Introduction
Gastric cancer (GC) is a malignant tumor that
originates in the epithelium of the gastric mucosa and is one of
the most common types of malignant tumors in the world (1). According to GLOBOCAN 2018, there were
>1,000,000 new cases of GC and ~783,000 deaths in 2018, thus
making it the cancer type with the fifth highest incidence rate and
the third highest mortality in the world (2). The poor five-year survival rate of GC is
primarily due the advanced stage of gastric tumors at the initial
diagnosis in the majority of patients, and thus limits treatment
opportunities (3). According to the
Cancer Staging Manual, 8th edition, of the American Joint Committee
on Cancer, only 30% of GC cases are diagnosed prior to metastasis,
and the five-year survival for pathological Tumor-Node-Metastasis
stage groups are between 68-80% for stage I, 46-60% for stage II,
8-30% for stage III and 5% for stage IV (4). Therefore, identifying potential
biomarkers for patients with early GC is critical for improving
patient outcomes.
In recent years, a variety of bioinformatics methods
have contributed greatly to the discovery of biomarkers associated
with tumor development, diagnosis and prognosis (5-8).
The combined use of multiple databases of biological information
for the analysis of cancer has also yielded certain breakthroughs.
Yong et al (9) used Gene
Expression Omnibus (GEO), Oncomine, Search Tool for Recurring
Instances of Neighbouring Genes (STRING) and other databases for
bioinformatic analysis, and concluded that PPP2CA may
function as an oncogene and a prognostic biomarker or therapeutic
target in the progression of colorectal cancer. Troiano et
al (10) used the GEO database
and Oncomine to examine the expression of BIRC5/Survivin in
oral squamous cell carcinoma and showed that Survivin expression
was upregulated compared with non-cancerous tissue. In addition,
immunohistochemistry staining showed that cytoplasmic expression of
Survivin was associated with poor overall survival in patients with
oral squamous cell carcinoma. It may be beneficial to use multiple
datasets and analysis tools to determine the potential mechanisms
underlying development and progression of GC, and to identify
potentially novel and specific diagnostic biomarkers for early
detection of GC to improve the survival of patients.
In the present study, the expression profiles from
four datasets (GSE13911, GSE19826, GSE54129 and GSE118916) in human
GC and normal gastric tissue samples were obtained from the GEO
database and analyzed to identify differentially expressed genes
(DEGs). Gene Ontology (GO) and pathway enrichment analysis were
performed to identify the biological functions and pathways of the
DEGs. STRING and Cytoscape were used to construct a protein-protein
interaction (PPI) network, and a total of six key genes were
selected from the PPI network and DEGs. The value of the key genes
was validated using the Oncomine and Kaplan-Meier platforms to
further increase the reliability of the results and confirm the
prognostic value of the key genes.
Materials and methods
Microarray data
The key word ‘gastric cancer’ was searched in the
GEO database (ncbi.nlm.nih.gov/geo/), and a total
of 9,224 datasets on human GC were retrieved. In the present study,
four gene expression profiles from the GEO database were used, as
they have not been studied together previously. The four datasets
were: GSE13911(11),
GSE19826(12), GSE54129 and
GSE118916(13). Among these,
GSE13911, GSE19826 and GSE54129 were based on the GPL570 platform
[(HG-U133_Plus_2) Affymetrix Human Genome U133 Plus 2.0 Array].
GSE118916 was based on the GPL15207 platform [(PrimeView)
Affymetrix Human Gene Expression Array].
Identification of DEGs
DEGs between GC samples and normal controls were
identified using the GEO2R online analysis tool (ncbi.nlm.nih.gov/geo/geo2r); |log FC|≥1.0 and
corrected P<0.05 were used as the cutoff criteria. The common
DEGs of the four gene expression profiles were screened using Wayne
analysis in Funrich (funrich.org/).
GO and KEGG enrichment analyses of
DEGs
After obtaining the common DEGs, GO (14,15) and
KEGG (16) analyses of the DEGs were
performed using the Database for Annotation Visualization and
Integrated Discovery (DAVID) online tool (17,18), with
P<0.01 used as the threshold for significance. GO was used to
identify the enrichment functions of three independent categories
of genes; biological process (BP), cellular component (CC) and
molecular function (MF). KEGG was used to search for the pathways
associated with the identified genes (19). Only the top 10 BP, CC and MF terms,
and the KEGG pathway with the smallest P-value were selected for
further examination in the present study. The figures were
generated using the OmicShare tools (omicshare.com/tools), a free
online platform for data analysis.
PPI network construction
To explore the interaction between DEGs, the DEGs
were analyzed using STRING (20) to
generate a PPI network. PPI pairs with a combined score >0.4
were extracted, and disconnected nodes in the network were hidden.
Subsequently, the PPI network was visualized using Cytoscape
(21) and the degree of each protein
node was calculated using the cytoHubba (22) plug-in in Cytoscape.
Identification of key genes
The two genes with the highest degree of
connectivity in the PPI network, the two genes with the largest
logFC values and the two genes with the smallest logFC among the
shared DEGs were selected and considered key genes.
Analysis of key genes in Oncomine
The Oncomine database (oncomine.org/) was
used to explore the mRNA expression differences of six key genes
between GC and normal gastric tissue. Oncomine is a chip-based gene
database and integrated data mining online cancer microarray
database designed to facilitate the discovery of novel biomarkers
from genome-wide expression analysis (23).
Survival analysis of key genes
The Kaplan-Meier plotter (24) is an online tool that can assess the
effect of 54,000 genes on survival in 21 types of cancer. The
largest datasets include breast (n=6,234), ovarian (n=2,190), lung
(n=3,452) and gastric cancer (n=1,440) cancer. The primary purpose
of the tool is to discover and validate biomarkers for survival.
Online survival analysis of the selected key genes based on the GC
database was performed using Kaplan-Meier Plotter. The hazard ratio
(HR) with 95% confidence intervals (CIs) and log-rank P-values were
calculated.
Results
Identification of DEGs
GSE13911 includes 38 GC samples and 31 normal
samples, GSE19826 contains 12 GC samples and 15 normal samples,
GSE54129 contains 111 GC samples and 21 normal samples, and
GSE118916 contains 15 GC samples and 15 normal samples (Table I). In GSE13911, there are 26
intestinal, 4 mixed, 6 diffuse and 2 unclassified gastric carcinoma
tissues, as well as 31 normal adjacent tissues. Unfortunately,
information on the histological subtypes were not available in the
other datasets. In the datasets, 1,001 upregulated and 2,304
downregulated DEGs were identified in GSE13911, 407 upregulated and
753 downregulated DEGs were identified in GSE19826, 1,852
upregulated and 2,083 downregulated DEGs were identified in
GSE54129, and 977 upregulated and 903 downregulated DEGs were
identified in GSE118916. Wayne analysis identified 99 common
upregulated genes and 172 common downregulated genes were obtained
from the 4 datasets (Table II;
Fig. 1).
 | Table IInformation for four gene expression
profiles from Gene Expression Omnibus. |
Table I
Information for four gene expression
profiles from Gene Expression Omnibus.
| Dataset ID | Gastric cancer | Normal | Total Number | Platform |
|---|
| GSE13911 | 38 | 31 | 69 | GPL570 |
| GSE19826 | 12 | 15 | 27 | GPL570 |
| GSE54129 | 111 | 21 | 132 | GPL570 |
| GSE118916 | 15 | 15 | 30 | GPL15207 |
 | Table IIThe differentially expressed genes
identified from the four gene expression profiles, between gastric
cancer and normal tissues. |
Table II
The differentially expressed genes
identified from the four gene expression profiles, between gastric
cancer and normal tissues.
| Differentially
expressed genes | Gene terms |
|---|
| Upregulated | INHBA CST1 COL11A1
FAP COL10A1 FNDC1 COL8A1 SERPINH1 CDH3 THBS2 CLDN1 TNFRSF11B SPP1
COL1A2 SFRP4 SULF1 CPXM1 BMP1 MFAP2 COL1A1 CTHRC1 BGN RARRES1
IGF2BP3 THBS4 COL6A3 SRPX2 OSR2 HOXB7 TIMP1 ASPN THY1 FKBP10 PRRX1
SDS APOE PMEPA1 COL12A1 GPNMB FBN1 ADAM12 C3 APOC1 COL5A1 SPARC
EPHB2 NID2 CMTM3 PLEKHO1 TNFRSF10B EHD2 FN1 MMP11 COCH AMIGO2
COL5A2 OLFML2B KLHL23 SPOCK1 CDH11 TWIST1 RAB31 SULF2 FGD6 VCAN
ITGBL1 PCOLCE HAVCR2 THBS1 DNM1 IGFBP7 PLAU TMEM158 COL3A1 FLNA
EDNRA LEF1 LIPG FZD2 GXYLT2 S100A10 LGALS1 NRP2 SIRPA ANTXR1 CD9
LIF COL4A2 TGM2 COL6A1 PDPN KCNJ8 ACTN1 GPR161 ZAK RCN3 BAG2
BHLHE40 COL4A1 |
| Downregulated | ATP4A ATP4B KCNE2
AQP4 GIF LIPF GKN1 GKN2 DPCR1 PGC SOSTDC1 ESRRG MUC6 SST FBP2 CPA2
VSIG1 CXCL17 PDIA2 CCKBR TMED6 CHGA TFF2 PSCA FUT9 CA9 SCNN1G
GUCA2B C16orf89 SLC26A9 KLK11 CWH43 DNER PSAPL1 CNTN3 ALDH3A1 GATA5
SCGB2A1 UGT2B15 RDH12 CLIC6 NRG4 CLDN18 CAPN9 SLC16A7 SSTR1 FBXL13
TCN1 VSIG2 AKR1B10 B3GNT6 FOLR1 MUM1L1 CHGB MAL TRIM50 AKR7A3
KIAA1324 PAIP2B SULT2A1 PTPRZ1 ARX LIFR ALDH1A1 HYAL1 BEX5 CA2
CYP2C18 ME1 SCNN1B ADH7 GCNT2 ACER2 FMO5 HPGD RASSF6 TFF1 TMEM171
CA4 KCNJ16 LDHD KCNJ15 GABRB3 HOMER2 TMPRSS2 LYPD6B KLHDC7A
ARHGAP42 PLAC8 IGFBP2 CAPN13 SYTL5 PDGFD RNASE1 RORC CYP2C9 EPN3
PBLD METTL7A ZBTB7C UBL3 SH3RF2 RNASE4 ARHGEF37 ALDH6A1 RAB27B
SULT1B1 PKIB PXMP2 GPRC5C RIMBP2 ATP8A1 FAM20A PIGR GOLM1 CYP3A5
FAM46C C9orf152 COBLL1 FA2H SORBS2 DGKD SGK2 TMEM220 ANG PLLP MYCN
C1orf116 FGD4 SLC41A2 ADAM28 MAGI1 GRAMD1C IQGAP2 GULP1 SYTL2 DHRS7
OASL RNF128 DBT ELL2 RAB27A NOSTRIN NEDD4L PPFIBP2 AKR1C3 PELI2
SMPD3 PTPRN2 RASEF TMEM92 ABCC5 GALNT12 LMO4 NTN4 TMEM116 ID4
ELOVL6 ALDOB EPB41L4B CD36 GALNT5 SH3BGRL2 MAGI3 MICALL1 HIPK2 MAOA
WWC1 SLC7A8 CDC14B FAM107B SUCLG2 |
GO and KEGG pathway enrichment
analyses of DEGs
GO and KEGG pathway enrichment analyses of the DEGs
was performed using the online tool DAVID, and the results are
presented in Table III. GO analysis
showed that in BP, the DEGs were primarily enriched for the GO
terms: ‘extracellular matrix organization’, ‘collagen catabolic
process’, ‘cell adhesion’, ‘collagen fibril organization’ and
‘digestion’ (Table III; Fig. 2A). CC analysis revealed that the DEGs
were significantly enriched for the terms: ‘extracellular space’,
‘extracellular matrix’, ‘extracellular exosome’, ‘extracellular
region’ and ‘endoplasmic reticulum lumen’ (Table III; Fig.
2B). For MF, the DEGs were enriched for the GO terms:
‘platelet-derived growth factor binding’, ‘collagen binding’,
‘extracellular matrix binding’, ‘inward rectifier potassium channel
activity’ and ‘SMAD binding’ (Table
III; Fig. 2C). According to KEGG
pathway analysis, the DEGs were primarily enriched for the pathway
terms: ‘ECM-receptor interaction’, ‘protein digestion and
absorption’, ‘focal adhesion’, ‘amoebiasis’ and ‘gastric acid
secretion’ (Table III; Fig. 2D).
 | Table IIIGO term and KEGG pathway enrichment
analyses of the 271 differentially expressed genes. |
Table III
GO term and KEGG pathway enrichment
analyses of the 271 differentially expressed genes.
| Category | Term | Description | Count | P-Value |
|---|
| BP term | GO:0030198 | Extracellular
matrix organization | 23 |
1.28x10-13 |
| BP term | GO:0030574 | Collagen catabolic
process | 14 |
7.06x10-12 |
| BP term | GO:0007155 | cell adhesion | 30 |
3.59x10-11 |
| BP term | GO:0030199 | Collagen fibril
organization | 9 |
7.87x10-08 |
| BP term | GO:0007586 | Digestion | 10 |
3.19x10-07 |
| BP term | GO:0035987 | Endodermal cell
differentiation | 7 |
2.13x10-06 |
| BP term | GO:0001501 | Skeletal system
development | 11 |
3.42x10-05 |
| BP term | GO:0008202 | Steroid metabolic
process | 7 |
3.60x10-05 |
| BP term | GO:0071230 | Cellular response
to amino acid stimulus | 7 |
6.04x10-05 |
| BP term | GO:0006805 | Xenobiotic
metabolic process | 8 |
1.45x10-04 |
| BP term | GO:0042060 | Wound healing | 8 |
1.70x10-04 |
| BP term | GO:0006081 | Cellular aldehyde
metabolic process | 4 |
4.70x10-04 |
| BP term | GO:0030277 | Maintenance of
gastrointestinal epithelium | 4 |
6.20x10-04 |
| BP term | GO:0010107 | Potassium ion
import | 5 |
6.98x10-04 |
| BP term | GO:0007584 | Response to
nutrient | 7 |
7.50x10-04 |
| BP term | GO:0002576 | Platelet
degranulation | 8 |
7.99x10-04 |
| BP term | GO:0060021 | Palate
development | 7 |
8.64x10-04 |
| BP term | GO:0010812 | Negative regulation
of cell-substrate adhesion | 4 | 0.001003 |
| BP term | GO:0001503 | Ossification | 7 | 0.001131 |
| BP term | GO:0030168 | Platelet
activation | 8 | 0.001523 |
| BP term | GO:0051216 | Cartilage
development | 6 | 0.001703 |
| BP term | GO:0010628 | Positive regulation
of gene expression | 12 | 0.001721 |
| BP term | GO:0001523 | Retinoid metabolic
process | 6 | 0.001977 |
| BP term | GO:0016525 | Negative regulation
of angiogenesis | 6 | 0.002125 |
| BP term | GO:0055114 | Oxidation-reduction
process | 19 | 0.002857 |
| BP term | GO:0032964 | Collagen
biosynthetic process | 3 | 0.003084 |
| BP term | GO:0008284 | Positive regulation
of cell proliferation | 16 | 0.003752 |
| BP term | GO:0001649 | Osteoblast
differentiation | 7 | 0.004274 |
| BP term | GO:0022617 | Extracellular
matrix disassembly | 6 | 0.005144 |
| BP term | GO:0071711 | Basement membrane
organization | 3 | 0.005647 |
| BP term | GO:0050891 | Multicellular
organismal water homeostasis | 3 | 0.005647 |
| BP term | GO:0001525 | Angiogenesis | 10 | 0.005716 |
| BP term | GO:0042476 | Odontogenesis | 4 | 0.007007 |
| BP term | GO:0010575 | Positive regulation
of vascular endothelial growth factor production | 4 | 0.007007 |
| BP term | GO:0050909 | Sensory perception
of taste | 4 | 0.008568 |
| BP term | GO:0001937 | Negative regulation
of endothelial cell proliferation | 4 | 0.008568 |
| BP term | GO:0040037 | Negative regulation
of fibroblast growth factor receptor signaling pathway | 3 | 0.008901 |
| BP term | GO:0042572 | Retinol metabolic
process | 4 | 0.009418 |
| CC term | GO:0005615 | Extracellular
space | 63 |
9.65x10-17 |
| CC term | GO:0031012 | Extracellular
matrix | 28 |
2.46x10-14 |
| CC term | GO:0070062 | Extracellular
exosome | 87 |
1.68x10-12 |
| CC term | GO:0005576 | Extracellular
region | 61 |
4.86x10-12 |
| CC term | GO:0005788 | Endoplasmic
reticulum lumen | 20 |
4.73x10-11 |
| CC term | GO:0005581 | Collagen
trimer | 15 |
5.56x10-11 |
| CC term | GO:0005604 | Basement
membrane | 9 |
1.82x10-05 |
| CC term | GO:0005578 | Proteinaceous
extracellular matrix | 22 |
3.57x10-10 |
| CC term | GO:0016324 | Apical plasma
membrane | 16 |
2.29x10-05 |
| CC term | GO:0009986 | Cell surface | 20 |
3.51x10-04 |
| CC term | GO:0005887 | Integral component
of plasma membrane | 34 | 0.004256 |
| CC term | GO:0005886 | Plasma
membrane | 79 | 0.004569 |
| CC term | GO:0030141 | Secretory
granule | 6 | 0.004319 |
| CC term | GO:0031093 | Platelet alpha
granule lumen | 5 | 0.008125 |
| CC term | GO:0031090 | Organelle
membrane | 6 | 0.008522 |
| MF term | GO:0048407 | Platelet-derived
growth factor binding | 6 |
2.55x10-07 |
| MF term | GO:0005518 | Collagen
binding | 8 |
2.37x10-05 |
| MF term | GO:0050840 | Extracellular
matrix binding | 6 |
3.05x10-05 |
| MF term | GO:0005242 | Inward rectifier
potassium channel activity | 4 | 0.002802 |
| MF term | GO:0046332 | SMAD binding | 5 | 0.003328 |
| MF term | GO:0005201 | Extracellular
matrix structural constituent | 12 |
2.77x10-09 |
| MF term | GO:0001758 | Retinal
dehydrogenase activity | 3 | 0.004132 |
| MF term | GO:0005178 | Integrin
binding | 11 |
2.77x10-06 |
| MF term | GO:0005509 | Calcium ion
binding | 27 |
1.47x10-05 |
| MF term | GO:0008201 | Heparin
binding | 12 |
2.07x10-05 |
| MF term | GO:0016491 | Oxidoreductase
activity | 9 | 0.008547 |
| MF term | GO:0008083 | Growth factor
activity | 8 | 0.009105 |
| KEGG pathway | hsa04512 | ECM-receptor
interaction | 16 |
5.16x10-11 |
| KEGG pathway | hsa04974 | Protein digestion
and absorption | 14 |
7.73x10-09 |
| KEGG pathway | hsa04510 | Focal adhesion | 18 |
2.67x10-07 |
| KEGG pathway | hsa05146 | Amoebiasis | 10 |
1.63x10-04 |
| KEGG pathway | hsa04971 | Gastric acid
secretion | 8 |
4.23x10-04 |
| KEGG pathway | hsa04151 | PI3K-Akt signaling
pathway | 17 |
7.35x10-04 |
| KEGG pathway | hsa00830 | Retinol
metabolism | 7 | 0.00124 |
| KEGG pathway | hsa00982 | Drug
metabolism-cytochrome P450 | 7 | 0.001703 |
| KEGG pathway | hsa00980 | Metabolism of
xenobiotics by cytochrome P450 | 7 | 0.002628 |
| KEGG pathway | hsa05204 | Chemical
carcinogenesis | 7 | 0.003889 |
PPI network construction
Based on the STRING prediction results, a PPI
network with 211 nodes and 741 sides was constructed in Cytoscape
(Fig. 3), and the number of segments
connected to each gene in the figure represents its degree.
Identification of six key genes
The two genes with the most nodes were FN1
and COL1A1. In the PPI network, FN1 was the most
prominent, with the highest degree of connectivity at 52. The
degree of connectivity of COL1A1 is 43 (Table IV). Expression of these two genes is
upregulated in GC tissues. Additionally, of those DEGs shared among
the four gene expression profiles, the two DEGs with the largest
logFC and the two DEGs with the smallest logFC values were
selected. The higher the logFC in the upregulated DEGs, the greater
the increase in expression of the gene. Similarly, the lower the
logFC values in the downregulated DEGs, the greater the decrease in
expression of the gene. When sorting DEGs according to logFC, the
logFC of GSE19826 was used as the standard, as chip GSE19826
represented a homogenous cancer tissue population at each
Tumor-Node-Metastasis stage (25),
which increases the accuracy of the expression profile (Table V). The two DEGs with the largest logFC
values were INHBA (logFC=4.35) and CST1 (logFC=4.18)
(Table VI). The two DEGs with the
smallest logFC values were ATP4A (logFC=-6.46) and
ATP4B (logFC=-5.91) (Table
VII). Therefore, these six genes were selected as key
genes.
 | Table IVThe 10 genes with the largest degree
of connectivity in the protein-protein-interaction network. |
Table IV
The 10 genes with the largest degree
of connectivity in the protein-protein-interaction network.
| Rank | Gene | Degree |
|---|
| 1 | FN1 | 52 |
| 2 | COL1A1 | 43 |
| 3 | COL1A2 | 38 |
| 4 | COL3A1 | 37 |
| 5 | FBN1 | 35 |
| 6 | BGN | 32 |
| 6 | COL5A2 | 32 |
| 8 | TIMP1 | 31 |
| 9 | SPARC | 30 |
| 10 | THBS2 | 28 |
 | Table VThe expression data from GSE19826 in
gastric cancer. |
Table V
The expression data from GSE19826 in
gastric cancer.
| Tissue type | Accession no. | Title | Stage |
|---|
| Noncancer
tissue | GSM495051 | CB2008210-1N | n/a |
| Gastric cancer
tissue | GSM495052 | CB2008210-1T | II |
| Noncancer
tissue | GSM495053 | CB2008210-2N | n/a |
| Gastric cancer
tissue | GSM495054 | CB2008210-2T | IV |
| Noncancer
tissue | GSM495055 | CB2008210-3N | n/a |
| Gastric cancer
tissue | GSM495056 | CB2008210-3T | I |
| Noncancer
tissue | GSM495057 | CB2008210-4N | n/a |
| Gastric cancer
tissue | GSM495058 | CB2008210-4T | II |
| Noncancer
tissue | GSM495059 | CB2008210-5N | n/a |
| Gastric cancer
tissue | GSM495060 | CB2008210-5T | III |
| Noncancer
tissue | GSM495061 | CB2008210-6N | n/a |
| Gastric cancer
tissue | GSM495062 | CB2008210-6T | IV |
| Noncancer
tissue | GSM495063 | CB2008210-7N | n/a |
| Gastric cancer
tissue | GSM495064 | CB2008210-7T | IV |
| Noncancer
tissue | GSM495065 | CB2008210-9N | n/a |
| Gastric cancer
tissue | GSM495066 | CB2008210-9T | III |
| Noncancer
tissue | GSM495067 | CB2008210-12N | n/a |
| Gastric cancer
tissue | GSM495068 | CB2008210-12T | II |
| Noncancer
tissue | GSM495069 | CB2008210-13N | n/a |
| Gastric cancer
tissue | GSM495070 | CB2008210-13T | I |
| Noncancer
tissue | GSM495071 | CB2008210-14N | n/a |
| Gastric cancer
tissue | GSM495072 | CB2008210-14T | III |
| Noncancer
tissue | GSM495073 | CB2008210-15N | n/a |
| Gastric cancer
tissue | GSM495074 | CB2008210-15T | I |
| Normal gastric
tissue | GSM495075 | CB2008210-3C | n/a |
| Normal gastric
tissue | GSM495076 | CB2008210-5C | n/a |
| Normal gastric
tissue | GSM495077 | CB2008210-9C | n/a |
 | Table VIThe 10 genes with the largest logFC
values in GSE19826. |
Table VI
The 10 genes with the largest logFC
values in GSE19826.
| Rank | Name | LogFC |
|---|
| 1 | INHBA | 4.35 |
| 2 | CST1 | 4.18 |
| 3 | COL11A1 | 4.11 |
| 4 | FAP | 3.91 |
| 5 | COL10A1 | 3.72 |
| 6 | FNDC1 | 3.27 |
| 6 | COL8A1 | 3.17 |
| 8 | SERPINH1 | 2.97 |
| 9 | CDH3 | 2.95 |
| 10 | THBS2 | 2.94 |
 | Table VIIThe 10 genes with the smallest logFC
values in GSE19826. |
Table VII
The 10 genes with the smallest logFC
values in GSE19826.
| Rank | Name | LogFC |
|---|
| 1 | ATP4A | -6.46 |
| 2 | ATP4B | -5.91 |
| 3 | KCNE2 | -5.88 |
| 4 | AQP4 | -5.81 |
| 5 | GIF | -5.75 |
| 6 | LIPF | -5.53 |
| 6 | CHIA | -5.51 |
| 8 | GKN1 | -5.49 |
| 9 | GKN2 | -5.44 |
| 10 | DPCR1 | -4.83 |
Analysis of the six key genes in
Oncomine
The Oncomine database was used to confirm the
expression of the six key genes in 20 different types of cancer.
The results showed that there were statistically significant
differences in their expression. In the Oncomine database, there
were no studies showing low expression of FN1,
COL1A1, INHBA or CST1 in GC, but there were
six, eight, seven and four studies showing increased expression,
respectively. For ATP4A and ATP4B, the reverse was
observed with no studies showing high expression, but seven and six
studies, respectively, showing decreased expression (Fig. 4).
After comparing the expression levels of these six
genes in cancerous and normal gastric tissue, the expression levels
of FN1, COL1A1, INHBA and CST1 in GC
tissues were significantly higher compared with the control group,
and the expression levels of ATP4A and ATP4B in GC
tissues were significantly lower compared with the control group
(Table VIII; Fig. 5).
 | Table VIIIAdditional information for the six
key genes shown in Figure 5. |
Table VIII
Additional information for the six
key genes shown in Figure 5.
| Author, year | Gene | Normal tissue
samples | Gastric cancer
samples | P-value | Fold Change | Published
journal | (Refs.) |
|---|
| Chen et al,
2003 | FN1 | 28 | 8 |
5.73x10-14 | 7.441 | Molecular Biology
of The Cell | (26) |
| Cui et al,
2011 | COL1A1 | 80 | 80 |
1.81x10-15 | 3.201 | Nucleic Acids
Research | (28) |
| Cui et al,
2011 | INHBA | 80 | 80 |
5.17x10-13 | 3.043 | Nucleic Acids
Research | (28) |
| Cho et al,
2011 | CST1 | 19 | 31 |
3.17x10-13 | 21.525 | Clinical Cancer
Research | (27) |
| Cho et al,
2011 | ATP4A | 19 | 20 |
4.73x10-17 | -100.911 | Clinical Cancer
Research | (27) |
| D'Errico et
al, 2009 | ATP4B | 31 | 26 |
6.15x10-19 | -246.630 | European Journal of
Cancer | (11) |
In addition, meta-analyses of the six key genes in
GC in the Oncomine database also supported the findings that
expression of FN1, COL1A1, INHBA and
CST1 is upregulated in GC, whereas expression of
ATP4A and ATP4B is downregulated in GC (11,12,26-28).
The studies and references involved are shown in Fig. 6. In the meta-analyses, P=-0.000,
FC≥2.0 and gene rank ≤300 were selected as the cutoff criteria.
Survival analysis of the six key
genes
To identify the prognostic value of the six
potential key genes, overall survival curves based on differential
expression of the six key genes were plotted using Kaplan-Meier
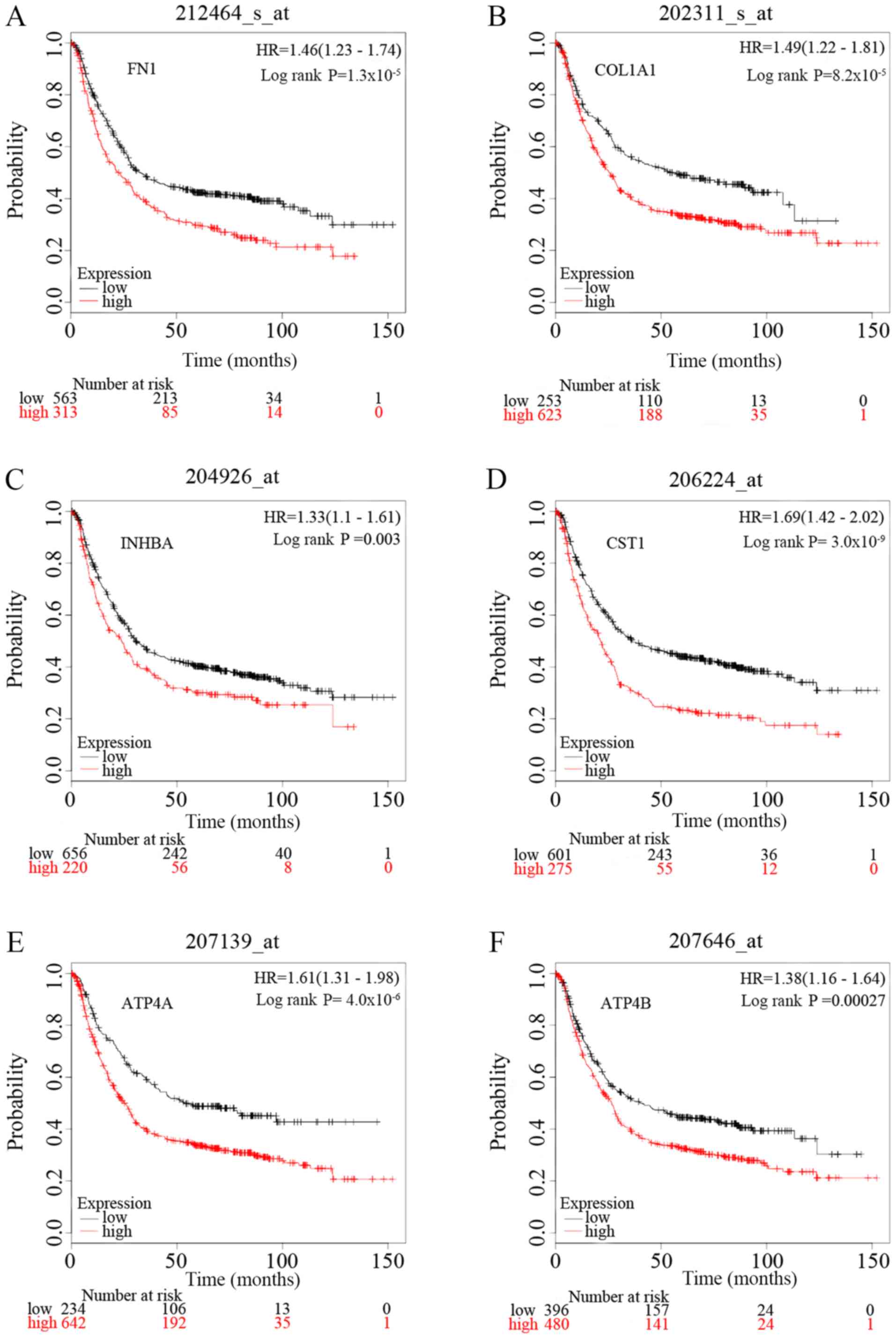
plotter (Fig. 7). There were 1,440
patients with GC on the Kaplan-Meier plotter platform who were
suitable for the analysis of overall survival. The curves indicate
that overexpression of the six key genes is significantly
associated with decreased overall survival times of patients with
GC. However, it is worth noting that ATP4A and ATP4B
were significantly downregulated in GC samples in the present
study.
Discussion
GC is a complex heterogeneous disease with high
incidence and mortality rates, and poses a serious threat to
afflicted patients. Therefore, it is important to identify
biomarkers that are meaningful for both diagnostic and prognostic
assessment (29).
In the present study, 271 DEGs were screened,
including 99 upregulated and 172 downregulated genes, by analyzing
four gene expression profiles containing a combined 176 GC tissue
samples and 82 normal gastric tissue samples. Of the causes of
cancer-associated deaths, 90% are the result of metastasis
(30). In the present study, GO
enrichment results showed that the occurrence and development of GC
was closely associated with metastasis. GO analysis indicated that
DEGs were primarily associated with extracellular matrix
organization, collagen catabolic process and cell adhesion.
Collagen is the primary component of the extracellular matrix and
of the interstitial microenvironment. Collagen can provide a
scaffold for tumor cell growth and induce migration of tumor cells
(31,32). There is evidence that collagen
synthesis increases in the presence of a gastric tumor (33). Zhou et al (32) reported that collagen components are
quantitatively and qualitatively reorganized in the tumor
microenvironment of GC, and collagen width was identified as a
useful prognostic indicator for GC (32). In addition, studies have shown that
changes in cell-cell adhesion and cell-matrix adhesion can promote
cancer cell metastasis (34). MF
analysis showed that the DEGs were significantly enriched in
platelet-derived growth factor binding. It has been demonstrated
that inhibition of platelet-derived growth factor receptor-a can
reduce the proliferation of gastrointestinal stromal tumor cells
with mutant v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene
homolog (KIT) by affecting the KIT-dependent transcription factor
ETV1(35).
KEGG pathway analysis showed that the DEGs were
primarily enriched in ECM-receptor interaction, protein digestion
and absorption, and focal adhesion. ECM-receptor interaction serves
a vital role in several types of cancer (36-38).
The interaction between membrane receptors of tumor cells and ECM
proteins serve an important role in tumor invasion and metastasis
(39), and ECM-receptor interaction
serve a crucial role in the process of tumor shedding, adhesion,
degradation, movement and hyperplasia (38). In addition, the non-steroidal
anti-inflammatory drug celecoxib may exhibit anti-GC effects by
inhibiting the expression of various proteins and inhibiting
leukocyte transendothelial migration and focal adhesion (40), which provides a possible mechanism for
future investigations of the role of focal adhesion in GC and
developing new anti-GC drugs.
The degree of connectivity of a gene in a PPI
network reflects its association with GC. The greater the
connectivity, the closer a gene is to the disease mechanism. The
logFC values of DEGs reflects the level of up or downregulation of
the gene. The higher the logFC values in the upregulated DEGs, the
greater the degree of upregulation of the gene, and the lower the
logFC values in the downregulated DEGs, the greater the degree of
downregulation (41-43).
Thus it was hypothesized that the DEGs with the highest and lowest
logFC values would be the genes most closely associated with
disease mechanisms.
In the present study, the two genes with the highest
degree of connectivity in the PPI network, and the two DEGs with
the largest and smallest logFC values, were all selected as key
genes. These were FN1, COL1A1, INHBA,
CST1, ATP4A and ATP4B. These six key genes
were verified in the Oncomine database. Expression of FN1,
COL1A1, INHBA and CST1 were upregulated in GC,
and expression of ATP4A and ATP4B were downregulated, consistent
with the results obtained from analysis of the GEO datasets.
Furthermore, survival analysis showed that upregulation of the six
key genes was significantly associated with worse overall survival,
and downregulation of ATP4A and ATP4B expression predicted a more
favorable prognosis for patients with GC, providing novel insights
into potential GC treatment strategies.
FN1 was the gene with the highest degree of
connectivity. It is expressed in a wide range of healthy
plasmalemmas, lamina propria mucosae and smooth-muscle cell layers,
and it is involved in a variety of cellular processes including
embryogenesis, blood coagulation, wound healing, host defense and
metastasis (44). As a glycoprotein
involved in cell adhesion and migratory processes, FN1 is
hypothesized to be associated with signaling pathways associated
with cancer (13). Expression of
FN1 is significantly increased in anti-chemotherapy
osteosarcoma cell lines and tissues, and is associated with a poor
prognosis (45). Knockdown of
FN1 gene expression results in reduced cell proliferation,
increased cellular senescence and apoptosis, and reduced migration
and invasion, by blocking the activation of the PI3K/AKT signaling
pathway (46). Furthermore,
downregulation of FN1 inhibits proliferation, migration and
invasion, and thus reduces progression of colorectal cancer
(47). The results of the present
study suggest that FN1 may be a potential biomarker and
therapeutic target for diagnosis and treatment of GC, consistent
with previous studies (13,48,49), and
thus further confirming the significance of FN1 in GC.
COL1A1 is one of the most important components of
the ECM, and it is highly expressed in most connective tissues and
various human solid tumors (50). It
is also the primary component of type I collagen, which serves a
key role in tumor cell adhesion and invasion (51). A mechanistic study revealed that
COL1A1 and COL1A2 affects angiogenesis in GC, and their expression
is also significantly associated with progression of GC (52). In addition, Zhang et al
(53) further confirmed that
overexpression of COL1A1 promoted GC cell proliferation
in vitro. These previous studies support the use of
COL1A1 as a key potential GC biomarker in the present
study.
INHBA is a member of the transforming growth
factor-β (TGF-β) superfamily, which is closely associated with
tumor proliferation and expression is upregulated in lung cancer
(54), GC (12) and colon cancer (55), where INHBA expression is
closely associated with their prognosis. In a study of GC, Chen
et al (56) found that
INHBA gene silencing reduced migration and invasion of GC
cells by blocking the activation of the TGF-β signaling pathway.
They suggested that INHBA was a potential target for GC
therapy (56). Another study showed
that INHBA mRNA expression in GC may be a useful prognostic
biomarker for patients with stage II or III GC who receive adjuvant
chemotherapy with S-1(57). The
results of the present study support the conclusions drawn in these
previous studies.
Cystatin SN (CST1) is a member of the type 2
cystatin superfamily, the primary role of which is to limit the
proteolytic activity of cysteine proteases (58). The dysregulated expression of
CST1 is hypothesized to be involved in several types of
cancer, including cholangiocarcinoma (59), breast cancer (58), GC (60)
and colorectal cancer (61).
CST1 prevents cell aging and promotes cancer development by
affecting the activity of cathepsin B (62). However, CST1 has not been
analyzed using bioinformatics for survival prognosis in GC, to the
best of our knowledge. Using multiple databases, the present study
is the first to validate CST1 as a novel prognostic
biomarker and a potential therapeutic target for treatment of
GC.
ATP4A encodes the α subunit and ATP4B
encodes the β subunit of the gastric H+, K+-ATPase, respectively.
They regulate gastric acid secretion and, as a result, are targets
for acid reduction (63). Fei et
al (64) found that expression of
ATP4A and ATP4B were significantly downregulated in
patients with GC, but their expression was not significantly
correlated with overall survival (64). In the present study, downregulation of
ATP4A and ATP4B expression was associated with
favorable overall survival in patients with GC. Downregulation of
ATP4A and ATP4B mRNA expression in GC tissue is
associated with the development of GC (65). Correa's Cascade is inversely
associated with gastric acid secretion rate, the downregulation of
ATP4A and ATP4B mRNA expression begins in the early
stages of gastric mucosal lesions, and the expression of both is
gradually decreased as Correa's cascade progresses (66). In addition, Helicobacter pylori
(H. pylori) inhibits parietal acid secretion by
downregulating the expression of ATP4A and ATP4B in
gastric parietal cells prior to the formation of GC, suggesting
that H. pylori is closely associated with the development of
GC (67). Thus, it was hypothesized
that ATP4A and ATP4B may inhibit the formation of GC.
Survival analysis showed that ATP4A and ATP4B in GC
are adverse prognostic factors for patients with GC, suggesting
that upregulation is associated with progression of GC. However,
studies have reported that the expression of ATP4A and
ATP4B is not regulated by H. pylori in GC (68-70).
Other studies have shown significant decreases in the abundance of
Helicobacter and Neisseria, and significant increases
in Achromobacter, Citrobacter,
Phyllobacterium, Clostridium, Rhodococcus and
Lactobacillus in gastric carcinoma in comparison with
chronic gastritis (71,72). Additionally, the gastric microbiota
composition in patients with gastric carcinoma is significantly
different compared with patients with chronic gastritis (71). Therefore, it was hypothesized that the
formation of an altered gastric microbiota composition may result
in the expression of ATP4A and ATP4B to be passively
upregulated as GC progresses. Further research is required to more
accurately determine the biological function of ATP4A and
ATP4B in GC.
Although several genes were identified as promising
diagnostic and prognostic biomarkers for GC, the present study has
the following limitations. First, the present study lacked
experimental and clinical validation. Second, the possibility that
different histological types may affect the accuracy of results
cannot be eliminated. Thus, future bioinformatics analysis should
be designed such that samples can be stratified by histological
type. Finally, the sample size was relatively small for the RNA-Seq
experiments, which may result in inaccuracies or results which are
not completely representative of the wider populace. Therefore, it
is necessary to use larger samples to perform bioinformatics
analysis, and further experimental and clinical studies are
required.
In conclusion, the present study used bioinformatics
to analyze biological processes and signaling pathways closely
associated with GC occurrence and development and identified
FN1, COL1A1, INHBA and CST1 as
promising diagnostic and prognostic biomarkers for GC patients.
Additionally, the results of the survival analysis of ATP4A
and ATP4B were inconsistent with other international
studies. Therefore, further studies are required to assess the
effects of ATP4A and ATP4B on GC initiation and
development. Furthermore, experimental and clinical studies are
required to validate the findings of the present study and
determine the potential clinical value of these potential
biomarkers.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Key
R&D Program of China (grant nos. 2018YFC1704100 and
2018YFC1704106).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author upon reasonable
request.
Authors' contributions
WW and YH conceived of and designed the study. YH
and QZ performed the bioinformatics analysis and analyzed the data.
WW and QZ wrote the manuscript. WW and ZL revised the manuscript.
XZ contributed to the design of the study and revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shi J, Qu YP and Hou P: Pathogenetic
mechanisms in gastric cancer. World J Gastroenterol.
20:13804–13819. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Van Cutsem E, Sagaert X, Topal B,
Haustermans K and Prenen H: Gastric cancer. Lancet. 388:2654–2664.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
In H, Solsky I, Palis B, Langdon-Embry M,
Ajani J and Sano T: Validation of the 8th Edition of the AJCC TNM
staging system for gastric cancer using the national cancer
database. Ann Surg Oncol. 24:3683–3691. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Peng H, Deng Y, Wang L, Cheng Y, Xu Y,
Liao J and Wu H: Identification of potential biomarkers with
diagnostic value in pituitary adenomas using prediction analysis
for microarrays method. J Mol Neurosci. 69:399–410. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wu Y, Jamal M, Xie T, Sun J, Song T, Yin
Q, Li J, Pan S, Zeng X, Xie S and Zhang Q: Uridine-cytidine kinase
2 (UCK2): A potential diagnostic and prognostic biomarker for lung
cancer. Cancer Sci. 110:2734–2747. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen Z, Zhou Y, Luo R, Liu K and Chen Z:
Trophinin-associated protein expression is an independent
prognostic biomarker in lung adenocarcinoma. J Thorac Dis.
11:2043–2050. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li D, Lin C, Li N, Du Y, Yang C, Bai Y,
Feng Z, Su C, Wu R, Song S, et al: PLAGL2 and POFUT1 are regulated
by an evolutionarily conserved bidirectional promoter and are
collaboratively involved in colorectal cancer by maintaining
stemness. EBioMedicine. 45:124–138. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yong L, YuFeng Z and Guang B: Association
between PPP2CA expression and colorectal cancer prognosis tumor
marker prognostic study. Int J Surg. 59:80–89. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Troiano G, Guida A, Aquino G, Botti G,
Losito NS, Papagerakis S, Pedicillo MC, Ionna F, Longo F, Cantile
M, et al: Integrative histologic and bioinformatics analysis of
BIRC5/Survivin expression in oral squamous cell carcinoma. Int J
Mol Sci. 19(E2664)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
D'Errico M, de Rinaldis E, Blasi MF, Viti
V, Falchetti M, Calcagnile A, Sera F, Saieva C, Ottini L, Palli D,
et al: Genome-wide expression profile of sporadic gastric cancers
with microsatellite instability. Eur J Cancer. 45:461–469.
2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang Q, Wen YG, Li DP, Xia J, Zhou CZ, Yan
DW, Tang HM and Peng ZH: Upregulated INHBA expression is associated
with poor survival in gastric cancer. Med Oncol. 29:77–83.
2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Li L, Zhu Z, Zhao Y, Zhang Q, Wu X, Miao
B, Cao J and Fei S: FN1, SPARC, and SERPINE1 are highly expressed
and significantly related to a poor prognosis of gastric
adenocarcinoma revealed by microarray and bioinformatics. Sci Rep.
9(7827)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology. The gene
ontology consortium. Nat Genet. 25:25–29. 2000.PubMed/NCBI View Article : Google Scholar
|
|
15
|
The Gene Ontology Consortium: The Gene
Ontology Resource: 20 years and still GOing strong. Nucleic Acids
Res 47: D330.D338, 2019.
|
|
16
|
Kanehisa M: ‘Post-genome Informatics’,
Oxford University Press (2000). https://www.kanehisa.jp/docs/archive/PGI-contents.html.
|
|
17
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Huang da W, Sherman BT and Lempicki RA:
Bioinformatics enrichment tools: Paths toward the comprehensive
functional analysis of large gene lists. Nucleic Acids Res.
37:1–13. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xu W, Wang Y, Wang Y, Lv S, Xu X and Dong
X: Screening of differentially expressed genes and identification
of NUF2 as a prognostic marker in breast cancer. Int J Mol Med.
44:390–404. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Szklarczyk D, Gable AL, Lyon D, Junge A,
Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork
P, et al: STRING v11: Protein-protein association networks with
increased coverage, supporting functional discovery in genome-wide
experimental datasets. Nucleic Acids Res. 47:D607–D613.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chin CH, Chen SH, Wu HH, Ho CW, Ko MT and
Lin CY: cytoHubba: Identifying hub objects and sub-networks from
complex interactome. BMC Syst Biol 8 (Suppl 4): S11, 2014.
|
|
23
|
Liu Y, Cui S, Li W, Zhao Y, Yan X and Xu
J: PAX3 is a biomarker and prognostic factor in melanoma: Database
mining. Oncol Lett. 17:4985–4993. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nagy Á, Lánczky A, Menyhárt O and Győrffy
B: Validation of miRNA prognostic power in hepatocellular carcinoma
using expression data of independent datasets. Sci Rep.
8(9227)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Rausei S, Ruspi L, Galli F, Pappalardo V,
Di Rocco G, Martignoni F, Frattini F, Rovera F, Boni L and Dionigi
G: Seventh tumor-node-metastasis staging of gastric cancer:
Five-year follow-up. World J Gastroenterol. 22:7748–7753.
2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chen X, Leung SY, Yuen ST, Chu KM, Ji J,
Li R, Chan AS, Law S, Troyanskaya OG, Wong J, et al: Variation in
gene expression patterns in human gastric cancers. Mol Biol Cell.
14:3208–3215. 2003.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cho JY, Lim JY, Cheong JH, Park YY, Yoon
SL, Kim SM, Kim SB, Kim H, Hong SW, Park YN, et al: Gene expression
signature-based prognostic risk score in gastric cancer. Clin
Cancer Res. 17:1850–1857. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cui J, Chen Y, Chou WC, Sun L, Chen L, Suo
J, Ni Z, Zhang M, Kong X, Hoffman LL, et al: An integrated
transcriptomic and computational analysis for biomarker
identification in gastric cancer. Nucleic Acids Res. 39:1197–207.
2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Serra O, Galán M, Ginesta MM, Calvo M,
Sala N and Salazar R: Comparison and applicability of molecular
classifications for gastric cancer. Cancer Treat Rev. 77:29–34.
2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gilkes DM, Semenza GL and Wirtz D: Hypoxia
and the extracellular matrix: Drivers of tumour metastasis. Nat Rev
Cancer. 14:430–439. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Climent M, Pera M, Aymar I, Ramón JM,
Grande L and Nogués X: Bone health in long-term gastric cancer
survivors: A prospective study of high-dose vitamin D
supplementation using an easy administration scheme. J Bone Miner
Metab. 36:462–469. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhou ZH, Ji CD, Xiao HL, Zhao HB, Cui YH
and Bian XW: Reorganized collagen in the tumor microenvironment of
gastric cancer and its association with prognosis. J Cancer.
8:1466–1476. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Jang M, Koh I, Lee SJ, Cheong JH and Kim
P: Droplet-based microtumor model to assess cell-ECM interactions
and drug resistance of gastric cancer cells. Sci Rep.
7(41541)2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu X and Chu KM: E-cadherin and gastric
cancer: Cause, consequence, and applications. Biomed Res Int.
2014(637308)2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hayashi Y, Bardsley MR, Toyomasu Y,
Milosavljevic S, Gajdos GB, Choi KM, Reid-Lombardo KM, Kendrick ML,
Bingener-Casey J, Tang CM, et al: Platelet-derived growth factor
receptor-α regulates proliferation of gastrointestinal stromal
tumor cells with mutations in KIT by stabilizing ETV1.
Gastroenterology. 149:420–432.e16. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Rahbari NN, Kedrin D, Incio J, Liu H, Ho
WW, Nia HT, Edrich CM, Jung K, Daubriac J, Chen I, et al: Anti-VEGF
therapy induces ECM remodeling and mechanical barriers to therapy
in colorectal cancer liver metastases. Sci Transl Med.
8(360ra135)2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Andersen MK, Rise K, Giskeødegård GF,
Richardsen E, Bertilsson H, Størkersen Ø, Bathen TF, Rye M and
Tessem MB: Integrative metabolic and transcriptomic profiling of
prostate cancer tissue containing reactive stroma. Sci Rep.
8(14269)2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Bao Y, Wang L, Shi L, Yun F, Liu X, Chen
Y, Chen C, Ren Y and Jia Y: Transcriptome profiling revealed
multiple genes and ECM-receptor interaction pathways that may be
associated with breast cancer. Cell Mol Biol Lett.
24(38)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhu H, Chen H, Wang J, Zhou L and Liu S:
Collagen stiffness promoted non-muscle-invasive bladder cancer
progression to muscle-invasive bladder cancer. Onco Targets Ther.
12:3441–3457. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Jin GH, Xu W, Shi Y and Wang LB: Celecoxib
exhibits an anti-gastric cancer effect by targeting focal adhesion
and leukocyte transendothelial migration-associated genes. Oncol
Lett. 12:2345–2350. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
He WQ, Gu JW, Li CY, Kuang YQ, Kong B,
Cheng L, Zhang JH, Cheng JM and Ma Y: The PPI network and clusters
analysis in glioblastoma. Eur Rev Med Pharmacol Sci. 19:4784–4790.
2015.PubMed/NCBI
|
|
42
|
Miryala SK, Anbarasu A and Ramaiah S:
Discerning molecular interactions: A comprehensive review on
biomolecular interaction databases and network analysis tools.
Gene. 642:84–94. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Yan H, Zheng G, Qu J, Liu Y, Huang X,
Zhang E and Cai Z: Identification of key candidate genes and
pathways in multiple myeloma by integrated bioinformatics analysis.
J Cell Physiol. 234:23785–23797. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zhang H, Sun Z, Li Y, Fan D and Jiang H:
MicroRNA-200c binding to FN1 suppresses the proliferation,
migration and invasion of gastric cancer cells. Biomed
Pharmacother. 88:285–292. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Kun-Peng Z, Chun-Lin Z, Xiao-Long M and
Lei Z: Fibronectin-1 modulated by the long noncoding RNA
OIP5-AS1/miR-200b-3p axis contributes to doxorubicin resistance of
osteosarcoma cells. J Cell Physiol. 234:6927–6939. 2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Liao YX, Zhang ZP, Zhao J and Liu JP:
Effects of fibronectin 1 on cell proliferation, senescence and
apoptosis of human glioma cells through the PI3K/AKT signaling
pathway. Cell Physiol Biochem. 48:1382–1396. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Cai X, Liu C, Zhang TN, Zhu YW, Dong X and
Xue P: Down-regulation of FN1 inhibits colorectal carcinogenesis by
suppressing proliferation, migration, and invasion. J Cell Biochem.
119:4717–4728. 2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Yan P, He Y, Xie K, Kong S and Zhao W: In
silico analyses for potential key genes associated with gastric
cancer. PeerJ. 6(e6092)2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Jiang K, Liu H, Xie D and Xiao Q:
Differentially expressed genes ASPN, COL1A1, FN1, VCAN and MUC5AC
are potential prognostic biomarkers for gastric cancer. Oncol Lett.
17:3191–3202. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Sun S, Wang Y, Wu Y, Gao Y, Li Q,
Abdulrahman AA, Liu XF, Ji GQ, Gao J, Li L, et al: Identification
of COL1A1 as an invasion-related gene in malignant astrocytoma. Int
J Oncol. 53:2542–2554. 2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Liu J, Shen JX, Wu HT, Li XL, Wen XF, Du
CW and Zhang GJ: Collagen 1A1 (COL1A1) promotes metastasis of
breast cancer and is a potential therapeutic target. Discov Med.
25:211–223. 2018.PubMed/NCBI
|
|
52
|
Huang C, Yang X, Han L, Fan Z, Liu B,
Zhang C and Lu T: The prognostic potential of alpha-1 type I
collagen expression in papillary thyroid cancer. Biochem Biophys
Res Commun. 515:125–132. 2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Zhang QN, Zhu HL, Xia MT, Liao J, Huang
XT, Xiao JW and Yuan C: A panel of collagen genes are associated
with prognosis of patients with gastric cancer and regulated by
microRNA-29c-3p: An integrated bioinformatics analysis and
experimental validation. Cancer Manag Res. 11:4757–4772.
2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Seder CW, Hartojo W, Lin L, Silvers AL,
Wang Z, Thomas DG, Giordano TJ, Chen G, Chang AC, Orringer MB, et
al: Upregulated INHBA expression may promote cell proliferation and
is associated with poor survival in lung adenocarcinoma. Neoplasia.
11:388–396. 2009.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Yang H, Wu J, Zhang J, Yang Z, Jin W, Li
Y, Jin L, Yin L, Liu H and Wang Z: Integrated bioinformatics
analysis of key genes involved in progress of colon cancer. Mol
Genet Genomic Med. 7(e00588)2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Chen ZL, Qin L, Peng XB, Hu Y and Liu B:
INHBA gene silencing inhibits gastric cancer cell migration and
invasion by impeding activation of the TGF-β signaling pathway. J
Cell Physiol. 234:18065–18074. 2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Katayama Y, Oshima T, Sakamaki K, Aoyama
T, Sato T, Masudo K, Shiozawa M, Yoshikawa T, Rino Y, Imada T and
Masuda M: Clinical significance of INHBA gene expression in
patients with gastric cancer who receive curative resection
followed by adjuvant S-1 chemotherapy. In Vivo. 31:565–571.
2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Dai DN, Li Y, Chen B, Du Y, Li SB, Lu SX,
Zhao ZP, Zhou AJ, Xue N, Xia TL, et al: Elevated expression of CST1
promotes breast cancer progression and predicts a poor prognosis. J
Mol Med (Berl). 95:873–886. 2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Tian A, Pu K, Li B, Li M, Liu X, Gao L and
Mao X: Weighted gene coexpression network analysis reveals hub
genes involved in cholangiocarcinoma progression and prognosis.
Hepatol Res. 49:1195–1206. 2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Kim J, Bae DH, Kim JH, Song KS, Kim YS and
Kim SY: HOXC10 overexpression promotes cell proliferation and
migration in gastric cancer. Oncol Rep. 42:202–212. 2019.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Jiang J, Liu HL, Tao L, Lin XY, Yang YD,
Tan SW and Wu B: Let-7d inhibits colorectal cancer cell
proliferation through the CST1/p65 pathway. Int J Oncol.
53:781–790. 2018.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Oh SS, Park S, Lee KW, Madhi H, Park SG,
Lee HG, Cho YY, Yoo J and Dong Kim K: Extracellular cystatin SN and
cathepsin B prevent cellular senescence by inhibiting abnormal
glycogen accumulation. Cell Death Dis. 8(e2729)2017.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Lin S, Lin B, Wang X, Pan Y, Xu Q, He JS,
Gong W, Xing R, He Y, Guo L, et al: Silencing of ATP4B of ATPase
H+/K+ transporting beta subunit by intragenic
epigenetic alteration in human gastric cancer cells. Oncol Res.
25:317–329. 2017.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Fei HJ, Chen SC, Zhang JY, Li SY, Zhang
LL, Chen YY, Chang CX and Xu CM: Identification of significant
biomarkers and pathways associated with gastric carcinogenesis by
whole genome-wide expression profiling analysis. Int J Oncol.
52:955–966. 2018.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Lozano-Pope I, Sharma A, Matthias M, Doran
KS and Obonyo M: Effect of myeloid differentiation primary response
gene 88 on expression profiles of genes during the development and
progression of Helicobacter-induced gastric cancer. BMC Cancer.
17(133)2017.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Di Mario F and Goni E: Gastric acid
secretion: Changes during a century. Best Pract Res Clin
Gastroenterol. 28:953–65. 2014.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Saha A, Hammond CE, Beeson C, Peek RM Jr
and Smolka AJ: Helicobacter pylori represses proton pump expression
and inhibits acid secretion in human gastric mucosa. Gut.
59:874–881. 2010.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Friis-Hansen L: Achlorhydria is associated
with gastric microbial overgrowth and development of cancer:
Lessons learned from the gastrin knockout mouse. Scand J Clin Lab
Invest. 66:607–621. 2006.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Sáenz JB and Mills JC: Acid and the basis
for cellular plasticity and reprogramming in gastric repair and
cancer. Nat Rev Gastroenterol Hepatol. 15:257–273. 2018.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Vinasco K, Mitchell HM, Kaakoush NO and
Castaño-Rodríguez N: Microbial carcinogenesis: Lactic acid bacteria
in gastric cancer. Biochim Biophys Acta Rev Cancer.
1872(188309)2019.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Ferreira RM, Pereira-Marques J,
Pinto-Ribeiro I, Costa JL, Carneiro F, Machado JC and Figueiredo C:
Gastric microbial community profiling reveals a dysbiotic
cancer-associated microbiota. Gut. 67:226–236. 2018.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Wang LL, Liu JX, Yu XJ, Si JL, Zhai YX and
Dong QJ: Microbial community reshaped in gastric cancer. Eur Rev
Med Pharmacol Sci. 22:7257–7264. 2018.PubMed/NCBI View Article : Google Scholar
|