Introduction
Vitamin D deficiency may increase the risk of
development of type 2 diabetes (T2DM) (1-3),
given its inverse relationship with diabetes onset (4). Both insulin resistance and b cell
dysfunction are associated with vitamin D deficiency (5), and obesity can exacerbate vitamin D
deficiency through sequestering of vitamin D into adipose tissue
(6). Vitamin D deficiency in T2DM
has also been associated with microvascular complications, although
causality remains unclear (7).
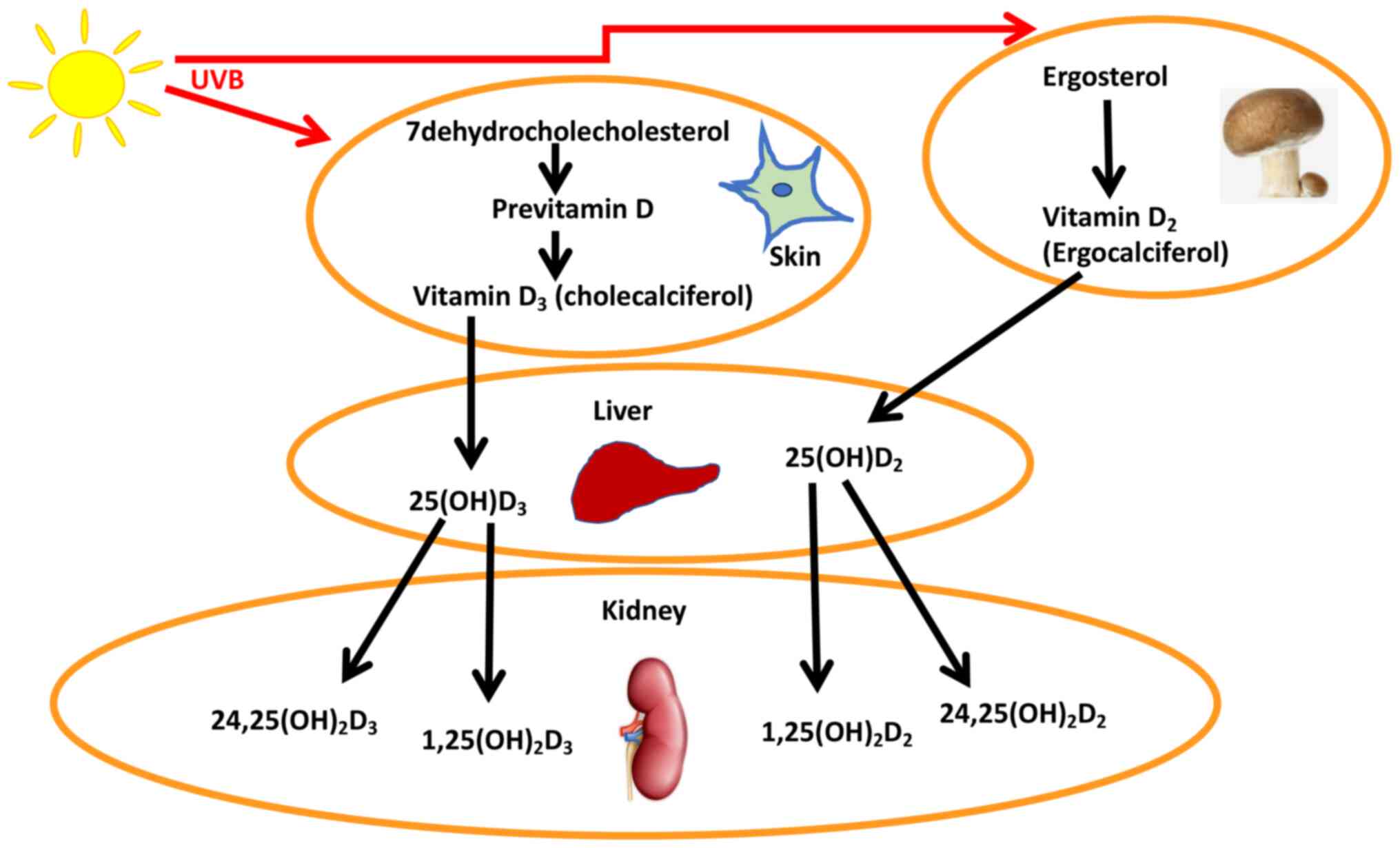
Vitamin D3 (also known as
cholecalciferol) is produced endogenously in the body, whereas
vitamin D2 is ingested in the diet as ergosterol, with
mushrooms and fungi being the primary sources, and is then
converted to ergocalciferol by ultraviolet light; multiple
25-hydroxylases then convert the ergosterol and cholecalciferol to
vitamin D2 (25(OH)D2) and vitamin
D3 (25(OH)D3), respectively (8,9). In the
kidney, vitamin D is converted to the active 1,25(OH)2D
form by 1a-hydroxylase, or to 24,25(OH)2D (10) (Fig.
1); 1,25(OH)2D is produced in extrarenal tissues and
may, and act locally (10).
Vitamin D2 is readily available as an
oral supplement to counter vitamin D deficiency, and is often
preferentially used over vitamin D3 as the latter is
more costly; therefore, the vitamin D levels and its metabolites
assayed and reported may be a composite of vitamin D2
and vitamin D3, as assays may not distinguish between
them (11).
In our previously study, the association between of
vitamin D3 deficiency and microvascular or
cardiovascular risk complications in patients with T2DM was
reported (12). Therefore, the aim
of the present study was to determine if total vitamin D was a
better predictor of complications in T2DM than vitamin
D3 alone.
Patients and methods
Study population
A total of 460 patients with T2DM (median age 55.2
years; age range, 30-90 years; 227 male and 233 female) were
recruited from patients attending the Hamad General Hospital
diabetes clinic, Qatar, between July 2013 and July 2015 (Table I). The criteria for inclusion in the
study were: Qatari ethnicity and ≥30 years old. T2DM was diagnosed
based on the World Health Organization guidelines (13). For inclusion in the T2DM cohort, one
or more of the following criteria had to be met: Fasting plasma
glucose of >7 mmol/l, HbA1c >6.5% or a diagnostic glucose
tolerance test. All diabetic patients had an estimated glomerular
filtration rate of >60 ml/min/kg to ensure that vitamin D levels
were not confounded by renal dysfunction. A total of 290 control
subjects were included in the present study (median age 46.1 years;
age range, 30-85 years; 151 male and 139 female). To be included in
the nondiabetic control group, a normal glucose tolerance test was
required. Criteria for exclusion were type 1 diabetes, gestational
diabetes or secondary diabetes due to steroid treatment. The T2DM
subjects underwent retinal photography, foot examination and
measurement of blood pressure.
 | Table IClinicopathological characteristics,
vitamin D levels and metabolite levels in the Control and Type 2
Diabetic cohorts. |
Table I
Clinicopathological characteristics,
vitamin D levels and metabolite levels in the Control and Type 2
Diabetic cohorts.
| Parameter |
Controlc |
Diabetesc | P-value |
|---|
| Age, years | 46 (30.0-85) | 55 (30-90) |
<0.001b |
| BMI,
kg/m2 | 30.1
(21.7-53.5) | 32.4
(17.0-61.0) |
<0.001b |
| HbA1c, % | 5.6 (4.4-9.0) | 7.9 (4.9-15.9) |
<0.001b |
| Glucose,
mmol/l | 5.2 (2.9-11.1) | 8.6 (2.3-29.0) |
<0.001b |
| Total
1,25(OH)2D, ng/dl | 0.044
(0.000-2.087) | 0.02
(0.000-0.189) |
<0.001b |
| Total 25(OH)D,
ng/dl | 19.58
(4.41-63.73) | 26.46
(0.00-61.21) |
<0.001b |
| Total 24,25(OH)D,
ng/dl | 0.387
(0.000-4.486) | 0.290
(0.000-7.772) |
<0.001b |
| Total 3epi25(OH)D,
ng/dl | 0.206
(0.000-7.564) | 0.326
(0.000-4.001) | 0.005a |
All study subjects had received vitamin
D2 supplements, 50,000 units weekly, prescribed for at
least the preceding 4 months. Diabetes subjects were all treated
with at least 2 antidiabetic medications that included metformin,
and whilst patients were prescribed insulin, compliance could not
be confirmed.
This study was approved by Weill Cornell
Institutional Review Board (approval no. IRB# 13-00063); all study
subjects provided written informed consent. Trial conduct was
undertaken in accordance with the International Conference on
Harmonisation-Good Clinical Practice (ICH GCP) and the Declaration
of Helsinki (14).
Study design
The study design has been previously described
(15). Briefly, patients were fasted
overnight, and subsequently, blood samples were collected, as well
as the baseline weight and blood pressure. Fasting venous blood was
collected in fluoride oxalate and serum gel tubes (BD Diagnostics;
Becton, Dickinson and Company). The samples were separated by
centrifugation at 2,000 x g for 15 min at 4˚C, and within 1 h of
collection, the aliquots were stored at -80˚C. Overnight urine
samples were also collected, aliquoted and stored at -80˚C, and
analyzed in batches. Blood pressure was measured using an automated
device (NPB-3900; Nellcor Puritan Bennett) during each visit. Blood
pressure measurements were performed after the subjects had been
seated quietly for at least 5 min, using the right arm which was
supported at heart level. For each measurement, three readings were
taken, at least 2 min intervals, and the mean of the three readings
was recorded (15).
Diabetic retinopathy was diagnosed using fundoscopy
and diabetic neuropathy was diagnosed based on the vibration
perception threshold (Neurothesiometer NU-1, Horwell-UK) of the
great toe being >25 V (16).
Coronary artery disease (CAD) was defined as a
history of myocardial infarction or angina, confirmed by coronary
angiography (17). Peripheral
arterial disease (PAD) was defined as a history of claudication or
pain at rest with evidence of artery stenosis on ultrasound or
lower limb angiography (18). Stroke
was defined as a sudden onset neurological deficit lasting >24 h
(19).
Serum vitamin D levels were measured using
isotope-dilution liquid chromatography tandem mass spectrometry
(LC-MS/MS) as described previously (12).
Statistical analysis
Statistical analysis was performed as described
previously (20). The sample size
used in the present study was based on a previous study, which
found that 51% of diabetics without microvascular complications and
80% with retinopathy exhibited vitamin D deficiency (7). Using the 49% of patients without
retinopathy as the comparison group, a sample size of 274 diabetic
patients was selected, which provides 80% statistical power to
detect a 68% prevalence of vitamin D deficiency in the retinopathy
group. When examining the mean differences in vitamin D, the 460
patients, assuming 40% (n=184) have retinopathy, this would yield
an harmonic mean of the sample size of ~132. Using this sample size
provided 95% power for a difference in vitamin D means of 0.35
deviations using a t-test, which was considered a moderate-sized
effect.
Data trends were visually and statistically
evaluated for normality. Non-parametric tests (Mann Whitney U) were
used on data that violated the assumptions of normality when tested
using a Kolmogorov-Smirnov Test (20). Statistical analysis was performed
using SPSS version 24.0. Values are reported as the median
(inter-quartile range).
Results
Baseline characteristics
The baseline characteristics for the T2DM and the
control cohorts are shown in Table
I. The diabetic patients were significantly older (P<0.001)
and had a higher BMI (P<0.001) compared to the control subjects.
The diabetic patients also had elevated HbA1c (P<0.001) and
fasting glucose levels compared with the control group
(P<0.001).
Vitamin D measurements
The levels of total 25(OH)D were significantly
higher compared with the 25(OH)D3 (P<0.001). The
levels of total 1,25(OH)2D and total
24,25(OH)2D did not differ compared with the levels of
1,25(OH)2D3 and
24,25(OH)2D3 (P>0.05).
The lower active total 1,25(OH)2D levels
were associated with hypertension and dyslipidemia in diabetic
patients (P=0.03) in comparison with the lower
1,25(OH)2D3 levels, which were associated
with diabetic retinopathy (P=0.006) hypertension and dyslipidemia
(both P=0.01) as well as CAD (P=0.012). There was no association
between either total 1,25(OH)2D or
1,25(OH)2D3 levels with diabetic neuropathy,
PAD or CAD. Total 25(OH)D levels were associated with both
hypertension and dyslipidemia (P<0.001) in comparison with
25(OH)D3 levels, which were associated with diabetic
retinopathy (P=0.03) and dyslipidemia (P=0.04). There was no
association between either total 25(OH)D or 25(OH)D3
levels with diabetic neuropathy, dyslipidemia, PAD, CAD or stroke.
Total 24,25(OH)2D levels were associated with
dyslipidemia (P=0.03) in accordance with
24,25(OH)2D3 levels (P<0.02). There was no
association between either total 24,25(OH)2D or
24,25(OH)2D3 with diabetic neuropathy,
diabetic retinopathy, hypertension, PAD, CAD or stroke. None of the
vitamin D metabolites were associated with diabetic neuropathy or
stroke (Table II).
 | Table IITotal vitamin D and vitamin
D3 level in patients with diabetes based microvascular
diabetic complications and cardiovascular complications. |
Table II
Total vitamin D and vitamin
D3 level in patients with diabetes based microvascular
diabetic complications and cardiovascular complications.
| Complication | Total 1,25 (OH)D,
ng/dld | P-value | Total 25(OH) D,
ng/dld | P-value | Total 24,25 (OH)D,
ng/dld | P-value | 1,25(OH) 2D3,
ng/dld | P-value | 25(OH) D3,
ng/dld | P-value | 24,25(OH) 2D3,
ng/dld | P-value |
|---|
| Diabetic
retinopathy | | 0.1 | | 0.1 | | 0.87 | | 0.006b | | 0.03a | | 0.97 |
|
No | 0.018
(0.000-0.043) | | 25.42
(18.23-35.90) | | 0.288
(0.187-0.604) | | 0.028
(0.010-0.047) | | 5.24
(3.07-12.02) | | 0.278
(0.185-0.587) | |
|
Yes | 0.025
(0.010-0.047) | | 30.82
(20.91-37.73) | | 0.302
(0.200-0.472) | | 0.015
(0.006-0.035) | | 7.50
(3.97-15.66) | | 0.302
(0.197-0.472) | |
| Diabetic
neuropathy | | 0.8 | | 0.3 | | 0.73 | | 0.48 | | 0.63 | | 0.77 |
|
No | 0.020
(0.000-0.044) | | 26.06
(18.05-36.25) | | 0.288
(0.189-0.506) | | 0.015
(0.000-0.040) | | 6.14
(3.34-14.00) | | 0.283
(0.188-0.506) | |
|
Yes | 0.020
(0.000-0.042) | | 28.56
(22.27-36.31) | | 0.310 (0.622) | | 0.013
(0.000-0.033) | | 6.18
(3.73-11.53) | | 0.271
(0.185-0.622) | |
| Hypertension | | 0.03a | |
<0.001c | | 0.29 | | 0.009b | | 0.46 | | 0.23 |
|
No | 0.030
(0.011-0.047) | | 22.65
(15.46-33.24) | | 0.297
(0.193-0.587) | | 0.021
(0.006-0.043) | | 6.39
(3.44-13.66) | | 0.295
(0.193-0.587) | |
|
Yes | 0.015
(0.000-0.042) | | 29.43
(20.77-38.82) | | 0.278
(0.187-0.518) | | 0.012
(0.000-0.032) | | 5.87
(3.31-13.88) | | 0.260
(0.187-0.518) | |
| Dyslipidemia | | 0.003b | | 0.009b | | 0.03a | | 0.003b | | 0.04a | | 0.02a |
|
No | 0.033
(0.014-0.054) | | 22.81
(16.00-31.29) | | 0.330
(0.221-0.638) | | 0.024
(0.009-0.045) | | 6.86
(3.84-14.47) | | 0.330
(0.221-0.638) | |
|
Yes | 0.016
(0.000-0.041) | | 28.29
(19.82-37.60) | | 0.267
(0.185-0.472) | | 0.013
(0.000-0.033) | | 5.30
(3.02-12.02) | | 0.260
(0.183-0.471) | |
| Peripheral artery
disease | | 0.19 | | 0.23 | | 0.7 | | 0.22 | | 0.55 | | 0.65 |
|
No | 0.019
(0.000-0.044) | | 26.48
(18.74-36.35) | | 0.289
(0.191-0.521) | | 0.014
(0.000-0.038) | | 6.06
(3.38-13.88) | | 0.283
(0.189-0.521) | |
|
Yes | 0.025
(0.017-0.050) | | 21.27
(14.07-32.78) | | 0.315
(0.144-0.622) | | 0.025
(0.012-0.050) | | 10.38
(4.06-15.40) | | 0.260
(0.144-0.622) | |
| Coronary artery
disease | | 0.99 | | 0.28 | | 0.44 | | 0.01b | | 0.75 | | 0.45 |
|
No | 0.021
(0.000-0.045) | | 25.98
(18.36-36.14) | | 0.295
(0.190-0.515) | | 0.028
(0.011-0.041) | | 6.28
(3.38-14.00) | | 0.289
(0.190-0.515) | |
|
Yes | 0.018
(0.005-0.039) | | 29.73
(21.65-36.97) | | 0.241
(0.189-0.604) | | 0.015
(0.005-0.027) | | 5.43
(3.21-11.69) | | 0.241
(0.187-0.604) | |
| Stroke | | 0.75 | | 0.28 | | 0.23 | | 0.05a | | 0.41 | | 0.24 |
|
No | 0.020
(0.000-0.044) | | 26.08
(18.42-36.27) | | 0.290
(0.192-0.541) | | 0.015
(0.000-0.040) | | 6.18
(3.38-14.00) | | 0.285
(0.191-0.541) | |
|
Yes | 0.023
(0.000-0.040) | | 30.13
(25.49-36.10) | | 0.218
(0.151-0.431) | | 0.011
(0.000-0.024) | | 4.92
(2.32-11.20) | | 0.218
(0.151-0.431) | |
Total 25(OH)D levels were significantly higher
compared with 25(OH)D3 (P<0.001), but those of
1,25(OH)2D and 24,25(OH)2D did not differ
between total and D3 metabolites. When the subjects with
25(OH)D deficiency (≤20 ng/ml) were compared to those who were
replete (≥30 ng/ml), there was no difference in hypertension,
dyslipidemia, retinopathy or neuropathy (data not shown). There was
no correlation between the estimated glomerular filtration rate and
any of the vitamin D metabolites (data not shown).
Discussion
Total 25(OH)D levels were significantly higher
compared with those of 25(OH)D3, and this reflects the
vitamin D2 supplementation that these patients were
taking. However, the levels of total 1,25(OH)2D and
total 24,25(OH)2D did not differ to those of
1,25(OH)2D3 and
24,25(OH)2D3. This result is in agreement
with a study on high dose vitamin D2 supplementation,
which showed that vitamin D2 was less efficacious at
raising serum 25(OH)D levels than vitamin D3, and that
vitamin D2 did not increase the 1,25(OH)2D
levels to the same degree that vitamin D3
supplementation did (21). This also
supports the notion that vitamin D3 is better than
vitamin D2 for treating vitamin D deficiency (21), and that vitamin D2
supplements may not sufficiently increase the levels of active
1,25(OH)2D.
Both 25(OH)D3 and
1,25(OH)2D3 were associated with diabetic
retinopathy, whereas neither total 25(OH)D nor total
1,25(OH)2D levels were associated with diabetic
retinopathy. It has been reported that in type 2 diabetes, vitamin
D deficiency is associated with development of microvascular
complications (22) and a recent
meta-analysis highlighted an association between vitamin D
deficiency and retinopathy (23);
however, these outcomes were not specifically correlated to either
total vitamin D or the vitamin D3 forms. These data may
also suggest why vitamin D and diabetes studies reported in the
literature are conflicting on the relationship of complications and
benefits of vitamin D supplementation if the effects of total
vitamin D differs from that of vitamin D3 (24).
Total vitamin D, in comparison with vitamin
D3 metabolites, showed very similar associations with
the other cardiovascular parameters, including dyslipidemia,
hypertension, PAD and stroke. The only additional significant
result was the association between
1,25(OH)2D3 and CAD, that was not seen with
total 1,25(OH)2D.
When the deficient and replete 25(OH)D populations
were compared, there was no difference between them, suggesting
that the serum 25(OH)D levels were not related to the development
of hypertension or dyslipidemia. These changes were not a result of
altered renal function, as there was no correlation with estimated
glomerular filtration rate.
There is increasing evidence showing that vitamin D
deficiency serves a role in the pathogenesis of type 2 diabetes
(25-27)
with evidence from epidemiological studies linking vitamin D
deficiency and insulin resistance (28,29). In
adults at high risk of developing type 2 diabetes, supplementation
with cholecalciferol has been shown to improve b cell function
(30) and 1,25(OH)2D may
also improve insulin sensitivity by activating peroxisome
proliferator-activated receptor δ (31). Conversely, long-term studies have
found that vitamin D and calcium supplementation do not offer
protection against the risk of diabetes development (22), and giving supplements to vitamin D
replete patients with T2DM had no effect on insulin resistance or
glycemic control (32); however,
differing meta-analyses have shown an improvement in HbA1c in
response to supplementation with vitamin D in some studies
(33,34), but not in others (35).
Both the total 24,25(OH)2D and
24,25(OH)2D3 levels were significantly
associated with dyslipidemia; 24,25(OH)2D may not be an
inactive metabolite, as it has been shown to suppress Apo A-1 in
hep G cells (36), and it may
exhibit a physiological role in growth plate formation (8); therefore, a direct effect on lipid
metabolism cannot be excluded.
The strength of the present cross-sectional study
was the homogeneous Qatari population studied and the number of
participants assessed using state-of-the-art measurements of
25(OH)D and metabolites, and these results may be generalizable to
other Qatari populations. However, this study was limited by its
cross-sectional design and that, whilst all subjects were
prescribed vitamin D2 supplements, it was not possible
to ascertain compliance. Additionally, the results may not be
generalizable to other ethnicities, for which a multi-center
approach with participation from institutes in several different
countries is required.
In conclusion, vitamin D3 metabolites
were associated with diabetic retinopathy, whereas total vitamin D
levels were not, suggesting that endogenous vitamin D3
metabolites are the better measure of diabetic microvascular
complications. However, both total vitamin D and vitamin
D3 metabolites were associated with cardiovascular risk
factors in patients with type 2 diabetes.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
AEB analyzed and interpretated the data as well as
wrote the manuscript. LHMA analyzed and interpretated the data. SRD
performed the statistical analysis. AL performed the vitamin D
measurements. EAA and AH participated in data analysis and
interpretation, as well as prepared the manuscript. SLA designed
the study and contributed to the discussion. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by Weill Cornell
Institutional Review Board (approval no. IRB# 13-00063). All study
subjects provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nakashima A, Yokoyama K, Yokoo T and
Urashima M: Role of vitamin D in diabetes mellitus and chronic
kidney disease. World J Diabetes. 7:89–100. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Husemoen LL, Thuesen BH, Fenger M,
Jorgensen T, Glumer C, Svensson J, Ovesen L, Witte DR and Linneberg
A: Serum 25(OH)D and type 2 diabetes association in a general
population: A prospective study. Diabetes Care. 35:1695–1700.
2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mathieu C: Vitamin D and diabetes: Where
do we stand? Diabetes Res Clin Pract. 108:201–209. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pittas AG, Dawson-Hughes B, Li T, Van Dam
RM, Willett WC, Manson JE and Hu FB: Vitamin D and calcium intake
in relation to type 2 diabetes in women. Diabetes Care. 29:650–656.
2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chiu KC, Chu A, Go VL and Saad MF:
Hypovitaminosis D is associated with insulin resistance and beta
cell dysfunction. Am J Clin Nutr. 79:820–825. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wortsman J, Matsuoka LY, Chen TC, Lu Z and
Holick MF: Decreased bioavailability of vitamin D in obesity. Am J
Clin Nutr. 72:690–693. 2000.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bajaj S, Singh RP, Dwivedi NC, Singh K,
Gupta A and Mathur M: Vitamin D levels and microvascular
complications in type 2 diabetes. Indian J Endocrinol Metab.
18:537–541. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bikle DD: Vitamin D metabolism, mechanism
of action, and clinical applications. Chem Biol. 21:319–329.
2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sakaki T, Kagawa N, Yamamoto K and Inouye
K: Metabolism of vitamin D3 by cytochromes P450. Front Biosci.
10:119–134. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Christakos S, Dhawan P, Verstuyf A,
Verlinden L and Carmeliet G: Vitamin D: Metabolism, molecular
mechanism of action, and pleiotropic effects. Physiol Rev.
96:365–408. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lhamo Y, Chugh PK and Tripathi CD: Vitamin
D Supplements in the Indian Market. Indian J Pharm Sci. 78:41–47.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Butler AE, Dargham SR, Latif A, Mokhtar
HR, Robay A, Chidiac OM, Jayyousi A, Al Suwaidi J, Crystal RG, Abi
Khalil C and Atkin SL: Association of vitamin D3 and its
metabolites in subjects with and without type 2 diabetes and their
relationship to diabetes complications. Ther Adv Chronic Dis.
11(2040622320924159)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Deckers JG, Schellevis FG and Fleming DM:
WHO diagnostic criteria as a validation tool for the diagnosis of
diabetes mellitus: A study in five European countries. Eur J Gen
Pract. 12:108–113. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Carlson RV, Boyd KM and Webb DJ: The
revision of the Declaration of Helsinki: Past, present and future.
Br J Clin Pharmacol. 57:695–713. 2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Dakroury Y, Butler AE, Dargham SR, Latif
A, Robay A, Crystal RG and Atkin SL: Association of differing
qatari genotypes with Vitamin D metabolites. Int J Endocrinol.
2020(7831590)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Young MJ, Breddy JL, Veves A and Boulton
AJ: The prediction of diabetic neuropathic foot ulceration using
vibration perception thresholds. A prospective study. Diabetes
Care. 17:557–560. 1994.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hansson GK: Inflammation, atherosclerosis,
and coronary artery disease. N Engl J Med. 352:1685–1695.
2005.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Campia U, Gerhard-Herman M, Piazza G and
Goldhaber SZ: Peripheral artery disease: Past, present, and future.
Am J Med. 132:1133–1141. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hu X, De Silva TM, Chen J and Faraci FM:
Cerebral vascular disease and neurovascular injury in ischemic
stroke. Circ Res. 120:449–471. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ahmed LHM, Butler AE, Dargham SR, Latif A,
Robay A, Chidiac OM, Jayyousi A, Al Suwaidi J, Crystal RG, Atkin SL
and Abi Khalil C: Association of vitamin D2 and
D3 with type 2 diabetes complications. BMC Endoc Disord.
20(65)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shieh A, Chun RF, Ma C, Witzel S, Meyer B,
Rafison B, Swinkels L, Huijs T, Pepkowitz S, Holmquist B, et al:
Effects of high-dose Vitamin D2 versus D3 on total and Free
25-Hydroxyvitamin D and markers of calcium balance. J Clin
Endocrinol Metab. 101:3070–3078. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
de Boer IH, Tinker LF, Connelly S, Curb
JD, Howard BV, Kestenbaum B, Larson JC, Manson JE, Margolis KL,
Siscovick DS, et al: Calcium plus vitamin D supplementation and the
risk of incident diabetes in the Women's Health Initiative.
Diabetes Care. 31:701–707. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Luo BA, Gao F and Qin LL: The association
between Vitamin D deficiency and diabetic retinopathy in type 2
diabetes: A meta-analysis of observational studies. Nutrients.
9(307)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Issa CM: Vitamin D and type 2 diabetes
mellitus. Adv Exp Med Biol. 996:193–205. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu E, Meigs JB, Pittas AG, Economos CD,
McKeown NM, Booth SL and Jacques PF: Predicted 25-hydroxyvitamin D
score and incident type 2 diabetes in the Framingham Offspring
Study. Am J Clin Nutr. 91:1627–1633. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gagnon C, Lu ZX, Magliano DJ, Dunstan DW,
Shaw JE, Zimmet PZ, Sikaris K, Grantham N, Ebeling PR and Daly RM:
Serum 25-hydroxyvitamin D, calcium intake, and risk of type 2
diabetes after 5 years: Results from a national, population-based
prospective study (the Australian Diabetes, Obesity and Lifestyle
study). Diabetes Care. 34:1133–1138. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lim S, Kim MJ, Choi SH, Shin CS, Park KS,
Jang HC, Billings LK and Meigs JB: Association of vitamin D
deficiency with incidence of type 2 diabetes in high-risk Asian
subjects. Am J Clin Nutr. 97:524–530. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Devaraj S, Jialal G, Cook T, Siegel D and
Jialal I: Low vitamin D levels in Northern American adults with the
metabolic syndrome. Horm Metab Res. 43:72–74. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Jorde R, Sneve M, Emaus N, Figenschau Y
and Grimnes G: Cross-sectional and longitudinal relation between
serum 25-hydroxyvitamin D and body mass index: The Tromso study.
Eur J Nutr. 49:401–407. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Mitri J, Dawson-Hughes B, Hu FB and Pittas
AG: Effects of vitamin D and calcium supplementation on pancreatic
beta cell function, insulin sensitivity, and glycemia in adults at
high risk of diabetes: The Calcium and Vitamin D for diabetes
mellitus (CaDDM) randomized controlled trial. Am J Clin Nutr.
94:486–494. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Dunlop TW, Vaisanen S, Frank C, Molnar F,
Sinkkonen L and Carlberg C: The human peroxisome
proliferator-activated receptor delta gene is a primary target of
1alpha,25-dihydroxyvitamin D3 and its nuclear receptor. J Mol Biol.
349:248–260. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Angellotti E, D'Alessio D, Dawson-Hughes
B, Nelson J, Cohen RM, Gastaldelli A and Pittas AG: Vitamin D
supplementation in patients with type 2 diabetes: The Vitamin D for
established type 2 diabetes (DDM2) study. J Endocr Soc. 2:310–321.
2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mirhosseini N, Vatanparast H, Mazidi M and
Kimball SM: The effect of improved serum 25-Hydroxyvitamin D status
on glycemic control in diabetic patients: A Meta-analysis. J Clin
Endocrinol Metab. 102:3097–3110. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wu C, Qiu S, Zhu X and Li L: Vitamin D
supplementation and glycemic control in type 2 diabetes patients: A
systematic review and meta-analysis. Metabolism. 73:67–76.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Krul-Poel YH, Ter Wee MM, Lips P and
Simsek S: Management of Endocrine Disease: The effect of vitamin D
supplementation on glycaemic control in patients with type 2
diabetes mellitus: A systematic review and meta-analysis. Eur J
Endocrinol. 176:R1–R14. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wehmeier K, Onstead-Haas LM, Wong NC,
Mooradian AD and Haas MJ: Pro-inflammatory signaling by
24,25-dihydroxyvitamin D3 in HepG2 cells. J Mol Endocrinol.
57:87–96. 2016.PubMed/NCBI View Article : Google Scholar
|