Introduction
Renal angiomyolipoma (RAML), also known as renal
hamartoma, is a benign tumor composed of mature adipose tissue,
smooth muscle and thick-walled blood vessels, which may impose a
significant morbidity or mortality due to its unique
characteristics and the complications subsequent to its treatment
(1). Clinically, RAML is often
misdiagnosed as renal cell carcinoma (RCC), and CT examination
combined with renal angiography can effectively identify RAML and
RCC (2,3). As RAML tends to grow quickly and is
often associated with complications including hemorrhage and pain,
active intervention is required. RAML is uncommon, with only 22
cases reported over the past 35 years (4,5). RAML
complicated with spontaneous rupture and hemorrhage is even more
rare in pregnant women. In the present case report, a case of RAML
in a 31-year-old pregnant woman with RAML of both kidneys during
her second pregnancy was described. The tumor measured ~21x12x10 cm
and grew quickly, causing spontaneous rupture and massive
hemorrhage at 20 weeks of gestational age. Emergency exploratory
laparotomy, left kidney resection and splenectomy were performed
under general anesthesia. A literature review was also performed,
and the composition, type classification, imaging characteristics
and various treatments currently available for this condition were
discussed, in an attempt to provide more information concerning the
diagnosis and treatment of this rare renal tumor.
Case report
A 31-year-old woman at 20 weeks of gestation age was
admitted to The First Hospital of Lanzhou University (Lanzhou,
China) with the complaint of left flank and abdominal pains for
>3 days. She was weak, anorexic and severely anemic with
episodes of nausea and vomiting. Physical examination revealed
tenderness over the left kidney and right lower abdominal regions
but with no rebound pain and muscle tension. A giant mass ~10x8 cm
was palpated in the left upper abdomen. Laboratory tests were
performed to obtain hemoglobin (63 g/l), platelet count (82x109/l),
serum urea (4.07 mmol/l) and serum creatinine (74.0 µmol/l) levels.
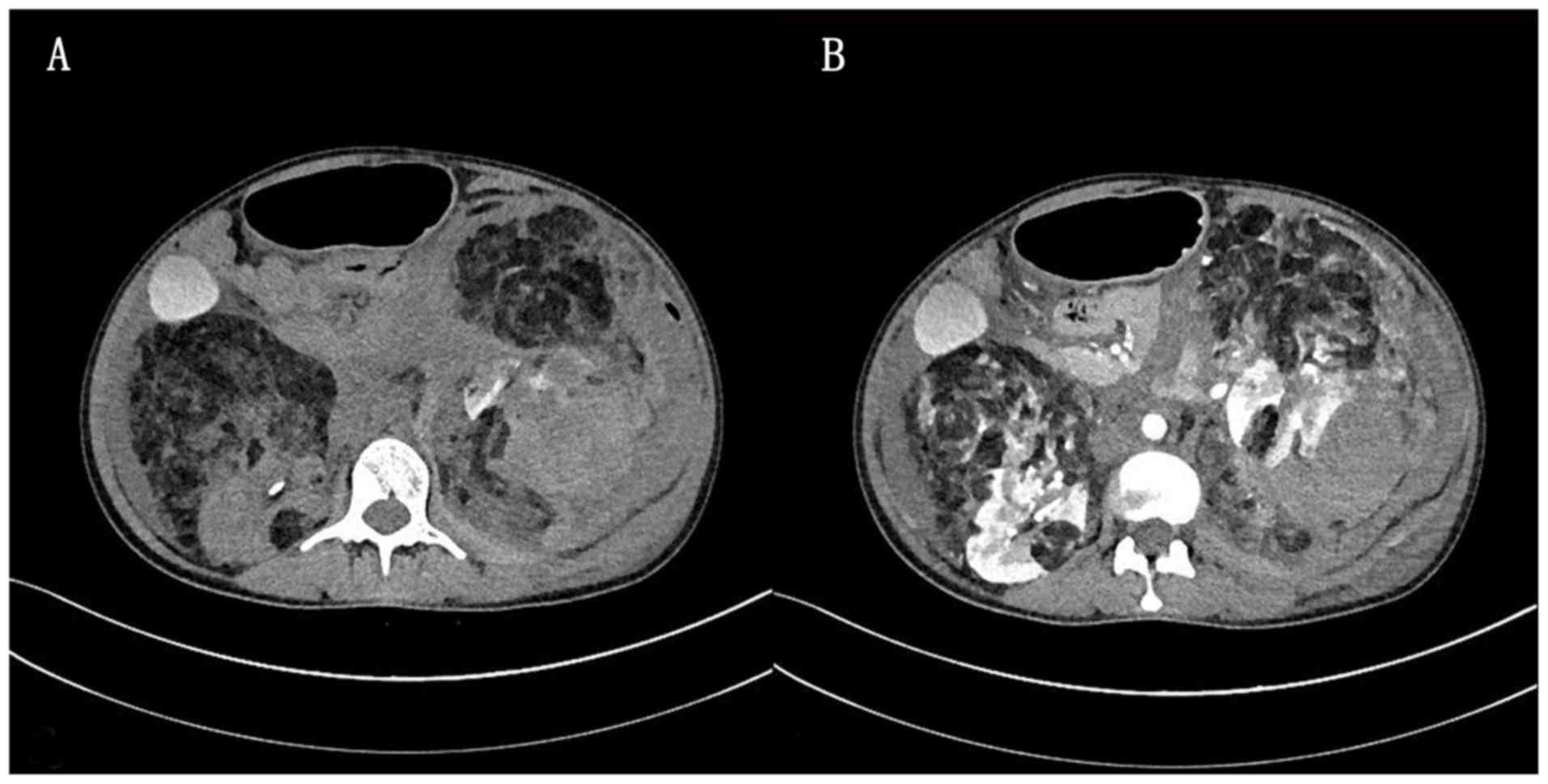
Computed tomography (CT) plain scan exhibited multiple cystic
masses in both kidneys, causing compression on and displacement of
the pancreas, spleen and surrounding intestines. The normal
morphology and structure of the left kidney had disappeared, and
the right kidney was abnormally shaped, with a large mixed density
shadow of the soft tissue and fat present (Fig. 1A). Contrast-enhanced CT scan revealed
enhancement of multiple vessels supplied by the bilateral renal
artery, fluids in the abdominal and pelvic cavities, destruction of
the bilateral renal structures, possible hamartoma, hemorrhage of
the left renal lesion, blood accumulation around the left kidney
and a small amount of gas accumulation (Fig. 1B). In addition, multiple abnormally
enhanced foci were observed in the liver, which were suspected to
be hemangioma and multiple cysts; an abnormal enhancement area was
observed in the left lobe of the liver, which was considered to be
an abnormal perfusion. Magnetic resonance imaging (MRI) was not
performed due to the nature of the emergency.
Emergency exploratory laparotomy, left kidney
resection and splenectomy were performed under general anesthesia.
Postoperative pathology revealed a well-defined grayish yellow and
soft mass ~3.0x3.0x2.0 cm on the surface of the 8.0x4.0x4.0 cm
resection specimen of the left kidney, and a second grayish yellow
and soft nodule ~21x12x10 cm at the renal hilum in the 17x12x9.0 cm
grey-yellow resected specimen (Fig.
2A). A splenectomic specimen measuring ~1x7.0x4.0 cm was also
removed (Fig. 2B). Postoperative
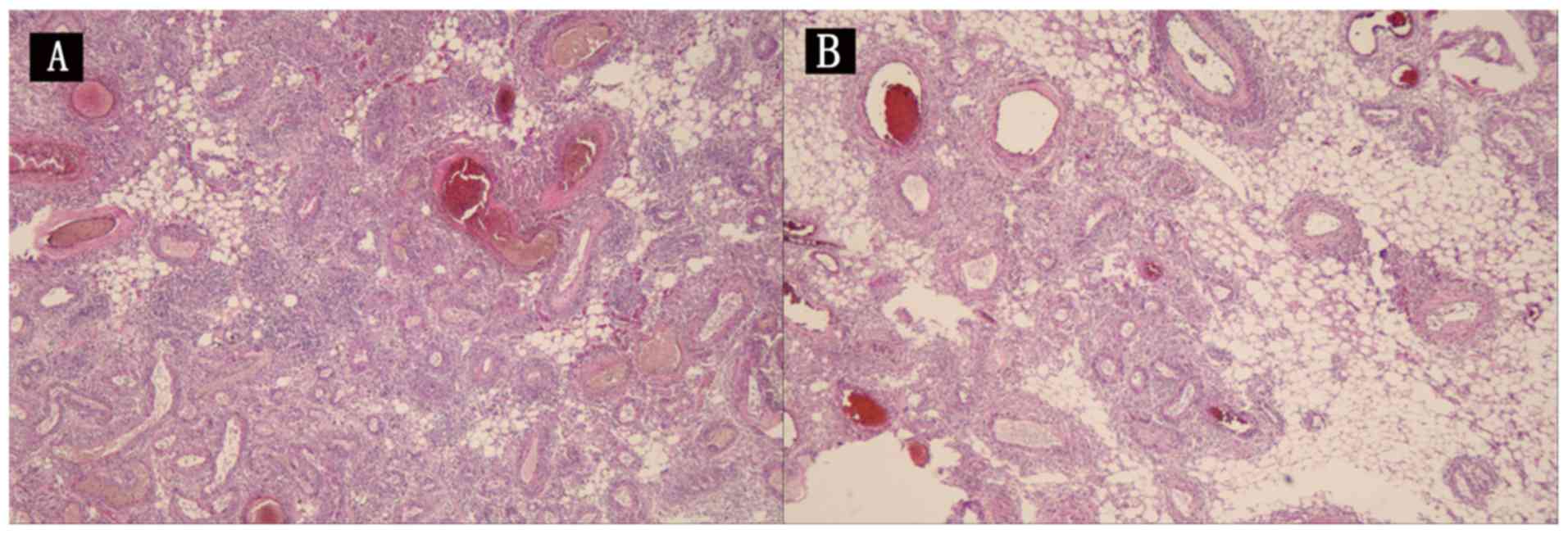
pathology of the left kidney revealed that the capsule of the tumor
tissue had disappeared due to swelling and infiltration. The tumor
parenchyma was composed of a large number of thick-walled blood
vessels, and a large amount of fat and a small amount of smooth
muscle. The tumor parenchyma cells were not atypical with rare
mitosis but hemorrhage and necrosis (Fig.
3A). The splenic capsule and part of the spleen were
hemorrhagic, with no tumor tissue observed (Fig. 3B). We performed immunohistochemical
analysis of paraffin-embedded sections (3 µm), which were fixed
with 10% neutral formaldehyde for 24 h at room temperature, and
subsequently rinsed with tap water. Sections were dewaxed with
xylene I and II for 20 min each, and rehydrated with a descending
alcohol series. In addition, samples were incubated with 3% H2O2 at
room temperature for 5-10 min to block endogenous peroxidase
activity and then washed with distilled water three times (5 min
per wash), followed by heating in a microwave for 10-15 while
immersed in PBS. Goat serum was then applied for 15 min at room
temperature. Subsequently, a primary antibody against S-100 was
applied (MAB-0697, Fuzhou Maixin Biotechnology Development Co.,
Ltd.) for 60 min at room temperature, and washed with PBS. A
secondary antibody was then applied (AP, KIT5101; Fuzhou Maixin
Biotechnology Development Co., Ltd.) for 15 min at room
temperature. We then performed counterstaining of the sections
using hematoxylin for 30 sec at room temperature; the sections were
sealed with an aqueous mounting agent. Sections were analyzed under
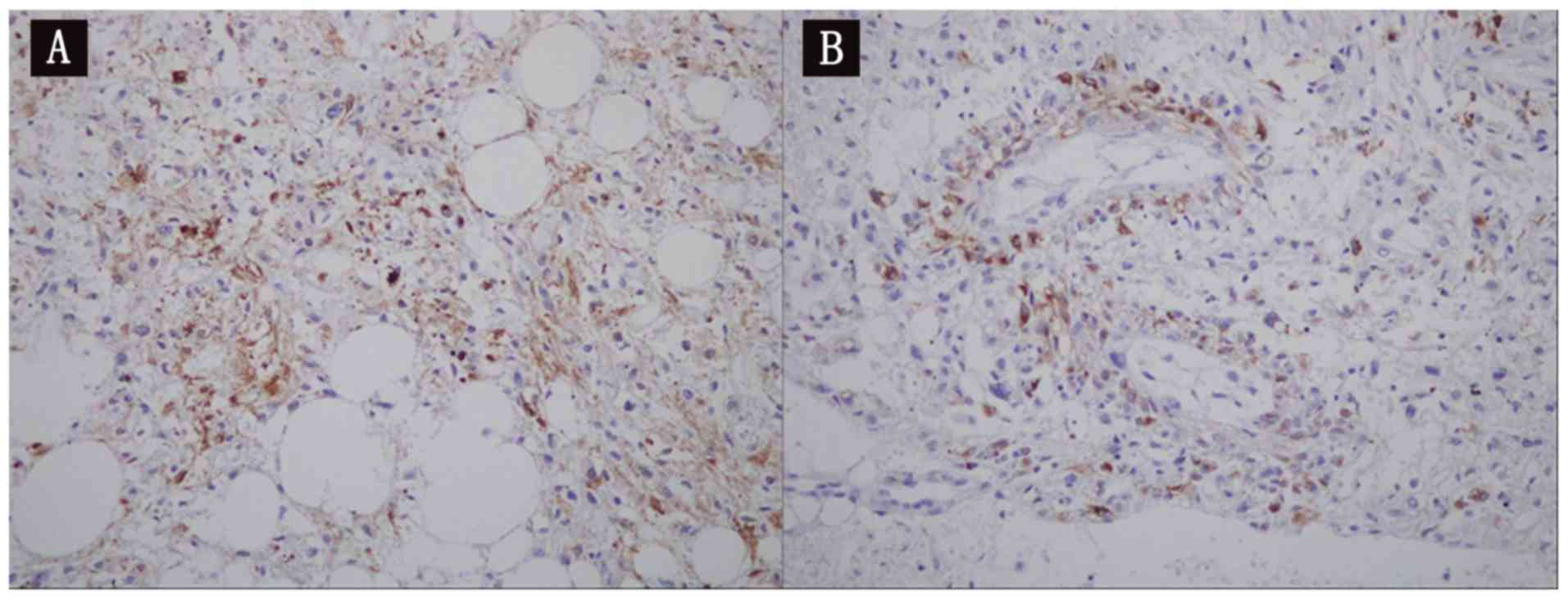
a light microscope at x200 magnification. Immunohistochemical
staining (alkaline phosphatase method) demonstrated cytokeratin
(-), vimentin (1+), S-100 protein (adipocyte+), human melanoma
black-45 (scattered lesion+), melanoma antigen recognized by
T-cells 1 (1+), antigen Ki-67 (<5%), platelet endothelial cell
adhesion molecule (vascular+), cluster of differentiation 34
(vascular+), and smooth muscle actin (partial+) staining patterns
(Fig. 4A and B). The results of
pathology and immunohistochemistry analyses supported the diagnosis
of renal hamartoma. Based on these examination results, the patient
was diagnosed with giant bilateral renal hamartoma with spontaneous
rupture and hemorrhage of the left kidney with involvement of the
spleen.
The patient was transferred from the operating room
to the Intensive Care Unit for subsequent observation, where she
delivered a dead fetus naturally 3 days following surgery, and
recovered well postoperatively. After 5 months, the patient was
re-admitted and underwent massive tumor resection of the right
kidney as part of further treatment, and recovered well
postoperatively.
Discussion
RAML is a benign mesenchymal tumor-like lesion
consisting of varying mixtures of smooth muscle, blood vessels and
mature fat. It was first described in 1880(5). It usually occurs in the kidneys but has
also been identified in the lung, liver, fallopian tube, vagina and
spermatic cord (6). Clinically, RAML
may be classified as two types: The tuberous sclerosis complex
(TSC) and the sporadic renal hamartoma (7). Patients with TSC-RAML usually have a
genetic family history, and the pathogenesis may be associated with
mutations in TSC complex subunit 1 and 2 genes. The sporadic renal
hamartoma is more common in adults (8). RAML may be diagnosed by ultrasonography,
CT and MRI. An overview of the relevant literature may assist in
understanding the imaging characteristics of renal hamartoma by
different examination methods. For example, renal hamartoma is
generally observed as a hyperechoic mass on ultrasound (9). It is difficult to differentiate giant
renal hamartoma from other retroperitoneal fatty lesions by
ultrasound alone. According to Luo et al (10) and Cheng et al (11) on the imaging characteristics of CT
about renal hamartoma, the majority of low fat hamartomas exhibit
high density or slightly high density on CT scan, and a few
demonstrate slightly low density and isodensity. Cheng et al
(11) demonstrated that low-fat renal
hamartoma usually exhibited homogeneous low signals on T2 and
iso-signal on T1 of MRI plain scan. When the proportion of the fat
component is <20%, it is difficult to demonstrate the imaging
features of renal hamartoma. This imaging feature in the clinical
diagnosis may assist in the diagnosis of renal hamartoma. However,
postoperative pathology remains the gold standard for the diagnosis
of renal hamartoma.
It is clear that early detection and diagnosis of
RAML may decrease the occurrence of serious complications. For
unruptured hamartoma tumors >4 cm, minimally invasive procedures
including arterial embolization, radiofrequency ablation,
cryoablation, mTOR inhibitors, and other novel therapies like
partial or total nephrectomy are recommended (12). Pregnancy with renal hamartoma with
spontaneous rupture bleeding is rare, and preoperative diagnosis is
difficult in pregnant women without presenting clinical symptoms,
which often cause misdiagnosis, thereby affecting the safety of the
mother and the fetus (13). Pregnancy
may promote tumor growth and increase the risk of tumor rupture,
which the majority of previous studies hypothesized was due to the
ubiquitous expression of estrogen and progesterone receptors in
RAML. With the elevation of estrogen and progesterone levels in
pregnant women, the tumor tends to grow quickly under stimulation
of tumor vascular proliferation, which increases the risk of
spontaneous rupture and bleeding of the tumor or surrounding
structures (14,15). With the exception of surgical
intervention, there is no successful treatment for renal hamartoma
complicated with spontaneous rupture and hemorrhage in pregnant
women.
To improve the survival rate of patients and
fetuses, prenatal examination in the first trimester of pregnancy
is important. When the diagnosis is confirmed, interventions
including renal artery embolization should be taken positively. At
present, for RAML complicated with spontaneous rupture and
hemorrhage, surgical resection or angioembolization appears to be
the only way to save the life of the patient.
Acknowledgements
The authors would like to thank Dr Yong-Lin Chen
(Department of Pathology, First Hospital of Lanzhou University) for
his help in the interpretation of pathological data.
Funding
The project was supported by the Natural Science
Foundation of Gansu Province (grant no. 1606RJZA125).
Availability of data and materials
All data relevant to the present study are available
from the corresponding author on reasonable request.
Authors' contributions
CMG participated in the collection of the data and
the drafting of the manuscript. CY, NX and YM contributed to
acquisition and analysis of data, YQM critically revised the
manuscript for important intellectual content. All authors have
read and approved the final version of this manuscript.
Ethics approval and consent to
participate
Informed consent was obtained from the patient
concerning the use of the imaging and other clinical data.
Patient consent for publication
Informed consent was obtained from the patient
concerning the use of the imaging and other clinical data. The
patient understood that her name and initials would not be
published and due efforts would be made to conceal her identity,
though anonymity cannot be guaranteed.
Competing interests
The authors declare that they have no competing
interests
References
|
1
|
Seyam RM, Alkhudair WK, Kattan SA,
Alotaibi MF, Alzahrani HM and Altaweel WM: The risks of renal
angiomyolipoma: Reviewing the evidence. J Kidney Cancer VHL.
4:13–25. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Catalano OA, Samir AE, Sahani DV and Hahn
PF: Pixel distribution analysis: Can it be used to distinguish
clear cell carcinomas from angiomyolipomas with minimal fat?
Radiology. 247:738–746. 2008. View Article : Google Scholar
|
|
3
|
He ZS, Zhang XC, Zhou LQ, et al: Diagnosis
and treatment of renal angiomyolipoma (report of 72 cases). Chin J
Urol. 23:135–137. 2002.
|
|
4
|
Preece P, Mees B, Norris B, Christie M,
Wagner T and Dundee P: Surgical management of haemorrhaging renal
angiomyolipoma in pregnancy. Int J Surg Case Rep. 7C:89–92.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang L, Liu YP and Huang WJ: Analysis of
ultrasonic misdiagnosis of atypical renal angiomyolipoma. Shanghai
Medical Imaging. 18(288)2009.
|
|
6
|
Abdulla M, Bui HX, del Rosario AD, Wolf BC
and Ross JS: Renal angiomyolipoma. DNA content and
immunohistochemical study of classic and multicentric variants.
Arch Pathol Lab Med. 118:735–739. 1994.PubMed/NCBI
|
|
7
|
Rijal JP, Dhakal P, Giri S and Dahal KV:
Tuberous sclerosis complex with autosomal dominant polycystic
kidney disease: a rare duo. BMJ Case Rep. 2014:bcr2014207471.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Seyam RM, Bissada NK, Kattan SA, Mokhtar
AA, Aslam M, Fahmy WE, Mourad WA, Binmahfouz AA, Alzahrani HM and
Hanash KA: Changing trends in presentation, diagnosis and
management of renal angiomyolipoma: comparison of sporadic and
tuberous sclerosis complex-associated forms. Urology. 72:1077–1082.
2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang LL, Dong AJ, Tang JN, Li MB, Yu JJ,
Sun PF and Meng GL: Brenge. Diagnosis and treatment of renal
hamartoma (40 cases). Mod Oncol. 26:233–237. 2018.
|
|
10
|
Luo J, Jing WB, Zhang DJ, Zhang J and Yang
Z: CT and MRI features of renal hamartoma. J Med Imaging
(Bellingham). 26:2043–2046. 2016.
|
|
11
|
Cheng XH, Zhou JZ, Yu ZJ and Zhang JC:
Clinical analysis of CT and MRI diagnosis of renal hamartoma and
renal cell carcinoma. J Med Imaging. 26:867–869. 2016.
|
|
12
|
Flum AS, Hamoui N, Said MA, Yang XJ,
Casalino DD, McGuire BB, Perry KT and Nadler RB: Update on the
diagnosis and management of renal angiomyolipoma. J Urol.
195:834–846. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Peng YC and Pan XT: Spontaneous rupture
and hemorrhage of pregnancy complicated with renal hamartoma: A
case report. Zhejiang Department of Trauma surgery. 22:403–404.
2017.
|
|
14
|
Gould Rothberg BE, Grooms MC and
Dharnidharka VR: Rapid growth of a kidney angiomyolipoma after
initiation of oral contraceptive therapy. Obstet Gynecol.
108:734–736. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Storm DW and Mowad JJ: Conservative
management of a bleeding renal angiomyolipoma in pregnancy. Obstet
Gynecol. 107:490–492. 2006.PubMed/NCBI View Article : Google Scholar
|