Introduction
The World Health Organization defines obesity as
accumulation of excessive fat in the body (1), which is most commonly caused by
overconsumption of fat-rich diets (2,3).
Consumption of excessive quantities of fat can result in the
accumulation of visceral fat and an increase in body weight
(2). Obesity has been cited as a
public health problem that is also associated with an increased
risk of metabolic diseases, such as cardiovascular disease,
diabetes mellitus, dyslipidaemia, metabolic syndrome and several
types of cancer (4,5), therefore, controlling it is essential
for improving personal health.
Papaya is widely grown in several regions around the
world, especially in central and South America, Asia and other
tropical countries. It is an economically significant crop, since
it is the fourth most traded tropical fruit following bananas,
mangoes and pineapples (6). Papaya
possesses several medicinal properties, including antioxidant,
anti-hypertensive and hepatoprotective properties (7). A previous study demonstrated that
papaya fruit aqueous extracts lowered lipid peroxidation, increased
glutathione levels, and increased the activity of catalase and
superoxide dismutase, as well as improving the immune status, as
reflected by an increase in IgG and IgM levels in acrylamide
intoxicated rats (8). Furthermore,
papaya leaf possesses hypoglycaemic and hypolipidemic effects on
rats fed a high cholesterol diet (9). Ripe papaya possess carotenoids and
vitamin C (7): β-carotene, a subtype
of carotenoids, exhibits an anti-hyperlipidaemic effect on
spontaneously hypertensive rats, and dietary β-carotene is
associated with a decreased serum lipid profile in
hypercholesterolemic rats fed a high cholesterol diet (10). In addition, accumulation of
β-carotene in 3T3-L1 adipocytes increases the expression of genes
associated with insulin sensitivity and reactive oxygen species
levels in insulin-resistant adipocytes (11).
Based on the properties of papaya extracts described
above, it was hypothesized that papaya may exhibit potential as a
treatment for obesity. In addition, there are several studies
regarding the anti-obesity effects of papaya, therefore, its
beneficial effects on obesity, inflammation and oxidative stress in
obese rats fed a high-fat (HF) diet were investigated. The doses of
papaya juice used in the present study can be easily implemented in
a nutritional diet for humans. Therefore, the use of the papaya
fruit may be a promising alternative dietary remedy against
obesity.
Materials and methods
Plant material and preparation
In the present study, two cultivars of papaya
(Holland and Khak-Dam) were used, due to their popularity and wide
consumption in Thailand. The papaya were purchased from a
supermarket in Phitsanulok, Thailand, and selected for their
uniformity of shape, size and external skin colour. The peel and
seeds were removed and the flesh was cut into small pieces and
crushed into a juice.
β-carotene and vitamin C
extraction
For extraction of vitamin C, 100 ml papaya juice was
mixed with 3% meta-phosphoric acid and 8% acetic acid in a
mechanical shaker at 180 rpm for 30 min, and then the mixture was
centrifuged at 9,000 x g for 10 min at 4˚C.
For β-carotene extraction, 10 ml papaya juice was
mixed with hexane, acetone and absolute ethanol using a magnetic
stirrer for 30 min, then 15 ml distilled water was added to the
mixture to separate by phase. Following separation, the supernatant
solution was stored at -80˚C.
High-performance liquid chromatography
(HPLC) analysis
Chromatographic analysis of vitamin C and β-carotene
in papaya juice was performed on an HPLC device (Shimadzu
LC-10ADvP; Bara Scientific Co., Ltd.) using a Luna 5u C18(2) 100A column (250x4.6 mm) (Phenomenex). In
vitamin C, the mobile phase was composed of methanol and buffer
solution (0.1 M KH2PO4) pH 4.4 (60:40). The
injected volume was 20 µl at a flow rate of 1.0 ml/min, with the
absorbance peak recorded at 245 nm.
In β-carotene, the mobile phase was composed of
acetonitrile, methanol and 2-propanol (20:30:50, v/v). The injected
volume was 50 µl at a flow rate of 1.0 ml/min, with the absorbance
peak recorded at 450 nm.
In vitro pancreatic lipase inhibition
assay
The in vitro pancreatic lipase assay was
slightly modified according to a previously described method
(12). Briefly, porcine pancreatic
lipase (Sigma-Aldrich; Merck KGaA) was dissolved in distilled water
to a final concentration of 1 mg/ml. The stock of 1% (w/v)
4-nitrophenyl laurate (Sigma-Aldrich; Merck KGaA) was used as the
lipase substrate and dissolved in 5 mM sodium acetate (pH 5.0)
containing 1% Triton X-100. To initiate the reaction, the reaction
mixture containing 80 µl assay buffer, 30 µl orlistat or papaya,
and 4-nitrophenyl laurate were mixed and incubated at 37˚C for 2 h
before centrifugation at 23,000 x g for 2.5 min at 25˚C. The
absorbance was measured at 400 nm. In a microplate reader (BioTek
Synergy HT; Bio-Tek Instruments, Inc.). The results are expressed
as percentage inhibition, and were calculated from
(Ablank-Asample)/Ablankx100, where Ablank is the absorbance of the
control and Asample is the absorbance of orlistat or papaya
juice.
Animals and experimental protocol
All experimental procedures were approved by the
Ethics Committee of the Centre for Animal Research, Naresuan
University (Phitsanulok, Thailand) (approval no. NUAE580174). A
total of 28 male Sprague Dawley rats weighing 80-100 g were
obtained from the National Laboratory Animal Centre, Mahidol
University (Bangkok, Thailand). The rats were kept in a temperature
controlled environment (22±10˚C) with a relative humidity of 55±10%
and a 12 h light-dark cycle. A commercial pellet diet and water
were provided ad libitum, and after 1 week of
acclimatization, the rats were fed either the normal diet or HF
diet for 8 weeks to induce obesity. The standard diets were
purchased from Perfect Companion Group Company. The HF diets were
prepared by mixing the control diet with 1.5% cholesterol, 20% palm
oil and 0.25% cholic acid as previously described (13). The rats were randomly divided into
four experimental groups as follows (n=7 rats/group): Group 1,
Control (C) group; these rats were fed a normal diet. Group 2, HF
diet group; these rats were fed a HF diet. Group 3, low-dose (HFL)
group; these rats were fed a HF diet with 0.5 ml/100 g of body
weight papaya juice. Group 4, high-dose (HFH) group; these rats
were fed a HF diet with 1.0 ml/100 g of body weight papaya
juice.
In week 8, the rats in the HFL and HFH groups
received papaya juice daily via oral feeding. Their food intake was
recorded daily, and their body weight was measured weekly
throughout the experiment. After 12 weeks, all the rats were fasted
for 12 h overnight, and then anesthetized with an intraperitoneal
injection of thiopental sodium (50 mg/kg). Cardiac puncture was
then performed to collect 10-12 ml blood. Death was confirmed by
the cessation of heartbeat and absence of reflexes. Epididymal,
perirenal and mesenteric adipose tissues were removed immediately,
weighed and stored at -80˚C until analysis. A portion of the
epididymal adipose tissue of each individual rat was fixed in 10%
neutral buffer formalin at room temperature for 24 h for
histological analysis.
Serum biochemical analysis
Blood was centrifuged at 800 x g for 30 min at 4˚C
to obtain the serum, and then frozen at -80˚C. The levels of serum
cytokines, including tumour necrosis factor-α (TNF-α; cat. no.
EZRTNFA; Sigma-Aldrich; Merck KGaA), interleukin-6 (IL-6; cat. no.
EZRIL6; Sigma-Aldrich; Merck KGaA), leptin (cat. no. EZRL-83K; EMD
Millipore) and insulin (cat. no. EZRMI-13K) were quantified using
ELISA kits. The levels of serum triglyceride (TG; Triglycerides
liquicolor mono; cat. no. 10720P), total cholesterol (TC;
Cholesterol liquicolor cat. no. 10017) and high-density lipoprotein
(HDL; HDL liquicolor cat. no. 10084) were measured using commercial
kits (all from HUMAN Gesellschaft fur Biochemica und Diagnostica
GmbH). Low-density lipoprotein (LDL) levels were determined using
as follows: LDL=TC-[HDL-(TG/5)].
Determination of lipid
peroxidation
Lipid peroxidation was determined by measuring
malondialdehyde (MDA) levels as previously described (14). Thiobarbituric acid (90 mM) and 3 M
trichloroacetic acid were added to the serum samples, and then
incubated at 95˚C for 60 min. The samples were immersed into an ice
bath for rapid cooling, and the peroxidation products formed in the
samples with thiobarbituric acid were measured at 532 nm with
malondialdehyde used as a standard, and the results are expressed
as nmol of thiobarbituric acid reactive substances (TBARS)/mg
protein.
Determination of superoxide dismutase
and glutathione reductase levels
Plasma superoxide dismutase and glutathione
reductase levels were measured spectrophotometrically using
commercial ELISA kits (superoxide dismutase assay kit, cat. no.
706002; glutathione reductase assay kit; cat. no. 703202; Cayman
Chemical Company) according to the manufacturer's protocol, and the
results are expressed as U/L.
Histological analysis
Epididymal adipose tissues were embedded in
paraffin, 8 µM sections were obtained, and stained with
haematoxylin for 2 min and eosin for 1 min at room temperature.
Images of the histological sections were obtained at magnifications
of x10 and x20. The size of the adipocytes were calculated using
Image-J version 1.50e (National Institutes of Health). The mean
area of the adipocytes was calculated in 20 adipocytes from 3
randomly selected fields of view per sample.
Western blot analysis
Protein extraction from adipose tissue was performed
by adding proteinase inhibitor (cat. no. 539131; EMD Millipore) and
RIPA lysis buffer (cat. no. R0278, Sigma-Aldrich; Merck KGaA) to
form a mixture, which was then homogenised by sonication on ice for
1 min. The supernatant was centrifuged at 20,000 x g for 15 min at
4˚C and the protein concentration from the epididymal adipose
tissue was measured using a bicinchoninic acid assay (cat. no.
23227; Thermo Fisher Scientific, Inc.). Total proteins were loaded
on a 15% SDS-gel, resolved using SDS-PAGE and transferred to a
polyvinylidene difluoride (PVDF) membrane using wet transfer.
Anti-PPARγ rabbit polyclonal antibody (cat. no. 07-466; EMD
Millipore) and anti-β-actin antibody (cat. no. AF7018; Affinity
Biosciences) was dissolved in 3% non-fat dry milk (1:1,000) and
incubated overnight at 4˚C, then washed with Tris-buffered saline
with Tween 20 buffer. Subsequently, peroxidase-conjugated
AffiniPure goat anti-Rabbit IgG (H+L) (cat. no. 111-035-003;
Jackson ImmunoResearch, PA, USA) was also dissolved in 3% non-fat
dry milk (1:1,000) for 2 h at room temperature.
Statistical analysis
All data are presented as the mean ± the standard
error of the mean. The data were analysed using GraphPad Prism
Version 6.0 (GraphPad Software, Inc.) and compared using a one-way
ANOVA with a Tukey's multiple comparison test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Quantification of β-carotene and
vitamin C levels in Holland and Khak-dam papaya juices
Quantification of β-carotene and vitamin C in the
two popular cultivars of papaya in Thailand; Holland and Khak-dam,
are presented in Table I. The
β-carotene content in the Holland papaya was 48.45±1.40 µg/g and
the vitamin C content was 255.90±4.56 µg/g. In the Khak-dam papaya,
the β-carotene content was 37.22±0.97 µg/g and the vitamin C was
156.60±2.10 µg/g.
 | Table IQuantification of β-carotene, vitamin
C and percentage of pancreatic lipase activity in Holland and
Khak-dam papaya juices. |
Table I
Quantification of β-carotene, vitamin
C and percentage of pancreatic lipase activity in Holland and
Khak-dam papaya juices.
| Compounds | Holland cultivar | Khak-dam
cultivar |
|---|
| β-carotene, µg/g | 48.45±1.40 |
37.22±0.97a |
| vitamin C, µg/g | 255.90±4.56 |
156.60±2.10a |
| Pancreatic lipase
inhibition, % | 40.37±3.87 | 26.41±3.49 |
Effect of papaya on pancreatic lipase
inhibition assay
The percentage of inhibitory activity on pancreatic
lipase using papaya juice from Holland cultivar was greater than
that of the Khak-Dam at the same fruit juice concentration
(40.37±3.87 and 26.41±3.49%, respectively) as shown in Table I. Based on the higher levels of
β-carotene, vitamin C and the percentage of pancreatic lipase
inhibition, the Holland cultivar was used for the animal
experiments.
Effects of the papaya juice on body
weight, food intake and adipose tissue weight
At the beginning of the experiment, the initial body
weight of all the experimental groups did not differ significantly
(Table II), whereas at the end of
12 weeks, the HF group exhibited significantly increased body
weight compared with the C group (Table
II). The final body weight and body weight gain in the
treatment group was significantly decreased by 7.73% in the HFL
group and 12.49% in the HFH group compared with the HF group, and
the food intake amongst the four groups did not differ
significantly. The mass of adipose tissues, including epididymal,
perirenal and mesenteric fat pads, were significantly decreased by
17, 10 and 6% in the HFL group, and decreased by 15, 18 and 11% in
the HFH group, respectively, compared with the HF group (Table II).
 | Table IIEffects of papaya juice on body
weight, food intake, tissue weight and biochemical parameters. |
Table II
Effects of papaya juice on body
weight, food intake, tissue weight and biochemical parameters.
| Factors | C | HF | HFL | HFH |
|---|
| Initial weight,
g | 218±29.83 | 236.5±33.26 | 215.5±34.31 | 221.83±35.01 |
| Final weight,
g | 465.83±11.13 |
536±33.24c |
509.33±33.57a |
471.33±15.04e |
| Weight gain, g | 247.8±8.36 |
299.5±16.26a | 293.8±8.64 |
249.5±14.92d |
| Food consumption,
g | 16.62±3.65 |
20.81±3.27c |
21.14±2.37c |
20.95±2.75c |
| Calorie intake,
kCal/day | 59.91±2.87 |
113.41±3.88c |
115.23±2.82c |
114.19±3.27c |
| Blood pressure,
mmHg | 132.45±12.33 | 134.83±11.76 | 133.27±16.23 | 129.2±17.93 |
| Plasma glucose,
mg/ml | 199.13±67.49 | 164.29±23.42 | 177.86±24.53 | 175.29±32.89 |
| Insulin, ng/dl | 0.41±0.15 |
5.58±1.02b |
1.83±0.97d | 3.58±1.82 |
| HOMA-IR | 4.61±1.59 |
59.55±13.03a | 22.58±15.15 | 38.04±23.69 |
| Leptin, ng/ml | 1.75±0.54 | 2.15±0.33 | 2.78±0.91 | 2.16±0.79 |
| Epididymal fat,
% | 0.78±0.08 | 0.83±0.1 |
0.69±0.05d |
0.71±0.06d |
| Perirenal fat,
% | 0.91±0.05 |
1.11±0.15a | 1±0.07 |
0.93±0.12d |
| Mesenteric fat,
% | 0.55±0.07 | 0.61±0.07 | 0.57±0.04 | 0.55±0.07 |
| Atherosclerosis
index | 0.26±0.11 |
0.67±0.08c |
0.7±0.12c |
0.66±0.06c |
| Serum triglyceride,
mg/dl | 52.89±4.86 |
96.90±15.68a | 82.63±5.94 | 68.88±7.01 |
| Serum cholesterol,
mg/dl | 73.45±3.19 |
274.0±38.84c |
211.4±34.29b |
216.4±23.00b |
| Serum LDL
cholesterol, mg/dl | 34.79±2.76 |
234.9±36.21c |
178.0±33.27b |
187.6±22.13b |
| Serum HDL
cholesterol, mg/dl | 28.09±0.96 |
19.76±2.11b |
16.88±2.06c |
15.00±1.43c |
| Serum TNF-α,
pg/dl | 47.28±2.82 | 45.69±3.95 | 45.57±3.22 | 46.89±2.01 |
| Serum IL-6,
pg/dl | 57.61±24.01 | 217.6±82.72 |
40.32±7.23d |
28.32±10.56d |
| MDA, nmol of
TBARS/mg protein | 1.52±0.19 |
3.20±0.34c |
1.51±0.21f |
1.40±0.26f |
| SOD, U/l | 21.88±0.53 | 15.96±1.68 |
28.80±2.65f |
30.41±2.02a,f |
| GR, U/l | 0.04±0.00 | 0.03±0.00 | 0.05±0.01 | 0.04±0.00 |
Effects of papaya juice on the serum
lipid profiles
The serum levels of TG, TC and LDL-C in the HF group
were significantly increased compared with the C group. In the HFL
and HFH groups, these parameters were notably lower. The serum
levels of HDL-C in the HF, HFL and HFH groups were significantly
lower compared with the C group (Table
II).
Effect of papaya on inflammatory
cytokines
There were no significant differences in the serum
levels of TNF-α amongst the C and treated groups. The serum levels
of IL-6 in the HF group were significantly increased compared with
the C group, and the levels in the HFL and HFH groups were
significantly reduced compared with the HF group (Table II).
Effect of papaya on serum leptin and
insulin levels
The serum levels of leptin did not differ
significantly between the C and treated groups. The insulin levels
in the HF group were significantly increased when compared with the
C group, and the HFL and HFH groups exhibited reduced insulin
levels compared with the HF group (Table II).
Effect of papaya on lipid
peroxidation
Using the TBARS method, the results of lipid
peroxidation from polyunsaturated fatty acids was used to determine
the degree of lipid oxidation. The results showed that the total
MDA content was significantly increased in the HF group when
compared with the C group, whereas in the rats treated with papaya
juice, the total MDA content was significantly decreased compared
with the HF group (Table II).
Effect of papaya on serum antioxidant
status
SOD activity was significantly reduced in the HF
group compared with the C group, and was significantly increased in
the HFL group when compared with the HF group. In the HFH group,
SOD activity was significantly increased compared with both the C
and HF groups (Table II), while the
activity of GR showed no difference in any of the groups (Table II).
Effect of papaya on epididymal adipose
tissue
Histological examination of the epididymal adipose
tissue showed that the adipocyte size of the epididymal fat in the
HF, HFL and HFH groups were enlarged when compared with the C
group, and this effect was visible by the naked-eye. Furthermore,
the epididymal adipocyte size was quantified by analysing images,
which showed that the size in the HF group was significantly higher
when compared with the C group, and the HFH group exhibited reduced
adipocyte hypertrophy compared with the HF group (Fig. 1).
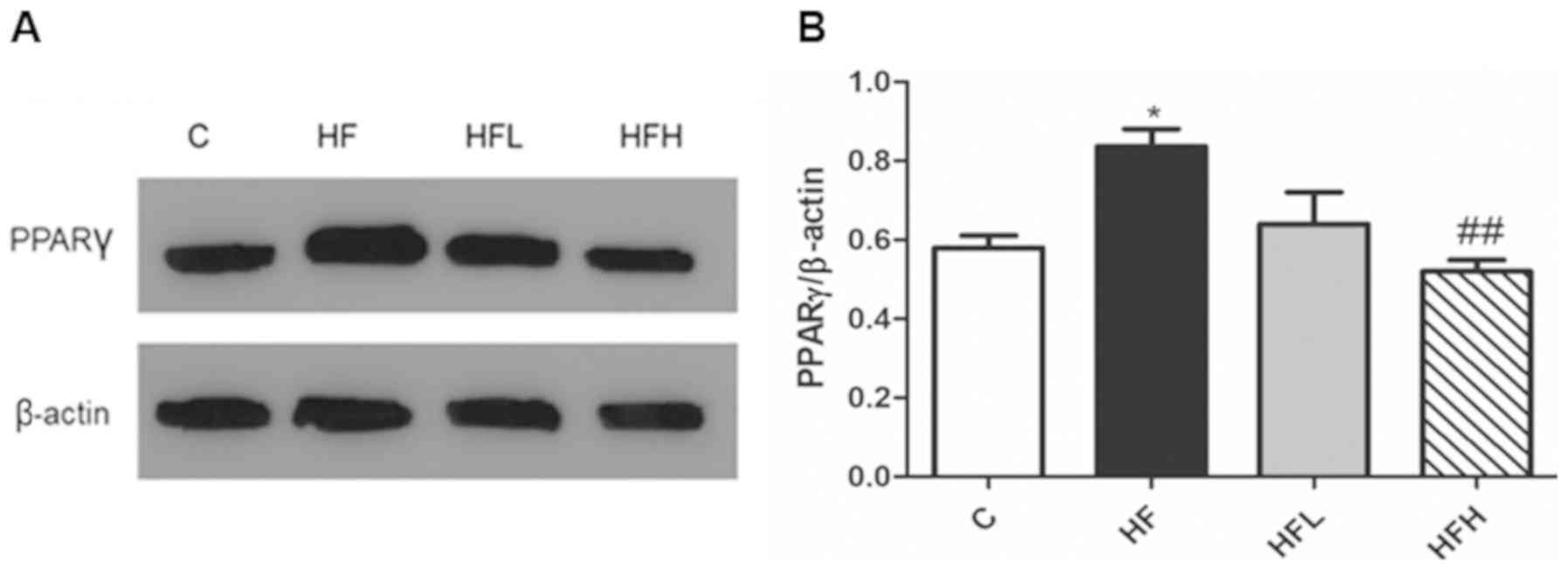
Effect of papaya on PPARγ expression
in the epididymal adipose tissue
PPARγ is one of the key regulators of lipid
metabolism (15), and thus was
measured in the epididymal adipose tissue using western blotting.
The protein expression levels of PPARγ in the HF group was
significantly increased compared with the C group, however,
supplementation of papaya juice (1.0 ml/100 g body weight)
significantly decreased PPARγ expression to levels close to that
observed in the C group (Fig.
2).
Discussion
Chronic consumption of a HF diet has been shown to
increase the prevalence of obesity (3). The largest component of dietary fats
are TGs, and these are hydrolysed to free fatty acids and
monoglycerides by pancreatic lipase, a key enzyme involved in the
digestion of fat (16). Free fatty
acids from triglycerides are transported to the blood system and
delivered to the adipose tissue and liver, leading to lipid
accumulation and the development of obesity. Inhibition of
pancreatic lipase reduces digestion and the absorption of fat
(16,17). Therefore, it is one of the most
common treatments for obesity. In the present study, the results
showed that papaya inhibited pancreatic lipase activity in the
in vitro study. This effect is beneficial in inhibiting or
delaying the digestion of lipids and, consequently, the absorption
of fatty acid (17). Thus, the
effects of papaya on a HF diet induced obese rats was assessed. The
results of the present study were similar to previous studies,
which showed that a HF diet increased body and adipose tissue
weight, and caused obesity (3,18). In
addition, in the present study, it was shown that a HF diet was
associated with hyperlipidaemia, which results in elevated levels
of lipids in the blood, such as triglycerides, total cholesterol
and/or LDL-cholesterol. Treatments using either 0.5 or 1.0 ml
papaya juice showed that it significantly decreased body and adipose
tissue weight, whilst also reducing TG, total cholesterol and LDL
cholesterol levels in the serum. The reduction of serum lipid
profiles indicated that papaya may decrease lipid transport to
blood circulation, which resulted in the reduction of lipid
accumulation in tissues. These results support the hypothesis that
papaya may reduce the extent of obesity induced by a HF diet, by
inhibiting intestinal absorption of dietary fat via the inhibition
of pancreatic lipase activity. Additionally, the results of the
present study showed that adipose tissue weight was decreased,
resulting in a reduction of body weight.
In the present study, the histological results of
the epididymal adipose tissue showed that the size of adipocytes
were smaller in the HF diet fed rats treated with papaya compared
with the HF group. Interestingly, papaya efficiently reduced both
adipocyte hypertrophy and adipose tissue weight. Lipid accumulation
in adipose tissue is a major cause of the production of ROS, which
leads to oxidative stress. A previous study reported that obese
mice exhibited increased release of H2O2 from
white adipose tissue, whereas no increase was found in the muscles
and the aorta (19). A study in
3T3-L1 murine adipocytes exposed to H2O2,
found that the adipocytes produced ROS (20). Similarly, an increase in adipocyte
tissue resulted in an increase in free radical levels in HF diet
induced obesity as observed in the present study. MDA levels in the
serum were increased in the obese rats and decreased in the rats
treated with papaya. A previous study reported that polyphenol-rich
extracts from papaya reduced the production of ROS and the
secretion of IL-6 in adipose cells exposed to
H2O2 (21).
The mRNA expression levels of TNF-α increased in the white adipose
tissue of obese mice, and also increased the expression levels of
IL-6 and MCP-1 in 3T3-L1 adipocytes exposed to ROS by incubation
with H2O2 (19,20),
therefore, these results demonstrate that ROS production is
associated with adipocytokines.
Obesity is caused by the accumulation of free fatty
acids in the adipose tissue which can result in enlarged adipocytes
and/or hypertrophy. Adipogenesis is the process of adipocyte
differentiation that transforms preadipocytes to adipocytes, and is
dependent on peroxisome PPAR-γ, which is a transcription factor
(15). The results of the present
study were similar to previous studies, which showed that a HF diet
increased the expression of PPARγ, both at the mRNA and protein
expression levels, and also resulted in enlarged adipocytes and/or
hypertrophy (22). One of the
characteristics of obesity is low-grade chronic inflammation in
which adipose tissue releases several inflammatory mediators
(23). As the adipocytes enlarge,
the blood supply to them is reduced, causing hypoxia. Adipose
tissue is not only a lipid storage site, but also functions as a
key endocrine organ. Therefore, during hypoxia, macrophages filter
into the adipocytes and stimulate secretion of pro-inflammatory
cytokines and adipocytokines, such as TNF-α and IL-6(24). In the present study, TNF-α and IL-6
levels in the serum were measured using ELISA. The results showed
that the HF diets resulted in a marked increase in serum IL-6
levels. The elevated IL-6 levels were significantly decreased when
the diet of the rats was supplemented with 0.5 or 1.0 ml papaya
juice, compared with the untreated HF diet fed rats. In addition, a
previous study showed that β-carotene decreased pro-inflammatory
cytokines such as nitric oxide, TNF-α and IL-1 in mice (25). In the present study, the results of
HPLC analysis found that papaya contains both vitamin C and
β-carotene. Therefore, vitamin C and/or β-carotene from papaya may
likely underlie the reduction of pro-inflammatory cytokines.
Obesity can induce systemic oxidative stress through
various biochemical mechanisms, including reducing antioxidant
defence or increasing chronic inflammation (26). Papaya is a good source of antioxidant
phytochemicals, such as vitamin C, carotenoids and vitamin E, which
serve as antioxidants reducing oxidative stress (27,28). MDA
was measured as a biomarker of oxidative stress and activity of the
antioxidant system. The results of the present study showed that
papaya improved the imbalance of oxidative stress generation and
ability to detoxify or repair the damage caused by decreased levels
of MDA and increased levels of antioxidants. These results are
similar to several studies which have shown the potent antioxidant
properties of papaya (8,29). Furthermore, papaya reduced the size
of the adipocytes, and the expression of PPARγ in obese rats, which
resulted in decreased levels of pro-inflammatory cytokines in the
serum, decreased levels of MDA and increased anti-oxidant levels.
The beneficial effects of several natural products on reducing
obesity are attributed to the presence of significant quantities of
bioactive compounds, which possess antioxidant and
anti-inflammatory properties (18).
Additionally, these bioactive compounds significantly decrease the
levels of TBARS, and increase SOD and glutathione reductase levels.
As a rich source of antioxidant activity, papaya can decrease the
serum levels of TBARS, which cause oxidative damage to lipid
components in cell membranes (14).
In addition, an increase in the activity of SOD was observed when
papaya was administered. SOD is one of the first lines of defence
in the detoxification of products resulting from oxidative stress
(30). An increase in SOD activity
following supplementation of papaya, may imply that papaya can
stimulate SOD, and this may result in counteracting the effects of
potentially harmful substances.
Based on these findings, it was shown that papaya
juice reduced lipid absorption as well as the anti-obesity,
anti-dyslipidaemia and anti-inflammatory effects in obese rats. The
proposed schematic is shown in Fig.
3. In conclusion, papaya juice may be a promising alternative
treatment and/or dietary supplement for obese individuals.
 | Figure 3Schematic diagram of the potential
mechanism of the observed anti-obesity effects of Carica
papaya in HF fed rats. Papaya may prevent high-fat diet induced
obesity, by inhibiting intestinal absorption of dietary fat via the
inhibition of pancreatic lipase activity. A HF diet induced
adipocyte hypertrophy, lipid accumulation and increased MDA levels,
whereas supplementation of papaya juice reversed all these
parameters. Papaya significantly increased serum superoxide
dismutase and decreased serum IL-6 levels. Papaya juice may have
regulated fat storage mediated via the PPARγ pathway in the adipose
tissue. The results demonstrated that papaya juice exhibits
potential for use as a nutritional supplement to reduce the risks
of obesity. SOD, superoxide dismutase; GR, glutathione reductase;
MDA, malondialdehyde; TNF-α, tumour necrosis factor-α; HDL,
high-density lipoprotein; VLDL, very low-density lipoprotein; IDL,
intermediate-density lipoprotein; LDL, low-density lipoprotein; TG,
triglyceride; TC, total cholesterol; FA, fatty acid; MG,
monoglyceride; HF, high-fat; HFL, HF diet treated with 0.5 ml
papaya juice/100 g body weight; HFH, HF diet treated with 1 ml
papaya juice/100 g body weight. |
Acknowledgements
We would like to thank Dr Tantip Boonsong,
Department of Biochemistry, Faculty of Medical Science, Naresuan
University for her technical guidance in determining protein
expression levels, and Mr. Kevin Roebl and Mr. Peter Barton at the
Division of International Affairs and Language Development,
Naresuan University for assistance revising our manuscript.
Funding
This study was supported by the Thailand research
fund (grant no. RDG5820017, ST) and partially supported by the
National Research Council of Thailand (grant no. 2562/20, WD) and
Centre of Excellence for Innovation in Chemistry (PERCH-CIC),
Ministry of Higher Education, Science, Research and Innovation.
Availability of data and materials
The datasets used and/or analysed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
POE and WD performed the experiments, analyzed,
interpreted the data and wrote the manuscript. WM and IP conceived
and designed the study, supervised the study, interpreted the data
and drafted the manuscript. ST designed and supervised the study,
interpreted the data, discussed the results, wrote and revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All experimental procedures were approved by the
Ethics Committee of the Centre for Animal Research, Naresuan
University (Phitsanulok, Thailand) (approval no. NUAE580174).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
World Health Organization (WHO): World
Health Statistics. WHO, Geneva, 2013. http://www.who.int/gho/publications/world_health_statistics/en/index.html.
|
|
2
|
Hariri N and Thibault L: High-fat
diet-induced obesity in animal models. Nutr Res Rev. 23:270–99.
2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Schrauwen P and Westerterp KR: The role of
high-fat diets and physical activity in the regulation of body
weight. Br J Nutr. 84:417–27. 2000.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hruby A and Hu FB: The epidemiology of
obesity: A big picture. Pharmacoeconomics. 33:673–689.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pi-Sunyer X: The medical risks of obesity.
Postgrad Med. 121:21–33. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Food and Agriculture Orgnaization of the
United Nations: Banana, mango, and pineapple, leading fruit
produced and traded worldwide. FAO, Rome, 2007. https://agris.fao.org/agris-search/search.do?recordID=PH2009000126.
|
|
7
|
Saeed F, Arshad MU and Pasha I:
Nutritional and Phyto-therapeutic potential of papaya (Carica
Papaya Linn.): An overview. Int J Food Prop. 17:1637–1653.
2014.
|
|
8
|
Mohamed Sadek K: Antioxidant and
immunostimulant effect of Carica papaya linn. aqueous
extract in acrylamide intoxicated rats. Acta Inform Med.
20:180–185. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zetina-Esquivel AM, Tovilla-Zárate CA and
Guzman-Garcia C: Effect of Carica papaya leaf extract on
serum lipids and liver metabolic parameters of rats fed a high
cholesterol diet. Health. 7:1196–1205. 2015.
|
|
10
|
Silva LS, de Miranda AM, de Brito
Magalhães CL, Dos Santos RC, Pedrosa ML and Silva ME: Diet
supplementation with beta-carotene improves the serum lipid profile
in rats fed a cholesterol-enriched diet. J Physiol Biochem.
69:811–820. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kameji H, Mochizuki K, Miyoshi N and Goda
T: β-Carotene accumulation in 3T3-L1 adipocytes inhibits the
elevation of reactive oxygen species and the suppression of genes
related to insulin sensitivity induced by tumor necrosis factor-α.
Nutrition. 26:1151–1156. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
McDougall GJ, Kulkarni NN and Stewart D:
Berry polyphenols inhibit pancreatic lipase activity in vitro. Food
Chem. 115:193–199. 2009.
|
|
13
|
Malakul W, Thirawarapan S, Suvitayavat W
and Woodman OL: Type 1 diabetes and hypercholesterolaemia reveal
the contribution of endothelium-derived hyperpolarizing factor to
endothelium-dependent relaxation of the rat aorta. Clin Exp
Pharmacol Physiol. 35:192–200. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tsai MC and Huang TL: Thiobarbituric acid
reactive substances (TBARS) is a state biomarker of oxidative
stress in bipolar patients in a manic phase. J Affect Disord.
173:22–26. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gregoire FM, Smas CM and Sul HS:
Understanding adipocyte differentiation. Physiol Rev.
78(783)1998.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mukherjee M: Human digestive and metabolic
lipases-a brief review. J Mol Catalysis B Enzymatic. 22:369–376.
2003.
|
|
17
|
Lunagariya NA, Patel NK, Jagtap SC and
Bhutani KK: Inhibitors of pancreatic lipase: State of the art and
clinical perspectives. Excil J. 13:897–921. 2014.PubMed/NCBI
|
|
18
|
Rochlani Y, Pothineni NV, Kovelamudi S and
Mehta JL: Metabolic syndrome: Pathophysiology, management, and
modulation by natural compounds. Ther Adv Cardiovasc Dis.
11:215–225. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Furukawa S, Fujita T, Shimabukuro M, Iwaki
M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M and
Shimomura I: Increased oxidative stress in obesity and its impact
on metabolic syndrome. J Clin Invest. 114:1752–1761.
2004.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Lee H, Lee YJ, Choi H, Ko EH and Kim JW:
Reactive oxygen species facilitate adipocyte differentiation by
accelerating mitotic clonal expansion. J Biol Chem.
284:10601–10609. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Somanah J, Bourdon E and Bahorun T:
Extracts of Mauritian Carica papaya (var. solo) protect
SW872 and HepG2 cells against hydrogen peroxide induced oxidative
stress. J Food Sci Technol. 54:1917–1927. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hosooka T, Noguchi T, Kotani K, Nakamura
T, Sakaue H, Inoue H, Ogawa W, Tobimatsu K, Takazawa K, Sakai M, et
al: Dok1 mediates high-fat diet-induced adipocyte hypertrophy and
obesity through modulation of PPAR-gamma phosphorylation. Nat Med.
14:188–193. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Makki K, Froguel P and Wolowczuk I:
Adipose tissue in obesity-related inflammation and insulin
resistance: Cells, cytokines, and chemokines. ISRN Inflamm.
2013(139239)2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ye J: Emerging role of adipose tissue
hypoxia in obesity and insulin resistance. Int J Obes (Lond).
33:54–66. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bai SK, Lee SJ, Na HJ, Ha KS, Han JA, Lee
H, Kwon YG, Chung CK and Kim YM: Beta-carotene inhibits
inflammatory gene expression in lipopolysaccharide-stimulated
macrophages by suppressing redox-based NF-kappaB activation. Exp
Mol Med. 37:323–334. 2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Manna P and Jain SK: Obesity, oxidative
stress, adipose tissue dysfunction, and the associated health
risks: Causes and therapeutic strategies. Metab Syndr Relat Disord.
13:423–444. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Asghar N, Naqvi SA, Hussain Z, Rasool N,
Khan ZA, Shahzad SA, Sherazi TA, Janjua MR, Nagra SA, Zia-Ul-Haq M
and Jaafar HZ: Compositional difference in antioxidant and
antibacterial activity of all parts of the Carica papaya
using different solvents. Chem Cent J. 10(5)2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Septembre-Malaterre A, Stanislas G,
Douraguia E and Gonthier MP: Evaluation of nutritional and
antioxidant properties of the tropical fruits banana, litchi,
mango, papaya, passion fruit and pineapple cultivated in Reunion
French Island. Food Chem. 212:225–233. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Amer J, Goldfarb A, Rachmilewitz EA and
Fibach E: Fermented papaya preparation as redox regulator in blood
cells of beta-thalassemic mice and patients. Phytother Res.
22:820–828. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Lee S, Keirsey KI, Kirkland R, Grunewald
ZI, Fischer JG and de La Serre CB: Blueberry supplementation
influences the Gut Microbiota, inflammation, and insulin resistance
in High-fat-diet-fed Rats. J Nutr. 148:209–219. 2018.PubMed/NCBI View Article : Google Scholar
|