1. Introduction
Food safety constitutes a key priority for public
health. Unsafe foodstuffs containing harmful micro-organisms
(bacteria, viruses, parasites etc.) or chemical compounds, may
cause various illnesses and even death (1). Unsafe food has always been considered
a major issue for public health throughout history. It is
estimated, that the majority of foodborne diseases that cost the
lives of ~1.8 million individuals each year occur as a result of
contaminated water or food (2).
The diseases caused by bacterial pathogens can be
classified into two types: Foodborne infection and foodborne
intoxication (3). Foodborne
infections result from the development of bacteria in the human
body (4). Bacteria are transmitted
through contaminated food, multiply within the intestines, and
excrete toxins that affect the proper function of the tissues or
other organs. Foodborne intoxication is an illness caused by the
ingestion of food contaminated by microbial toxins even though the
microorganisms responsible for the production of toxins may have
already been destroyed (5).
Therefore, intoxication is caused by toxins produced by bacteria in
the food (3,4,6). The
World Health Organization defines foodborne intoxication as an
‘illness caused by the ingestion of toxins produced by bacteria
in the food as a natural by-product of their metabolic
processes’ (7). Related terms
regarding toxins include bacterial toxins, phytotoxins produced by
algae, mycotoxins produced by fungi, and poisons produced by
animals (8).
Clostridium botulinum (Cl.
botulinum) is a bacterium that produces a dangerous toxin
that has been reported to be one of the most lethal substances
known to man (9-11).
Foodborne botulism constitutes an acute paralytic disease caused by
a toxin produced by the bacterium. The disease may be caused by
ingesting food that contains this toxin or due to the development
of spores within the intestines of young children (3,12,13).
Clostridium took the name botulinum from the Latin
‘botulus’ which means lunch meat, salami, sausage, etc. The
corresponding ancient Greek word is ‘Αλλάς’ from which the
word allantiasis comes (14).
Growing consumer demand for traditional food
products, such as cheese products, poses significant challenges for
the food industry (15). Greece has
a long tradition in the production and consumption of cheese
products. It is ranked among the countries with the highest
consumption of cheese worldwide (16). Cheeses are widely consumed all over
the world and they have often been involved in outbreaks of
foodborne illnesses (17).
Consumers consider local dairy products, such as cheese, as a
cost-effective solution, which may occasionally expose the
population to the risk of foodborne botulism (15).
The risk of Cl. botulinum in cheese products
is still observed today. Recently, a well-known food company in the
United Kingdom and Ireland recalled its products (cheese spread in
tubes) due to possible infection with Cl. botulinum. Company
officials report that they were in contact with local public health
inspectors and the UK Food Standard Agency (FSA) (18). The UK FSA issued a press release on
16th of June 2020 informing consumers about the recall of products
and the risk of consuming related products (19). However, the risk of foodborne
botulism associated with the consumption of dairy products has not
been discussed in detail (20). The
aim of this review is to highlight the occurrence of foodborne
botulism caused by cheese products and their significance to public
health.
2. Foodborne botulism: An overview
Human botulism can occur in six different forms:
Foodborne botulism caused by the ingestion of food contaminated
with bacterial toxins, wound botulism caused by Cl.
botulinum colonization of a wound and the production of toxins
in the wound, infant botulism caused by Cl. botulinum
intestinal colonization and the production of toxins, and
unclassified botulism that is even rarer and is characterized by
intestinal colonization and the production of toxins in the
intestine of adults (21).
Iatrogenic botulism due to improper neurotoxin administration in
the systemic circulation instead of the predefined therapeutic goal
and inhalation botulism caused by toxin inhalation in the form of
aerosol, have also been observed (22).
Foodborne botulism is an acute and potentially fatal
disease (11,23). Exotoxins of Cl. botulinum are
proteins, and they are very toxic to the host (10). They are large polypeptides of
similar structure and their effects manifest as neuromuscular
blockade that usually leads to flaccid paralysis (24). Botulinum toxin is one of the most
potent neurotoxins. It is a 150 kDa polypeptide [consisting of a
100-kDa heavy chain joined to a 50-kDa light chain via a disulfide
bond] with three separate domains: N, middle and C. The C domain
binds to the pre-synaptic membrane, the N domain is a specific
polypeptidase and the middle domain facilitates the light chain
into the cytosol. The most remarkable aspect is the affinity of
this substance for the most active synapses (25,26).
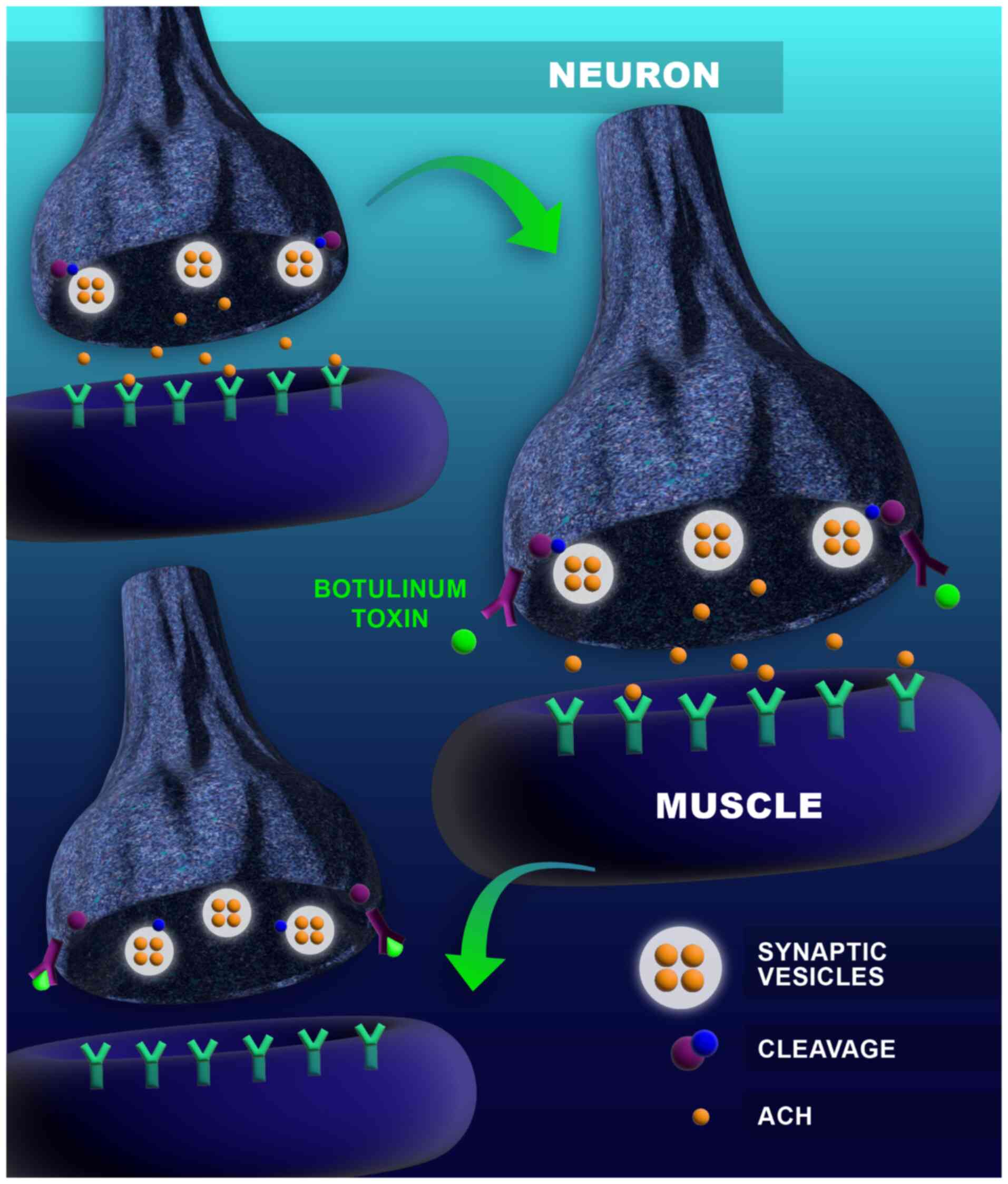
The mechanism of action relies on blocking
acetylcholine at the neuromuscular junction, which blocks the
transmission of the nervous impulse resulting in secondary flaccid
paralysis (25,27). The average lethal dose
(LD50) is 1 ng of toxin per a Kg of body weight
(22); it is, however, relatively
rare (11,13,28).
This intoxication is caused by the ingestion of Cl.
botulinum neurotoxins that have already been formed in the
contaminated food (9). Foodborne
botulism is not spread from one individual to another (11). The mechanism of action of the toxin
is represented in Fig. 1.
Visual difficulty (blurred or double vision),
dysphagia and dry mouth are usually the first symptoms. These
symptoms can also lead to symmetrical flaccid paralysis (3,23). The
original symptoms may also involve nausea, vomiting, abdominal pain
and diarrhea (7). Fever is not
observed unless infectious complications occur (3,7). After
the onset of neurological symptoms, constipation is common.
Symptoms usually occur within 12-36 h after exposure to toxins
(from 6 h to 8 days) (5,7,23).
Extensive paralysis of respiratory muscles may lead to respiratory
insufficiency and death within 3 days if no supportive therapy is
received (7,23). Death may occur within 20 h to 1 week
following the consumption of suspicious food (12). The removal of contaminated food from
the intestine either by subclasses or by inducing vomiting is
considered critical. Ventilatory support may be deemed necessary
for 2-8 weeks, although cases of ventilatory support for a longer
period have been reported (22).
The most typical signs and symptoms of foodborne botulism are
described in Table I.
 | Table ITypical signs and symptoms of
foodborne botulism with toxin productiona. |
Table I
Typical signs and symptoms of
foodborne botulism with toxin productiona.
| Medical
history | Negative for
infectious diseases |
|---|
| First clinical
evaluation | Normal mental
status, afebrile |
| | Gastrointestinal
manifestations |
| | Xerostomia/sore
throat and/or dysphagia |
| | Weakness |
| | Normal cardiac
activity (rarely bradycardia) |
| | Anxiety |
| Physical
examination | Symmetric
descending neurological alterations |
| | Symmetric
oculobulbar signs |
Treatment in addition to supportive care, intubation
and mechanical ventilatory support when deemed necessary may
include the administration of a botulinum antitoxin. Early
administration of antitoxin may mitigate the extent and severity of
paralysis, and in some cases, may prevent respiratory paralysis and
in other cases, reduce the duration of mechanical support and
hospitalization in intensive care (29,30).
The administration of antibiotics is not indicated in foodborne
botulism (7,22). Vaccination with a toxoid form of
botulinum toxin applies to all types of botulism (29,31).
Recognizing an agent that causes the disease is the
necessary condition for the choice of appropriate therapy. PCR is
the primary method for the confirmation of Cl. botulinum
presence or absence (32,33). Another opportunity for detecting
botulism is to detect the residual levels of the toxin in the blood
plasma or blood. Though direct assay of botulinum toxin appears to
be an ideal means of diagnosis of botulism, it is not simple to
achieve in reality. Any method relying on direct detection of
botulinum toxin has very low limits of detection. A number of
immunochemical tests such as ELISA (34), chromatography, mass spectrometry
(35) and single-step test strips
(lateral flow immunochromatographic assays) (36) are suitable analytical methods for
botulinum toxin detection.
3. Clostridium botulinum as a
pathogen
Clostridium botulinum is an anaerobic
Gram-positive spore-forming bacillus (10). Bacterial spores are heat-resistant
and can survive in foods even after intense heat-treatment.
According to the antigenic specificity of the toxin produced by
each bacterial strain seven types (A-G) have been identified
(Clostridium Argentinense) (37). Types A, B, E and F are involved in
human botulism. Types C and D are involved in the majority of
botulism events in animals (29,38).
Some associated Clostridium species, such as Clostridium
baratii and Clostridium butyricum, can also produce
botulinum toxins (3,23,39).
All toxins cause a clinically similar syndrome (3).
The strains that produce the toxins can be divided
into proteolytic and nonproteolytic strains (40). The optimum temperature for toxin
development and production by proteolytic strains is 35˚C, and for
nonproteolytic it is 26-28˚C. It is worth noting that
nonproteolytic strains B, E and F can produce toxins at cooler
temperatures (3-4˚C) (40,41). Its bacterial spores are widely
spread within the environment and can be traced in the dust, on the
ground, in the water that has not undergone treatment and in the
peptic system of animals and fishes (5,37,42).
They can be found in cultivated and forest lands, river, lake and
coastal sediments, as well as in gills and the internal organs of
crabs and other shellfishes (9).
Usually, the spores survive cooking and food
preparation. The combination of environmental conditions however,
that allow for spore formation (anaerobic environment, non-acidic
pH and low salt and sugar concentration) are rarely observed in
food, which explains the low number of foodborne botulism cases
(29,39). The marginal conditions for bacterial
growth are indicated in Table
II.
 | Table IIMarginal growth conditions for Cl.
botulinuma. |
Table II
Marginal growth conditions for Cl.
botulinuma.
| Growth
conditions | Proteolytic
strains | Nonproteolytic
strains |
|---|
| Minimum water
activity value (AW) | 0.935 | 0.970 |
| Minimum pH
value | 4.6 | 5.0 |
| Maximum pH
value | 9.0 | 9.0 |
| Maximum NaCl
concentration | 10% | 5% |
| Minimum temperature
value | 10-12˚C | 3.3˚C |
| Maximum temperature
value | 50˚C | 45˚C |
4. Clostridium botulinum in
foods
Any food that facilitates spore germination and
botulinum toxin production allows spores to survive through food
preparation, if not subjected to heat treatment before consumption
(43). Almost every type of food
lacking sufficient acidity (pH >4.6) can lead to the development
and production of bacterial toxins. The toxins are typically
sensitive and can be destroyed by heating to 85˚C for 5 min
(5). Spores are destroyed in
conditions of wet sterilization at 120˚C for 5 min (9). Spore germination and toxin production
are achieved when foods are exposed to conditions of an anaerobic
environment, pH >4.6, low salt and sugar concentration and
temperatures from 4-12˚C (21).
Most cases of foodborne botulism are related to the
consumption of canned foods (such as tuna fish), homemade foods or
containerized food available on the market (such as canned
vegetables) (15).
5. Epidemiological data on foodborne
botulism
Foodborne botulism occurs worldwide (37). It is a disease with a high mortality
rate. Before 1950, 60% of botulinum cases in America resulted in
death. Later the mortality rate was reduced to <10%, due to the
production of antitoxins and the provision of intensive supportive
care (44). More recent data in the
United States (2016) refer to 205 confirmed cases of botulinum
intoxication, 14% of which were associated with foodborne botulism
(45). The majority of botulinum
cases that have been reported in the United States are related to
insufficiently processed canned foods, home-canned foods and
occasionally products that are available on the market (37).
In Europe, the botulinum rate is generally low with
~200 cases per year (0.03 cases per 100,000 persons). The highest
rate of cases over the last 10 years has been reported in Poland
and Lithuania (13). Recent cases
of foodborne botulism have been reported in 2015 in France and
Slovakia. In France 3 botulinum cases were reported, involving the
consumption of Bolognese sauce at a restaurant and one case
involved Cl. baratii in minced meat. In Slovakia botulinum
toxin (type A) was detected in three hummus products (46). In Greece botulism constitutes one of
the rarest diseases for which declaration is mandatory, and the
statistical data from the National Agency for Public Health (ΕΟΔΥ)
did not reveal any cases of food-borne botulism in the recent years
(47).
6. Cheese making data
Codex Alimentarius defines cheese as the fresh or
mature product obtained by draining following coagulation of the
whole, partly skimmed or nonfat milk or buttermilk, or a mixture of
some or all of these products (48). The Greek Food and Drinks Code
defines ripened cheeses that are produced from milk, as products
obtained from curd-ripening (maturation) free of whey-to the
desired degree each time-that have been prepared by using rennet
(rennet effect) or other enzymes that function accordingly in milk,
partly skimmed milk or a mixture of those, and a mixture of those
with milk cream (49,50).
Cheese products may contain several types of
microorganisms at all stages from manufacturing to the consumption
thereof. These may be lactic-acid bacteria (Lactococcus,
Streptococcus, Lactobacillus) developed during the
production of cheese and used at the stages of fermentation and
ripening (maturation), microorganisms that may enter milk from
environmental contamination (Micrococcus,
Streptococcus, Proteus, Pseudomonas,
Bacillus etc.), yeasts and moulds. The microbial population
may undergo constant qualitative and quantitative changes depending
on several factors, such as water activity, microbial competition,
degree of acidity, temperature and salt concentration. Under normal
conditions of maturation, the initial microbial load steadily
decreases. If the milk used for cheese-making is not pasteurized,
the rich microbial flora can be transmitted to the product. If the
changes in the microbial flora at the ripening stage for cheeses
that are ripened are not constant and they do not ensure cheese
sanitation, then the consumption of this cheese may prove dangerous
to the consumer (50). Only
high-temperature pasteurization can neutralize types a and b
botulinum toxin (51).
A low number of botulism cases related to dairy
products, produced domestically or at food-businesses, have been
reported. Milk contamination may originate from the manufacturing
environment or while adding ingredients that may transmit Cl.
botulinum spores. The maintenance conditions of dairy products
also constitutes a key factor involved in the possible development
of vegetative forms (of microorganisms) and the production of
toxins (51). Cheese and other
dairy products are the cause of botulism, (<1% in the United
States) (21,52). Τhe rare presence of botulinum spores
in dairy products has been observed. In a study relating to the
presence of Cl. botulinum spores, involving a limited number
of cheese samples, such as Edam and Cheddar, as well as spreadable
cheese (40 and 10 samples, respectively) the results were all
negative (53,54).
7. A brief history of foodborne botulism
outbreaks transmitted by cheese products
The cases of botulism that have been reported
(Table III) occurred in France
and Switzerland following the consumption of ripened cheese, such
as Brie, contaminated with Cl. botulinum toxin type Β. The
epidemiological investigation of these cases demonstrated the
involvement of the straw on which the products were ripened
(54). Cheese contamination from
straw was investigated experimentally by Blllon et al
(55) in a research paper published
in 1980. The storage of soft cheese on inoculated straw (1,000
spores' type Β/cm2) resulted in the production of the
toxin on cheese surfaces, but not in the cheese body. The toxin was
found to be unstable and disappeared in the subsequent ripening
stages.
 | Table IIIBotulinum cases from the
consumption of cheese products between 1912-2019. |
Table III
Botulinum cases from the
consumption of cheese products between 1912-2019.
| First author,
year | Year | Type of Cl.
botulinum | Product
involved | Area | Outbreaks | Cases | Deaths | (Refs.) |
|---|
| Collins-Thompson
and Wood, 1992 | 1912 | - | Cottage cheese | California,
USA | 1 | 7 | 2 | (54) |
| | 1914 | - | Neufchatel | California,
USA | 1 | 2 | 2 | |
| | 1914 | Β | Cottage cheese | New York, USA | 1 | 3 | 3 | |
| | 1935 | - | Curd cheese | California,
USA | 1 | 3 | 0 | |
| | 1939 | Α | Cottage | New York, USA | 1 | 3 | 0 | |
| | 1951 | Β | Liederkranz | California,
USA | 0 | 1 | 1 | |
| | 1973 | Β | Brie | Marseilles,
France | 1 | 22 | - | |
| | 1973 | Β | Brie | Lausanne,
Switzerland | 1 | 43 | - | |
| | 1974 | Α | Cheese spread | Buenos Aires,
Argentina | 1 | 6 | 3 | |
| Townes et
al, 1996 | 1993 | Α | Cheese sauce | Georgia, USA | 1 | 8 | 1 | (57) |
| Aureli et
al, 1996 | 1996 | Α | Mascarpone | Calabria and
Campania, Italy | 4 | 8 | 1 | (58) |
| Pourshafie et
al, 1998 | 1997 | Α | Intangible | Northern Province,
Iran | 1 | 27 | 1 | (60) |
| Rosen et al,
2018 | 2018 | - | Nacho cheese
sauce | California,
USA | 1 | 10 | 1 | (71) |
| Kamaloddini and
Kheradmand, 2021 | 2019 | - | Local dairy
cheese | Iran | 0 | 1 | - | (15) |
| Total | | | | | 15 | 144 | 15 | |
An outbreak of botulism from toxin type Α, involving
a commercially available, spreadable onion cheese, was reported in
Buenos Aires. Specific intrinsic parameters, such as water activity
(aw=0.97) and pH value (5.6-6.1) of this product facilitated
germination and production of Cl. botulinum toxins type A
(54). An experimental study by
Briozzo et al (56)
indicated that substates with similar pH values to those of
spreadable cheeses facilitate the development of Cl.
botulinum and the production of the toxin at a lower water
activity value of water activity (aw)=0.965 and not at 0.949.
A rather rare outbreak of foodborne botulism cases
was observed in 1993 in Georgia, United States, resulting from the
consumption of cheese sauce at a restaurant, contaminated during
handling. After the laboratory investigation of the suspicious
food, it was found that the contaminated food was a canned cheese
sauce. A laboratory investigation of two samples of containerized
cheese from the same batch showed negative results. Cl.
botulinum type Α was detected in cultures obtained from the
suspected can. Experimental inoculation studies for this particular
cheese sauce demonstrated the development of botulinum toxin at an
ambient temperature (22˚C) but not at refrigeration temperatures
(5˚C) (57).
In 1996, eight cases of botulism from Cl.
botulinum type A involving an industrially produced Mascarpone
cheese (spreadable cheese) were reported in South Italy (Campania
and Calabria). The patients consumed the suspicious cheese itself
or as an additive to a tiramisu dessert, which had not been
subjected to heat-treatment. The toxin was detected in samples from
the remains of tiramisu that had been consumed by the patients and
in mascarpone cheese samples that were collected from the points-of
sale that supplied the cheese to the other patients (58). Experimental analysis on inoculation
of bacterial spores in Mascarpone cheese demonstrated that
non-compliance with temperature regulations throughout maintenance
favors the production of this toxin (59).
Studies on botulism from Cl. botulinum type Α
caused by the consumption of cheese were also conducted in Iran.
Toxin type Α was detected in cheese and 37% of serum and stool
samples from the patients and the bacterium were isolated from
cultures for clinical samples and cheese (60). Furthermore a case of foodborne
botulism was recently observed in Iran caused by the consumption of
a local product (15).
8. Control measures
Control measures for the manufacturing
of cheese products
The majority of botulism cases that have been
observed in dairy products are related to cheese or cheese products
(20). Cheese products are not
regularly involved in botulism incidents; it is, however, necessary
to take into consideration some inhibitory factors throughout the
production process. European regulation allows for the production
of some cheese products from unpasteurized milk (61). The intensity of heat-treatment
throughout the production and cheese maintenance temperature
constitute basic inhibitory factors (52). An apparent cause of botulism cases
that has been observed is inadequate cooling. Unlike milk, which
can be easily spoiled without cooling, cheese products can be
spoiled without obvious macroscopic features and maintaining the
refrigeration chain may not be deemed necessary by consumers or
sales persons in order to ensure safety and quality of products
(20). Water activity and salinity
factors also inhibit microorganisms. The development of proteolytic
strains of Cl. botulinum is inhibited at aw values
<0.935, while for non-proteolytic strains of Cl.
botulinum the corresponding minimum growth rate is 0.937. The
salinity levels for the production of cheese may also have a
negative influence on the production of toxins, but it does not
constitute a sufficient means for the prevention of toxin
production alone (20,52). Various additives are extensively
used in foods to control the risk of botulism. The main additives
used are nitrates and nitrites (52). More specifically, it has been
observed that the addition of nitrites to curd prevents the risk of
botulinum toxin growth, therefore the Food and Agriculture
Organization/World Health Organization allows the addition of
nitrates (≤200 ppm) in the production of several types of cheese
(50). European legislation allows
the use of nitrites and nitrates in some dairy products (i.e.,
matured/ripened cheese and whey, as well as cheese products, so
that maximum permitted levels are defined as amounts added during
manufacture and not as amount of residue in the final product
(62). The Greek Food Code limits
the use of potassium nitrate [E251] and sodium nitrate in hard,
semi-hard and semi-soft cheeses, as well as in dairy-based cheese
analogues, and sets the maximum amount that may be added during
manufacture to 150 mg/kg (49).
Despite the advantages from their addition to products, their use
has raised concerns since the 1970'sdue to the fact that
nitrates/nitrites react with secondary amines resulting to the
formation of N-nitrosamines, substances that exhibit carcinogenic
activity (63,64).
Control measures regarding home
production of food
Due to the emergence of several epidemic outbreaks,
originating primarily from the consumption of home-made canned food
(65), it is recommended to follow
the directives below: Canned foods, including those produced for
private domestic consumption must be subjected to inspection before
use. Moreover, container swelling/bulging, damaged containers and
foods with an abnormal flavor should not be consumed as they are
indicative of bacterial growth that produces gases inside the
package.
If they are intended for consumption, canned foods
of low acidity must be heated to at least 80˚C for 30 min. The
products that contain oils with garlic or herbs must be properly
cooled during storage. For the safe preparation of canned products,
and in order to ensure that all appropriate requirements of time,
temperature and pressure are met to prevent the development of
vegetative forms and spores of the microorganism; thus it is
necessary to use pressure-cooking equipment. Spores can be
inactivated by heat at extremely high temperatures of 116-121˚C
(66).
Although foodborne botulism is not regularly
associated with canned products available at the market, their
content must not be consumed if expired or the packaging is
damaged. If the canned foods are produced for private domestic
consumption, it is suggested to use nitrites in brine solution to
reduce the growth of Cl. botulinum. During food storage the
appropriate temperatures must be met. Cooked/baked foods must be
kept warm (>57˚C) and cooled (<5˚C) in order to prevent
germination of spores and the consecutive development of toxins
(67). Conditions of personal and
environmental hygiene must be preserved (68). For the best implementation of
preventative measures, hygiene training of the food handlers
regarding the home preparation of food and especially canned food
is required to understand the conditions of destruction of the
spores of the bacterium (14).
9. Conclusions
Foodborne botulism remains an important public
health concern. Although rarely observed, it remains important for
environmental health. Domestically canned foods are still among the
primary causes of the manifestation of the disease. It is of prime
importance that suspected foodborne botulism cases are immediately
reported to public health services.
Cheese products are not regularly involved in the
occurrence of botulism, due to high risk of the disease; however,
it is necessary to take under consideration some bacterial growth
inhibitors throughout the production process. The intensity of heat
treatment during production, the regulation of acidity and the
maintenance of cheese products' temperatures are considered key
inhibitory factors. Water activity and salinity factors are
inhibitory to the development of Cl. botulinum, but salinity
is not a sufficient means for the prevention of toxin production
alone. Moreover, the use of nitrates and nitrites prevents the risk
of botulinum toxin formation throughout the preparation of several
cheese products. Therefore, further research is required to
evaluate the development of spore-forming bacteria in different
temperatures regarding their composition, packaging and storage, to
improve the safety of cheese products.
Finally, given the severity of cases of botulism,
even one incident is considered as an emergency for public health
as it may signal the beginning of an outbreak. Public health
authorities at the national and international level must be
informed in the case of suspected foodborne botulism to investigate
possible causes, and to examine the need for further research and
possible precautionary measures.
Acknowledgements
The authors would like to express special thanks to
Mrs. Asimina Velecheri (Department of Biomedical Sciences, Sector
of Medical Laboratories, University of West Attica, Athens, Greece)
for the 3D graphic design of Fig.
1.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
EC conducted the review, conceived the subject of
review based on the literature, collected the relevant data and
wrote the manuscript. PP and DK drafted and reviewed the
manuscript. IM and AM edited and reviewed the manuscript. ACL and
NK provided scientific input. Data authentication is not
applicable. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
World Health Organization (WHO): Food
safety Key facts. WHO, Geneva, 2017.
|
|
2
|
World Health Organization (WHO): Five keys
to safer food manual. WHO, Geneva, 2006.
|
|
3
|
Chin J (ed): Control of communicable
diseases manual. 17th edition. American Public Health Association,
Washington, DC, p624, 2000.
|
|
4
|
World Health Organization (WHO): Foodborne
disease outbreaks: Guidelines for investigation and control. WHO,
Geneva, 2008.
|
|
5
|
Vagiona-Arvanitidou M: Hygiene. 2nd
edition. University Studio Press, Thessaloniki, pp384-390,
2009.
|
|
6
|
Trichopoulos D: Preventive medicine.
Health education, social medicine, public hygiene. Parisianou
Maria, Athens, 1986. ISBN: 9789609025409.
|
|
7
|
Robinson JP: Public health response to
biological and chemical weapons: WHO guidance. World Health
Organization, Geneva, 2004.
|
|
8
|
Madsen JM: Bio Warfare and Terrorism:
Toxins and Other Mid-Spectrum Agents. In: Encyclopedia of
Toxicology. 2nd edition. Academic Press, pp273-279, 2005.
|
|
9
|
Katsougiannopoulos VC: Hygiene and social
medicine-Social medicine. Kyriakidis Bros-Publications S.A,
Thessaloniki, 2003.
|
|
10
|
Kalkani-Bousiakou H: General microbiology,
2007. ISBN: 9789602868997.
|
|
11
|
World Health Organization (WHO):
Botulism-key facts. WHO, Geneva, 2018.
|
|
12
|
Roukas C: Population hygiene. Organization
for the Publishing of Textbooks, Athens, Greece, 1994. http://libsearch.teiep.gr/Record/00462127.
|
|
13
|
European Centre for Disease Prevention and
Control: Facts about botulism, 2020. europa.eu.
|
|
14
|
Katsougiannopoulos VC: Hygiene and social
medicine-hygiene. 2nd edition. Kyriakidis Bros-Publications S.A,
Thessaloniki, 2003.
|
|
15
|
Kamaloddini MH and Kheradmand HR: A
foodborne botulism occurrence in Mashhad: Clostridium
botulinum in local cheese. J Emerg Pract Trauma. 7:66–68.
2021.
|
|
16
|
Panagou EZ, Nychas GJE and Sofos JN: Types
of traditional Greek foods and their safety. Food Control.
29:32–41. 2013.
|
|
17
|
Possas A, Bonilla-Luque OM and Valero A:
From cheese-making to consumption: Exploring the microbial safety
of cheeses through predictive microbiology models. Foods.
10(355)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
News Desk: Primula recalls cheese due to
Clostridium botulinum. Food Safety News, 2020. https://www.foodsafetynews.com/2020/06/primula-recalls-cheese-due-to-clostridium-botulinum/.
|
|
19
|
Food Standards Agency (FSA) UK: Primula
recalls all primula cheese tubes because of Clostridium
botulinum, 2020. https://www.food.gov.uk/print/pdf/node/4361.
|
|
20
|
Lindström M, Myllykoski J, Sivelä S and
Korkeala H: Clostridium botulinum in cattle and dairy
products. Crit Rev Food Sci Nutr. 50:281–304. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sobel J: Botulism. Clin Infect Dis.
41:1167–1173. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
National Public Health Organization
(EODY): Botulism, 2021. https://eody.gov.gr/disease/allantiasi/.
|
|
23
|
Centers for disease control and prevention
(CDC) US: Botulism, 2020. https://www.cdc.gov/botulism/index.html.
|
|
24
|
Sugiyama H: Clostridium botulinum
neurotoxin. Microbiol Rev. 44:419–448. 1980.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Dan Spinu A, Gabriel Bratu O, Cristina
Diaconu C, Maria Alexandra Stanescu A, Bungau S, Fratila O,
Bohiltea R and Liviu Dorel Mischianu D: Botulinum toxin in low
urinary tract disorders-over 30 years of practice (Review). Exp
Ther Med. 20:117–120. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Rosen O, Feldberg L, Yamin TS, Dor E,
Barnea A, Weissberg A and Zichel R: Development of a multiplex
Endopep-MS assay for simultaneous detection of botulinum toxins A,
B and E. Sci Rep. 7(14859)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jankovic J: Botulinum toxin: State of the
art. Mov Disord. 32:1131–1138. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sobel J, Tucker N, Sulka A, McLaughlin J
and Maslanka S: Foodborne botulism in the United States, 1990-2000.
Emerg Infect Dis. 10:1606–1611. 2004.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Charizani F: Infections and preventive
measures. Papazisis, Athens, Greece, 2004. ISBN: 960-02-1804-8.
|
|
30
|
Rao AK, Sobel J, Chatham-Stephens K and
Luquez C: Clinical guidelines for diagnosis and treatment of
botulism, 2021. MMWR Recomm Rep. 70:1–30. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Khouri JM, Motter RN and Arnon SS: Safety
and immunogenicity of investigational recombinant botulinum
vaccine, rBV A/B, in volunteers with pre-existing botulinum toxoid
immunity. Vaccine. 36:2041–2048. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Maikanov B, Mustafina R, Auteleyeva L,
Wiśniewski J, Anusz K, Grenda T, Kwiatek K, Goldsztejn M and
Grabczak M: Clostridium botulinum and clostridium
perfringens occurrence in Kazakh honey samples. Toxins (Basel).
11(472)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mariano V, Nardi A, Gradassi S, De Santis
P, Anniballi F, Bilei S, Scholl F, Auricchio B, Bielli C, Culicchi
M and Casali De Rosa GL: A severe outbreak of botulism in cattle in
Central Italy. Vet Ital. 55:57–62. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sarita R, Ponmariappan S, Sharma A, Kamboj
DV and Jain AK: Development of immunodetection system for botulinum
neurotoxin serotype E. Indian J Med Res. 147:603–610.
2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kalb SR, Baudys J and Barr JR: Detection
of the HA-33 protein in botulinum neurotoxin type G complex by mass
spectrometry. BMC Microbiol. 15(227)2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ching KH, Lin A, McGarvey JA, Stanker LH
and Hnasko R: Rapid and selective detection of botulinum neurotoxin
serotype-A and -B with a single immunochromatographic test strip. J
Immunol Methods. 380:23–29. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Adams MR and Motarjemi Y: Basic food
safety for health workers. World Health Organization, 1999.
|
|
38
|
Food and Drug Administration (FDA) U.S.:
Hazard analysis and risk-based preventive controls for human food:
Draft guidance for industry, 2016. https://www.fda.gov/media/100002/download.
|
|
39
|
Centers for Disease Control and Prevention
(CDC): Botulism in the United States, 1899-1996. Handbook for
epidemiologists, clinicians, and laboratory workers. CDC, Atlanta,
GA, 1998.
|
|
40
|
Poggas N: Environmental microbiology,
2007. http://postgraduate.med.uth.gr/gr/graduate/grad1/docs/08_Attachment_28.zip.
|
|
41
|
Food and Drug Administration (FDA): Fish
and fishery products hazards and controls guidance. Clostridium
botulinum Toxin Formation, 2001. https://www.fda.gov/food/laboratory-methods-food/bam-chapter-17-clostridium-botulinum#:~:text=Clostridium%20botulinum%20is%20an%20anaerobic,toxin%2Dcontaining%20foods%20are%20ingested.
|
|
42
|
European Centre for Disease Prevention and
Control (ECDC): Facts about botulism. European Centre for Disease
Prevention and Control, 2017. https://www.ecdc.europa.eu/en/botulism/facts.
|
|
43
|
U.S. Food and Drug Administration (FDA):
Bad bug book. Foodborne pathogenic microorganisms and natural
toxins handbook Listeria monocytogenes. FDA, Silver Spring, MD,
2006. http://www.cfsanfda.gov/~mow/chap6.html.
|
|
44
|
Jackson KA, Mahon BE, Copeland J and Fagan
RP: Botulism mortality in the USA, 1975-2009. Botulinum J. 3:6–17.
2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Centers for Disease Control and Prevention
(CDC): National botulism surveillance summary 2016. CDC, Atlanta,
GA, 2016.
|
|
46
|
European Centre for Disease Prevention and
Control (ECDC): Botulism-annual epidemiological report for 2015,
2018. https://www.ecdc.europa.eu/en/publications-data/botulism-annual-epidemiological-report-2015.
|
|
47
|
National Public Health Organization:
Epidemiological data of botulism in Greece 2004-2020, 2021.
https://eody.gov.gr/wp-content/uploads/2021/09/allantiasi-2004-2020-gr.pdf.
|
|
48
|
Codex Alimentarius: General standard for
cheese (Codex-Stan 283-1978). Codex Alimentarius: Rome, 1978.
https://www.fao.org/fao-who-codexalimentarius/sh-proxy/es/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B283-1978%252FCXS_283e.pdf.
|
|
49
|
General Chemical State Laboratory of
Greece. Greek food code-cheese products, 2014. https://www.aade.gr/sites/default/files/2020-03/83-iss3.pdf.
|
|
50
|
Mantis A, Papageorgiou D, Fletouris D and
Angelidis A: Hygiene and technology of milk and its products.
Kyriakidis Bros-Publications S.A, Thessaloniki, 2018.
|
|
51
|
Rasetti-Escargueil C, Lemichez E and
Popoff MR: Public health risk associated with botulism as foodborne
zoonoses. Toxins (Basel). 12(17)2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Doyle CJ, Gleeson D, Jordan K, Beresford
TP, Ross RP, Fitzgerald GF and Cotter PD: Anaerobic sporeformers
and their significance with respect to milk and dairy products. Int
J Food Microbiol. 197:77–87. 2015.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Insalata NF, Witzeman SJ, Fredericks GJ
and Sunga FC: Incidence study of spores of clostridium
botulinum in convenience foods. Appl Microbiol. 17:542–544.
1969.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Collins-Thompson DL and Wood DS: Control
in dairy products. In: Clostridium botulinum Ecology and Control in
Food. Hauschild AHW and Dodds KL (eds). Marcel Dekker, New York,
NY, pp261-277, 1992.
|
|
55
|
Blllon J, Guérin J and Sebald M: Etude de
la toxinogénèse de clostridium botulinum type B au cours de
la maturation de fromages à pâte molle. Lait. 60:329–342. 1980.
|
|
56
|
Briozzo J, de Lagarde EA, Chirife J and
Parada JL: Clostridium botulinum type A growth and toxin
production in media and process cheese spread. Appl Environ
Microbiol. 45:1150–1152. 1983.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Townes JM, Cieslak PR, Hatheway CL,
Solomon HM, Holloway JT, Baker MP, Keller CF, McCroskey LM and
Griffin PM: An outbreak of type A botulism associated with a
commercial cheese sauce. Ann Intern Med. 125:558–563.
1996.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Aureli P, Franciosa G and Pourshaban M:
Foodborne botulism in Italy. Lancet. 348(1594)1996.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Franciosa G, Pourshaban M, Gianfranceschi
M, Gattuso A, Fenicia L, Ferrini AM, Mannoni V, De Luca G and
Aureli P: Clostridium botulinum spores and toxin in
mascarpone cheese and other milk products. J Food Prot. 62:867–871.
1999.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Pourshafie MR, Saifie M, Shafiee A,
Vahdani P, Aslani M and Salemian J: An outbreak of food-borne
botulism associated with contaminated locally made cheese in Iran.
Scand J Infect Dis. 30:92–94. 1998.PubMed/NCBI View Article : Google Scholar
|
|
61
|
European Union: Commission Regulation (EC)
No 2073/2005 on microbiological criteria for foodstuffs, 2005.
|
|
62
|
European Commission: Regulation (EC) No
1333/2008 of the European parliament and of the council of 16
December 2008 on food additives. European Commission, Brussels,
2008.
|
|
63
|
Preussmann R and Stewart BW: N-nitroso
Carcinogens. In: Chemical carcinogens. Searle CE (ed). American
Chemical Society, Washington, DC, pp643-828, 1984.
|
|
64
|
Tu A (ed): Handbook of natural toxins:
Food poisoning. Routledge, 2018.
|
|
65
|
Saeidi S, Dadpour B, Jarahi L, Ghamsari AA
and Nooghabi MJ: Clinical predictive values in botulism: A 10-year
survey. Indian J Crit Care Med. 25:411–415. 2021.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Aminzadeh Z, Vahdani P and Mirzaei J: A
survey on 80 cases of botulism and its clinical presentations as a
public health concern. Iran J Clinic Infect Dis. 2:77–81. 2007.
|
|
67
|
Centers for Disease Control and Prevention
(CDC): Home-canned foods. Protect Yourself from Botulism. CDC,
Atlanta, GA, 2019.
|
|
68
|
Schneider KR, Chang A and Goodrich RM:
Preventing foodborne illness: Clostridium botulinum.
University of Florida Institute of Food and Agricultural Sciences
(IFAS) Extension, 2011. https://edis.ifas.ufl.edu/pdf/FS/FS10400.pdf.
|
|
69
|
Proverbio MR, Lamba M, Rossi A and Siani
P: Early diagnosis and treatment in a child with foodborne
botulism. Anaerobe. 39:189–192. 2016.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Lonati D, Schicchi A, Crevani M, Buscaglia
E, Scaravaggi G, Maida F, Cirronis M, Petrolini VM and Locatelli
CA: Foodborne botulism: Clinical diagnosis and medical treatment.
Toxins (Basel). 12(509)2020.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Rosen H, Kimura A, Mukhopadhyay R, Nash J,
Boetzer J, Poe A, Tecle S, McAuley K, Kasirye O, Garza A, et al:
1721. An outbreak of botulism associated with nacho cheese sauce
from a gas station in California. Open Forum Infect Dis. 5 (Suppl
1)(S53)2018.
|