. In terms of drug therapy for RA, two patients were
administered oral methotrexate (MTX; average dose, 3.00±1.41
mg/week), one was administered salazosulfapyridine (1 g/day), and
one was administered mizoribine (150 mg/day). Prednisolone (PSL)
was used in the treatment of all four patients (average dose,
3.63±2.14 mg/day). The patients had never been treated with
biological disease-modifying anti-rheumatic drugs or Janus kinase
inhibitors.
Synovial samples were collected from the patients,
who provided informed written consent to their involvement in the
study in accordance with the World Medical Association Declaration
of Helsinki Ethical Principles for Medical Research Involving Human
Subjects. The protocol, including consent procedures, was approved
by the Ethics Committee of Kobe University Graduate School of
Health Sciences (Kobe, Japan; approval no. 308). The tissue
specimens were minced and digested in Dulbecco's modified Eagle's
medium (DMEM; Merck KGaA, Darmstadt, Germany) containing 0.2%
collagenase (Merck KGaA) for 2 h at 37˚C with 5% CO2.
The dissociated cells were cultured in DMEM supplemented with 10%
fetal bovine serum (Merck KGaA) and 100 U/ml of
penicillin/streptomycin (Meiji Seika Pharma Co., Ltd., Tokyo,
Japan). Following incubation overnight and the removal of
non-adherent cells, the adherent cells were further incubated in
fresh medium. All experiments were performed using cells from
passages 3-4(20).
RNA extraction
Four individual cell lines (samples 1-4) of primary
cultured RA-FLS (2x106 cells/well) were incubated with
1.0 µg/ml of recombinant human TL1A protein (R&D Systems, Inc.,
Minneapolis, MN, USA) or were left untreated with OPTI-MEM medium
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 12 h at 37˚C
with 5% CO2. Following incubation, RNA was extracted
with QIAshredder (Qiagen GmbH, Hilden, Germany) and RNeasy Mini kit
(Qiagen GmbH) according to the manufacturer's protocol. The
extraction of total RNA was performed for each sample
separately.
Gene expression profiling and data
analysis
Gene expression was detected by microarray assay
(Human Genome U133 Plus 2.0, GeneChip® 3' Expression
Array; Thermo Fisher Scientific, Inc.). The labeling of RNA probes,
hybridization and washing were performed according to the
manufacturer's protocol.
Avadis 3.3 Prophetic software (Strand Life Sciences,
Bangalore, India) was used for statistical analysis (27). Differentially expressed genes were
extracted using a paired t-test with P<0.05 considered to
indicate a statistically significant difference and fold-change
>1.4, and ordered into hierarchical clusters using the Euclidean
algorithm as the distance measure and the complete algorithm as the
linkage method. Values are expressed as the mean ± standard
deviation unless otherwise indicated.
Results
Microarray analysis for gene
expression profiling of RA-FLS stimulated by TL1A
The microarray analysis performed in the present
study (Human Genome U133 Plus 2.0, GeneChip® 3'
Expression Array) detected the expression of 54,613 genes. The
entire microarray data obtained were deposited in the NCBI Gene
Expression Omnibus (GEO) and are accessible through GEO series
accession no. GSE118958 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=gse118958).
The microarray analysis revealed that TL1A
upregulated or downregulated the expression of various genes in
RA-FLS. The NCBI UniGene database (https://www.ncbi.nlm.nih.gov/UniGene/clust.cgi?ORG=Hs&CID=55682)
was used to identify the gene names, with gene symbols representing
abbreviations of the gene names. The fold change is the ratio of
each gene expression in the TL1A-stimulated group compared with
that in the control group. Among the 100 most differentially
upregulated genes by TL1A, 67 genes were annotated in the database,
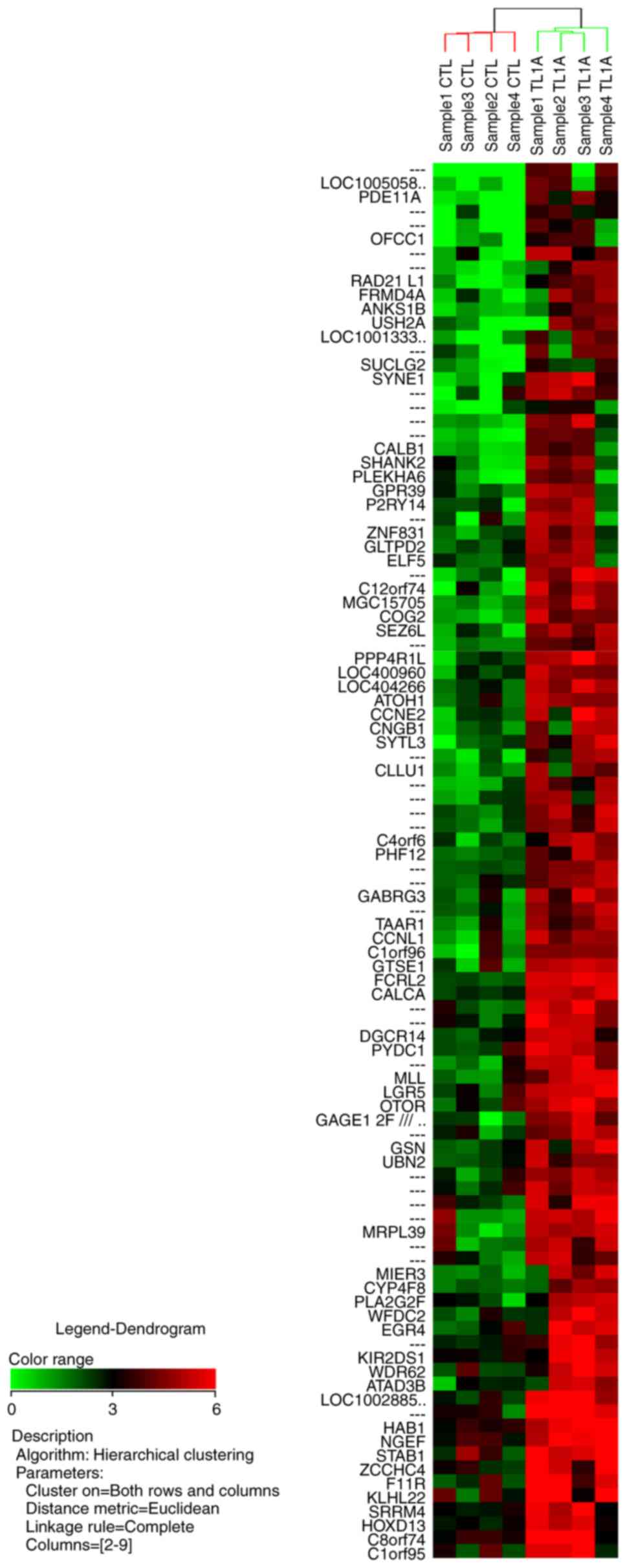
and 21 of these 67 genes upregulated by TL1A are shown in Table I. Gene annotations of 58 of the 100
most differentially downregulated genes by TL1A were also annotated
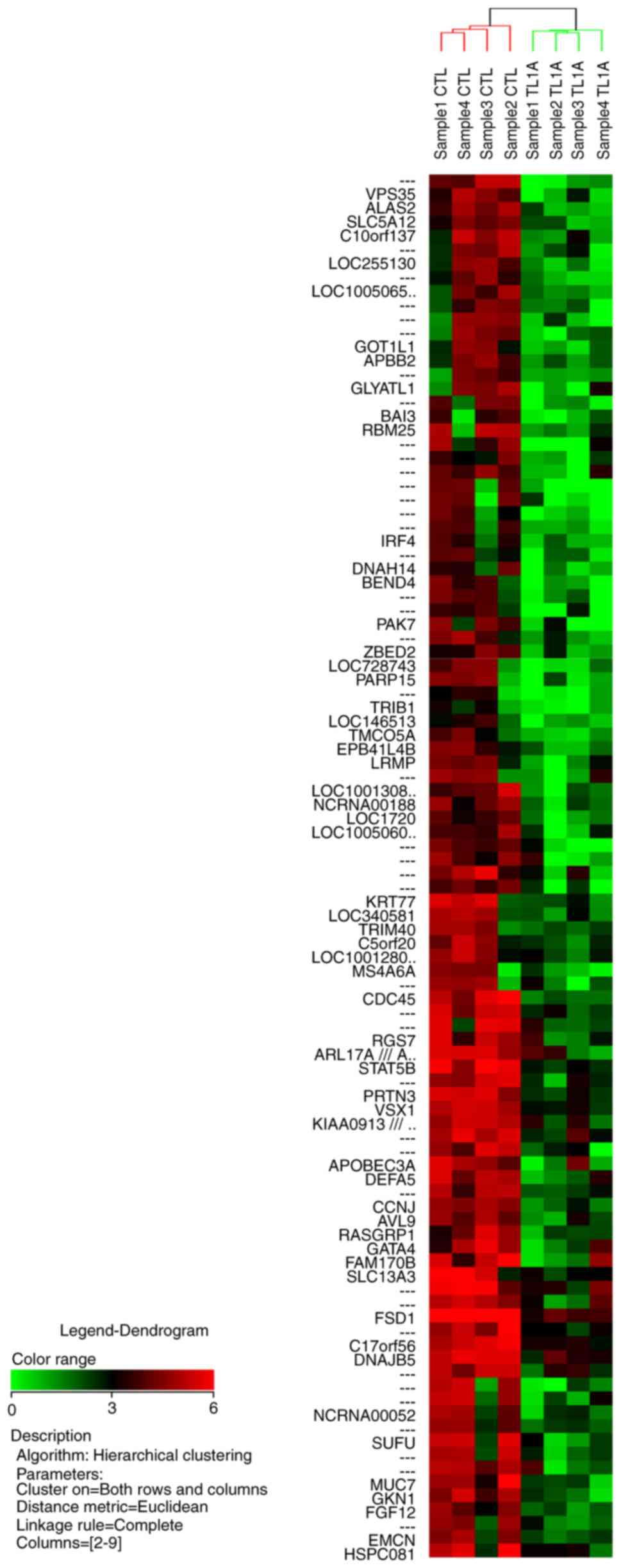
in the database, and 21 of the 58 genes downregulated by TL1A are
shown in Table II. The results of
hierarchical clustering analysis for the 100 most upregulated genes
and the 100 most downregulated genes are illustrated in Figs. 1 and 2,
respectively.
 | Table IList of the 21 genes upregulated by
tumor necrosis factor-like ligand 1A. |
Table I
List of the 21 genes upregulated by
tumor necrosis factor-like ligand 1A.
| Gene symbol | Fold-change | P-value | Gene name |
|---|
| SYNE1 | 15.3 | 0.013391 | Spectrin
repeat-containing, nuclear envelope 1 |
| PDE11A | 14.40000186 | 0.013724 | Phosphodiesterase
11A |
| MGC15705 | 12.6299996 | 0.006738 | Hypothetical
protein MGC15705 |
| COG2 | 12.61445783 | 0.016001 | Component of
oligomeric golgi complex 2 |
| FCRL2 | 11.97633136 | 0.000003 | Fc receptor-like
2 |
| RAD21L1 | 11.82758621 | 0.007302 | RAD21-like 1 (S.
pombe) |
| PPP4R1L | 10.1975316 | 0.005913 | Protein phosphatase
4, regulatory subunit 1-like |
| MIER3 | 9.72072035 | 0.048129 | Mesoderm induction
early response 1, family member 3 |
| LOC100505801 | 9.711538484 | 0.029304 | Hypothetical
LOC100505801 |
| C12orf74 | 9.576924282 | 0.008377 | Chromosome 12 open
reading frame 74 |
| CCNE2 | 9.118012422 | 0.039656 | Cyclin E2 |
| LOC100288507 | 9.064056744 | 0.009929 | Hypothetical
protein LOC100288507 |
| USH2A | 8.986842105 | 0.048673 | Usher syndrome 2A
(autosomal recessive, mild) |
| SEZ6L | 8.7578125 | 0.002512 | Seizure related 6
homolog (mouse)-like |
| LOC100133308 | 8.376812174 | 0.026850 | Ras suppressor
protein 1 pseudogene |
| MLL | 8.357512953 | 0.007109 | Myeloid/lymphoid or
mixed-lineage leukemia (trithorax homolog, Drosophila) |
| SYTL3 | 8.348148148 | 0.038552 | Synaptotagmin-like
3 |
|
GAGE12F///GAGE12G/// | 8.124137931 | 0.018128 | G antigen 12F///G
antigen 12G///G antigen 12I///G antigen 5/// |
|
GAGE12I///GAGE5///GAGE7 | | | G antigen 7 |
| FRMD4A | 7.891089109 | 0.044768 | FERM
domain-containing 4A |
| LOC404266 | 7.725807116 | 0.003327 | Hypothetical
LOC404266 |
| PYDC1 | 5.42662116 | 0.006955 | PYD (pyrin
domain)-containing 1 |
 | Table IIList of the 21 genes downregulated by
tumor necrosis factor-like ligand 1A. |
Table II
List of the 21 genes downregulated by
tumor necrosis factor-like ligand 1A.
| Gene symbol | Fold-change | P-value | Gene name |
|---|
| CDC45 | 0.08 | 0.006927 | Cell division cycle
45 homolog (S. cerevisiae) |
| STAT5B | 0.10 | 0.019873 | Signal transducer
and activator of transcription 5B |
| BEND4 | 0.11 | 0.026734 | BEN
domain-containing 4 |
| ALAS2 | 0.11 | 0.004251 | Aminolevulinate,
δ-, synthase 2 |
| LOC728743 | 0.11 | 0.031562 | Similar to
GLI-Kruppel family member HKR1 |
| LOC100130815 | 0.12 | 0.037456 | Hypothetical
LOC100130815 |
| LOC255130 | 0.12 | 0.020509 | Hypothetical
LOC255130 |
| RBM25 | 0.12 | 0.032102 | RNA-binding motif
protein 25 |
| PARP15 | 0.12 | 0.028820 | Poly (ADP-ribose)
polymerase family, member 15 |
| SUFU | 0.12 | 0.025031 | Suppressor of fused
homolog (Drosophila) |
| MUC7 | 0.12 | 0.045462 | Mucin 7,
secreted |
| VPS35 | 0.12 | 0.013976 | Vacuolar protein
sorting 35 homolog (S. cerevisiae) |
| GKN1 | 0.13 | 0.013159 | Gastrokine 1 |
| KRT77 | 0.13 | 0.030364 | Keratin 77 |
| GOT1L1 | 0.14 | 0.047977 |
Glutamic-oxaloacetic transaminase 1-like
1 |
| DEFA5 | 0.14 | 0.005483 | Defensin, α5,
Paneth cell-specific |
| SLC13A3 | 0.14 | 0.041769 | Solute carrier
family 13 (sodium-dependent dicarboxylate transporter), member
3 |
| C10orf137 | 0.15 | 0.030915 | Chromosome 10 open
reading frame 137 |
| PRTN3 | 0.15 | 0.001312 | Proteinase 3 |
| RASGRP1 | 0.15 | 0.038526 | RAS
guanyl-releasing protein 1 (calcium and DAG-regulated) |
| IRF4 | 0.22 | 0.027305 | Interferon
regulatory factor 4 |
Functional annotation
The 100 genes most regulated by TL1A were
significantly classified into 14 categories registered in the
Database for Annotation, Visualization and Integrated Discovery
bioinformatics database (https://david.ncifcrf.gov/) according to their
biological functions; alternative splicing, splice variant,
coenzyme A, regulation of cytokine production, Cyclin; N-terminal,
Cyclin, Pleckstrin homology-type, CYCLIN, transcription from RNA
polymerase II promoter, cyclin, compositionally biased
region:Ser-rich, postsynaptic membrane, positive regulation of
transcription from RNA polymerase II promoter, and synapse. The
regulated genes belonging to each cluster are listed in Table III.
 | Table IIIFunctions of the 100 most regulated
genes classified into 14 categories with statistical
significance. |
Table III
Functions of the 100 most regulated
genes classified into 14 categories with statistical
significance.
| Term | P-value | Genes |
|---|
| Alternative
splicing | 0.004007 | KIAA0913, ELF5,
C17ORF56, FCRL2, PDE11A, FGF12, CNGB1, LGR5, |
| | GLYATL1, C10ORF137,
CCNE2, PPP4R1L, ATAD3B, GSN, MIER3, |
| | KLHL22, MRPL39,
USH2A, ANKS1B, BEND4, CCNJ, AVL9, CCNL1, |
| | TRIM40, SEZ6L,
UBN2, PARP15, LRMP, WFDC2, PLA2G2F, EMCN, |
| | RAD21L1, DNAH14,
OFCC1, EPB41L4B, SUFU, CALCA, RASGRP1, |
| | MS4A6A, C1ORF96,
RBM25, NGEF, MLL, TMCO5A, PHF12, SHANK2, |
| | VSX1, SYNE1,
ARL17B, ARL17A, WDR62, STAB1, RGS7, SLC13A3, |
| | SYTL3, IRF4, APBB2,
DNAJB5, SLC5A12 |
| Splice variant | 0.00425 | KIAA0913, ELF5,
C17ORF56, FCRL2, PDE11A, FGF12, CNGB1, LGR5, |
| | GLYATL1, C10ORF137,
CCNE2, PPP4R1L, ATAD3B, GSN, MIER3, |
| | KLHL22, MRPL39,
USH2A, ANKS1B, BEND4, CCNJ, AVL9, CCNL1, |
| | TRIM40, SEZ6L,
UBN2, PARP15, LRMP, WFDC2, PLA2G2F, EMCN, |
| | RAD21L1, DNAH14,
OFCC1, EPB41L4B, SUFU, CALCA, RASGRP1, |
| | MS4A6A, C1ORF96,
RBM25, NGEF, MLL, TMCO5A, PHF12, SHANK2, |
| | VSX1, SYNE1,
ARL17B, ARL17A, WDR62, STAB1, RGS7, SLC13A3, |
| | SYTL3, IRF4, APBB2,
DNAJB5, SLC5A12 |
| Coenzyme A | 0.011118 | SAT1, ALAS2,
SUCLG2 |
| Regulation of
cytokine production | 0.013645 | CALCA, STAT5B,
GATA4, IRF4, PYDC1 |
| Cyclin,
N-terminal | 0.01396 | CCNE2, CCNJ,
CCNL1 |
| Cyclin | 0.020121 | CCNE2, CCNJ,
CCNL1 |
| Pleckstrin
homology-type | 0.025198 | ANKS1B, NGEF,
PLEKHA6, FRMD4A, EPB41L4B, APBB2 |
| Cyclin | 0.025934 | CCNE2, CCNJ,
CCNL1 |
| Transcription from
RNA polymerase II promoter | 0.031469 | ATOH1, MLL, ELF5,
GATA4, HOXD13 |
| Cyclin | 0.033221 | CCNE2, CCNJ,
CCNL1 |
| Compositionally
biased region: Ser-rich | 0.036248 | SYNE1, SRRM4,
KIAA0913, FRMD4A, C17ORF56, C5ORF20, UBN2 |
| Postsynaptic
membrane | 0.036429 | ANKS1B, GABRG3,
SYNE1, SHANK2 |
| Positive regulation
of transcription from RNA polymerase II promoter | 0.04068 | ATOH1, MLL, STAT5B,
GATA4, HOXD13, IRF4 |
| Synapse | 0.042545 | ANKS1B, GABRG3,
RAD21L1, SYNE1, APBB2, SHANK2 |
Discussion
Genome-wide gene expression cDNA microarrays provide
a useful way of investigating the pathophysiology of a variety of
diseases, including tumors (28-30),
immune-mediated diseases (31,32), and
inflammatory diseases (33-35).
Using microarrays, our previous study revealed the expression
profiles of genes in RA-FLS regulated by DcR3(22). Subsequently, based on that profile,
the significance of the regulation of IL-12B p40(23), tryptophan hydroxylase 1(24), and centrosomal protein 70 kDa
(25) by DcR3 in RA-FLS was
investigated in detail.
The present study is the first, to the best of our
knowledge, to demonstrate the expression profiles of genes in
RA-FLS regulated by TL1A. Among the genes in this profile, the
following genes were of note: Spectrin repeat-containing nuclear
envelope 1 (SYNE1), Fc receptor-like 2 (FCRL2), PYD (pyrin
domain)-containing 1 (PYDC1), cell division cycle 45 homolog
(CDC45), signal transducer and activator of transcription 5B
(STAT5B), and interferon regulatory factor 4 (IRF4), as these genes
were highly regulated by TL1A and belong to major functional
clustering categories.
SYNE1 in RA-FLS was upregulated by TL1A in this gene
expression profile. SYNE1 is a member of the spectrin family that
is expressed in various tissues (36,37). It is
reported to be associated with cytokinesis in HeLa cells (38), and the proliferation and apoptosis of
mesenchymal stem cells (39).
FCRL2 was upregulated in this profile and is a
member of the Fc receptor-like molecules superfamily. It is
predominantly expressed by memory B cells and can influence B-cell
signaling due to having both immunoreceptor tyrosine-based
activation and inhibitory motifs (40-42).
Jackson et al suggested that FCRL2 may serve as a negative
regulator of the memory B cell response (43). FCRL2 has been reported to be expressed
at high levels in B-cell chronic lymphocytic leukemia cells,
affecting disease progression and survival rates (42,44,45), and
is associated with the inflammatory marker and disease activity of
RA (46).
PYDC1 was upregulated in this profile.
PYD-containing proteins have been reported to be involved in the
activation of NF-κB and caspase-1, which regulates the processing
of IL-1β and IL-18, and is associated with inflammation and
apoptosis (47-49).
CDC45 was downregulated in this profile. CDC45
serves a critical role in DNA replication (50), and has been reported to be
overexpressed in cancer cells (51)
and cancer-derived cell lines (52).
The expression of CDC45 is significantly suppressed by the
knockdown of IL-1 receptor-associated kinase 1 in endometrial
carcinoma (53). The expression of
CDC45 is closely associated with proliferating cell populations in
cancer (52).
STAT5B was downregulated in this profile. STATs
regulate gene transcription to influence cellular functions,
including proliferation, apoptosis, differentiation, reproduction
and lipid metabolism, and have biological roles in several
diseases, including autoimmune disease (54-60).
The expression and activity of STATs can contribute to the onset,
progression and severity of RA (61).
IRF4 was downregulated in this profile. IRF4 has
been reported to be an RA risk locus, as identified by GWAS data
analysis (62). IRF4 is an IRF family
member of transcription factors and is associated with the
development and function of immune cells (63). Previous studies have found that IRF4
regulates autoimmunity (63,64). In addition, IRF4 regulates Th17 cell
differentiation and the production of IL-17, which are important
for modulation of autoimmunity, including RA (63,65).
Although neither SYNE1 nor CDC45 are reported to be
associated with RA directly, SYNE1 is associated with cytokinesis,
proliferation and apoptosis, and CDC45 serves a critical role in
the cell cycle of proliferating cells, which are important factors
in the pathogenesis of RA.
The limitations of the present study included its
small sample size and that it examined microarray data only,
without detecting mRNA or protein expression. The results of the
present study revealed a series of genes whose expression is
regulated by TL1A in RA-FLS using microarray analysis, however,
each gene revealed through microarray analysis requires
confirmation one by one with mRNA or protein analysis in a future
study. In addition to the expression analysis of each genes, how
these genes regulated by TL1A in RA-FLS are involved in combination
in the pathogenesis of RA also requires investigation in a future
study.
In conclusion, the present study is the first, to
the best of our knowledge, to report the expression profile of
genes in RA-FLS regulated by TL1A. The data demonstrate that TL1A
may regulate the gene expression of various key molecules in
RA-FLS, thus affecting the pathogenesis of RA, including
proliferation, regulation of B cells and T cells, inflammation, and
cytokine processing. Further investigations of the genes detected
in this profile may provide a deeper understanding of the
pathogenesis and novel targets for the treatment of RA.
Acknowledgements
The authors would like to thank Mrs. Kyoko Tanaka,
Ms. Minako Nagata and Ms. Maya Yasuda (Department of Orthopaedic
Surgery, Kobe University Graduate School of Medicine, Kobe, Japan)
for their technical assistance.
Funding
This study was supported by a Grant-in-Aid for
Scientific Research (KAKENHI; grant nos. 15K10473 and
18K09106).
Availability of data and materials
The datasets generated and analyzed during the
current study are available in the NCBI GEO repository and are
accessible through GEO series accession no. GSE118958, (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=gse118958).
Authors' contributions
KF was involved in conception and design, data
collection and analysis, manuscript writing and final approval of
the manuscript; YM was involved in conception and design, data
collection and analysis, manuscript writing and final approval of
the manuscript. TM was involved in conception and design, data
collection, and final approval of the manuscript. SH was involved
in conception and design, data collection and final approval of the
manuscript. RK was involved in conception and design, data
collection, and final approval of the manuscript.
Ethics approval and consent to
participate
The study protocol, including consent procedures,
was approved by the Ethics Committee of Kobe University Graduate
School of Health Sciences (approval no. 308).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chou CT, Yang JS and Lee MR: Apoptosis in
rheumatoid arthritis-expression of Fas, Fas-L, p53, and Bcl-2 in
rheumatoid synovial tissues. J Pathol. 193:110–116. 2001.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tak PP, Zvaifler NJ, Green DR and
Firestein GS: Rheumatoid arthritis and p53: How oxidative stress
might alter the course of inflammatory diseases. Immunol Today.
21:78–82. 2000.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yamanishi Y, Boyle DL, Rosengren S, Green
DR, Zvaifler NJ and Firestein GS: Regional analysis of p53
mutations in rheumatoid arthritis synovium. Proc Natl Acad Sci USA.
99:10025–10030. 2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bustamante MF, Garcia-Carbonell R,
Whisenant KD and Guma M: Fibroblast-like synoviocyte metabolism in
the pathogenesis of rheumatoid arthritis. Arthritis Res Ther.
19(110)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Migone TS, Zhang J, Luo X, Zhuang L, Chen
C, Hu B, Hong JS, Perry JW, Chen SF, Zhou JX, et al: TL1A is a
TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell
costimulator. Immunity. 16:479–492. 2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kamada N, Hisamatsu T, Honda H, Kobayashi
T, Chinen H, Takayama T, Kitazume MT, Okamoto S, Koganei K, Sugita
A, et al: TL1A produced by lamina propria macrophages induces Th1
and Th17 immune responses in cooperation with IL-23 in patients
with Crohn's disease. Inflamm Bowel Dis. 16:568–575.
2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bamias G, Martin C III, Marini M, Hoang S,
Mishina M, Ross WG, Sachedina MA, Friel CM, Mize J, Bickston SJ, et
al: Expression, localization, and functional activity of TL1A, a
novel Th1-polarizing cytokine in inflammatory bowel disease. J
Immunol. 171:4868–4874. 2003.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Prehn JL, Mehdizadeh S, Landers CJ, Luo X,
Cha SC, Wei P and Targan SR: Potential role for TL1A, the new
TNF-family member and potent costimulator of IFN-gamma, in mucosal
inflammation. Clin Immunol. 112:66–77. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Papadakis KA, Zhu D, Prehn JL, Landers C,
Avanesyan A, Lafkas G and Targan SR: Dominant role for TL1A/DR3
pathway in IL-12 plus IL-18-induced IFN-gamma production by
peripheral blood and mucosal CCR9+ T lymphocytes. J Immunol.
174:4985–4990. 2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cassatella MA, Pereira-da-Silva G, Tinazzi
I, Facchetti F, Scapini P, Calzetti F, Tamassia N, Wei P, Nardelli
B, Roschke V, et al: Soluble TNF-like cytokine (TL1A) production by
immune complexes stimulated monocytes in rheumatoid arthritis. J
Immunol. 178:7325–7333. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Prehn JL, Thomas LS, Landers CJ, Yu QT,
Michelsen KS and Targan SR: The T cell costimulator TL1A is induced
by FcgammaR signaling in human monocytes and dendritic cells. J
Immunol. 178:4033–4038. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang J, Wang X, Fahmi H, Wojcik S, Fikes
J, Yu Y, Wu J and Luo H: Role of TL1A in the pathogenesis of
rheumatoid arthritis. J Immunol. 183:5350–5357. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sethi G, Sung B and Aggarwal BB:
Therapeutic potential of VEGI/TL1A in autoimmunity and cancer. Adv
Exp Med Biol. 647:207–215. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bamias G, Siakavellas SI, Stamatelopoulos
KS, Chryssochoou E, Papamichael C and Sfikakis PP: Circulating
levels of TNF-like cytokine 1A (TL1A) and its decoy receptor 3
(DcR3) in rheumatoid arthritis. Clin Immunol. 129:249–255.
2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ma Z, Wang B, Wang M, Sun X, Tang Y, Li M,
Li F and Li X: TL1A increased IL-6 production on fibroblast-like
synoviocytes by preferentially activating TNF receptor 2 in
rheumatoid arthritis. Cytokine. 83:92–98. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shi G, Wu Y, Zhang J and Wu J: Death decoy
receptor TR6/DcR3 inhibits T cell chemotaxis in vitro and in vivo.
J Immunol. 171:3407–3414. 2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Pitti RM, Marsters SA, Lawrence DA, Roy M,
Kischkel FC, Dowd P, Huang A, Donahue CJ, Sherwood SW, Baldwin DT,
et al: Genomic amplification of a decoy receptor for Fas ligand in
lung and colon cancer. Nature. 396:699–703. 1998.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Tsuji S, Hosotani R, Yonehara S, Masui T,
Tulachan SS, Nakajima S, Kobayashi H, Koizumi M, Toyoda E, Ito D,
et al: Endogenous decoy receptor 3 blocks the growth inhibition
signals mediated by Fas ligand in human pancreatic adenocarcinoma.
Int J Cancer. 106:17–25. 2003.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yu KY, Kwon B, Ni J, Zhai Y, Ebner R and
Kwon BS: A newly identified member of tumor necrosis factor
receptor superfamily (TR6) suppresses LIGHT-mediated apoptosis. J
Biol Chem. 274:13733–13736. 1999.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hayashi S, Miura Y, Nishiyama T, Mitani M,
Tateishi K, Sakai Y, Hashiramoto A, Kurosaka M, Shiozawa S and
Doita M: Decoy receptor 3 expressed in rheumatoid synovial
fibroblasts protects the cells against Fas-induced apoptosis.
Arthritis Rheum. 56:1067–1075. 2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Takahashi M, Miura Y, Hayashi S, Tateishi
K, Fukuda K and Kurosaka M: DcR3-TL1A signalling inhibits
cytokine-induced proliferation of rheumatoid synovial fibroblasts.
Int J Mol Med. 28:423–427. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Fukuda K, Miura Y, Maeda T, Takahashi M,
Hayashi S and Kurosaka M: Decoy receptor 3 regulates the expression
of various genes in rheumatoid arthritis synovial fibroblasts. Int
J Mol Med. 32:910–916. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Fukuda K, Miura Y, Maeda T, Hayashi S and
Kurosaka M: Interleukin12B is upregulated by decoy receptor 3 in
rheumatoid synovial fibroblasts. Mol Med Rep. 13:3647–3652.
2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Maeda T, Miura Y, Fukuda K, Hayashi S and
Kurosaka M: Decoy receptor 3 regulates the expression of tryptophan
hydroxylase 1 in rheumatoid synovial fibroblasts. Mol Med Rep.
12:5191–5196. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Fukuda K, Miura Y, Maeda T, Hayashi S and
Kuroda R: Decoy receptor 3 down-regulates centrosomal protein 70
kDa specifically in rheumatoid synovial fibroblasts. Mod Rheumatol.
28:287–292. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Arnett FC, Edworthy SM, Bloch DA, McShane
DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH and Luthra
HS: The American Rheumatism Association 1987 revised criteria for
the classification of rheumatoid arthritis. Arthritis Rheum.
31:315–324. 1988.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Choi YJ and Yun HK: Transcriptional
profiles of Rhizobium vitis-inoculated and salicylic acid-treated
‘Tamnara’ grapevines based on microarray analysis. J Plant
Biotechnol. 43:37–48. 2016. View Article : Google Scholar
|
|
28
|
Chang YC, Chen TC, Lee CT, Yang CY, Wang
HW, Wang CC and Hsieh SL: Epigenetic control of MHC class II
expression in tumor-associated macrophages by decoy receptor 3.
Blood. 111:5054–5063. 2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Espinosa I, Catasus L, Canet B, D'Angelo
E, Muñoz J and Prat J: Gene expression analysis identifies two
groups of ovarian high-grade serous carcinomas with different
prognosis. Mod Pathol. 24:846–854. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Khan J, Simon R, Bittner M, Chen Y,
Leighton SB, Pohida T, Smith PD, Jiang Y, Gooden GC, Trent JM and
Meltzer PS: Gene expression profiling of alveolar rhabdomyosarcoma
with cDNA microarrays. Cancer Res. 58:5009–5013. 1998.PubMed/NCBI
|
|
31
|
Whitney LW, Becker KG, Tresser NJ,
Caballero-Ramos CI, Munson PJ, Prabhu VV, Trent JM, McFarland HF
and Biddison WE: Analysis of gene expression in mutiple sclerosis
lesions using cDNA microarrays. Ann Neurol. 46:425–428.
1999.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li J, Yang S, Lu S, Zhao H, Feng J, Li W,
Ma F, Ren Q, Liu B, Zhang L, et al: Differential gene expression
profile associated with the abnormality of bone marrow mesenchymal
stem cells in aplastic anemia. PLoS One. 7(e47764)2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
van der Pouw Kraan TC, van Gaalen FA,
Kasperkovitz PV, Verbeet NL, Smeets TJ, Kraan MC, Fero M, Tak PP,
Huizinga TW, Pieterman E, et al: Rheumatoid arthritis is a
heterogeneous disease: Evidence for differences in the activation
of the STAT-1 pathway between rheumatoid tissues. Arthritis Rheum.
48:2132–2145. 2003.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lee SK, Jeon EK, Kim YJ, Seo SH, Kim CD,
Lim JS and Lee JH: A global gene expression analysis of the
peripheral blood mononuclear cells reveals the gene expression
signature in psoriasis. Ann Dermatol. 21:237–242. 2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Heller RA, Schena M, Chai A, Shalon D,
Bedilion T, Gilmore J, Woolley DE and Davis RW: Discovery and
analysis of inflammatory disease-related genes using cDNA
microarrays. Proc Natl Acad Sci USA. 94:2150–2155. 1997.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Gough LL, Fan J, Chu S, Winnick S and Beck
KA: Golgi localization of Syne-1. Mol Biol Cell. 14:2410–2424.
2003.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Apel ED, Lewis RM, Grady RM and Sanes JR:
Syne-1, a dystrophin- and Klarsicht-related protein associated with
synaptic nuclei at the neuromuscular junction. J Biol Chem.
275:31986–31995. 2000.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Fan J and Beck KA: A role for the spectrin
superfamily member Syne-1 and kinesin II in cytokinesis. J Cell
Sci. 117:619–629. 2004.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yang W, Zheng H, Wang Y, Lian F, Hu Z and
Xue S: Nesprin-1 plays an important role in the proliferation and
apoptosis of mesenchymal stem cells. Int J Mol Med. 32:805–812.
2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Davis RS: Fc receptor-like molecules. Annu
Rev Immunol. 25:525–560. 2007.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Shabani M, Bayat AA, Jeddi-Tehrani M,
Rabbani H, Hojjat-Farsangi M, Ulivieri C, Amirghofran Z, Baldari CT
and Shokri F: Ligation of human Fc receptor like-2 by monoclonal
antibodies down-regulates B-cell receptor-mediated signalling.
Immunology. 143:341–353. 2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Nückel H, Collins CH, Frey UH, Sellmann L,
Dürig J, Siffert W and Dührsen U: FCRL2 mRNA expression is
inversely associated with clinical progression in chronic
lymphocytic leukemia. Eur J Haematol. 83:541–549. 2009.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Jackson TA, Haga CL, Ehrhardt GR, Davis RS
and Cooper MD: FcR-like 2 Inhibition of B cell receptor-mediated
activation of B cells. J Immunol. 185:7405–7412. 2010.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Li FJ, Ding S, Pan J, Shakhmatov MA,
Kashentseva E, Wu J, Li Y, Soong SJ, Chiorazzi N and Davis RS:
FCRL2 expression predicts IGHV mutation status and clinical
progression in chronic lymphocytic leukemia. Blood. 112:179–187.
2008.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Stamatopoulos B, Haibe-Kains B, Equeter C,
Meuleman N, Sorée A, De Bruyn C, Hanosset D, Bron D, Martiat P and
Lagneaux L: Gene expression profiling reveals differences in
microenvironment interaction between patients with chronic
lymphocytic leukemia expressing high versus low ZAP70 mRNA.
Haematologica. 94:790–799. 2009.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Khanzadeh A, Habibagahi Z, Hosseini A and
Amirghofran Z: Investigation of the human FCRL1, 2, and 4 gene
expressions in patients with rheumatoid arthritis. Rheumatol Int.
36:1149–1156. 2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Stehlik C, Krajewska M, Welsh K, Krajewski
S, Godzik A and Reed JC: The PAAD/PYRIN-only protein POP1/ASC2 is a
modulator of ASC-mediated nuclear-factor-kappa B and pro-caspase-1
regulation. Biochem J. 373:101–113. 2003.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Stehlik C: The PYRIN domain in signal
transduction. Curr Protein Pept Sci. 8:293–310. 2007.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Martinon F and Tschopp J: Inflammatory
caspases: Linking an intracellular innate immune system to
autoinflammatory diseases. Cell. 117:561–574. 2004.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Hopwood B and Dalton S: Cdc45p assembles
into a complex with Cdc46p/Mcm5p, is required for minichromosome
maintenance, and is essential for chromosomal DNA replication. Proc
Natl Acad Sci USA. 93:12309–12314. 1996.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Sun J, Shi R, Zhao S, Li X, Lu S, Bu H and
Ma X: Cell division cycle 45 promotes papillary thyroid cancer
progression via regulating cell cycle. Tumour Biol.
39(1010428317705342)2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Pollok S, Bauerschmidt C, Sänger J,
Nasheuer HP and Grosse F: Human Cdc45 is a proliferation-associated
antigen. FEBS J. 274:3669–3684. 2007.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wang Y, Duan X and Zhang Z: Interleukin-1
receptor-associated kinase 1 correlates with metastasis and
invasion in endometrial carcinoma. J Cell Biochem. 119:2545–2555.
2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Majri SS, Fritz JM, Villarino AV, Zheng L,
Kanellopoulou C, Chaigne-Delalande B, Grönholm J, Niemela JE,
Afzali B, Biancalana M, et al: STAT5B: A differential regulator of
the life and death of CD4+ effector memory T cells. J
Immunol. 200:110–118. 2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Darnell JE Jr: STATs gene regulation.
Science. 277:1630–1635. 1997.PubMed/NCBI View Article : Google Scholar
|
|
56
|
O'Shea JJ, Lahesmaa R, Vahedi G, Laurence
A and Kanno Y: Genomic views of STAT function in CD4+ T helper cell
differentiation. Nat Rev Immunol. 11:239–250. 2011.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Villarino AV, Kanno Y, Ferdinand JR and
O'Shea JJ: Mechanisms of Jak/STAT signaling in immunity and
disease. J Immunol. 194:21–27. 2015.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Grimley PM, Dong F and Rui H: Stat5a and
Stat5b: Fraternal twins of signal transduction and transcriptional
activation. Cytokine Growth Factor Rev. 10:131–157. 1999.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Kanai T, Jenks J and Nadeau KC: The STAT5b
pathway defect and autoimmunity. Front Immunol.
3(234)2012.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Nadeau K, Hwa V and Rosenfeld RG: STAT5b
deficiency: An unsuspected cause of growth failure,
immunodeficiency, and severe pulmonary disease. J Pediatr.
158:701–708. 2011.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Zare F, Dehghan-Manshadi M and Mirshafiey
A: The signal transducer and activator of transcription factors
lodge in immunopathogenesis of rheumatoid arthritis. Reumatismo.
67:127–137. 2015.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Okada Y, Wu D, Trynka G, Raj T, Terao C,
Ikari K, Kochi Y, Ohmura K, Suzuki A, Yoshida S, et al: Genetics of
rheumatoid arthritis contributes to biology and drug discovery.
Nature. 506:376–381. 2014.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Xu WD, Pan HF, Ye DQ and Xu Y: Targeting
IRF4 in autoimmune diseases. Autoimmun Rev. 11:918–924.
2012.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Biswas PS, Gupta S, Stirzaker RA, Kumar V,
Jessberger R, Lu TT, Bhagat G and Pernis AB: Dual regulation of
IRF4 function in T and B cells is required for the coordination of
T-B cell interactions and the prevention of autoimmunity. J Exp
Med. 209:581–596. 2012.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Hwang ES: Transcriptional regulation of T
helper 17 cell differentiation. Yonsei Med J. 51:484–491.
2010.PubMed/NCBI View Article : Google Scholar
|